p38γ MAPK Inflammatory and Metabolic Signaling in Physiology and Disease
Abstract
1. Introduction
2. p38γ Signaling in Inflammation
3. p38γ Signaling in Metabolism
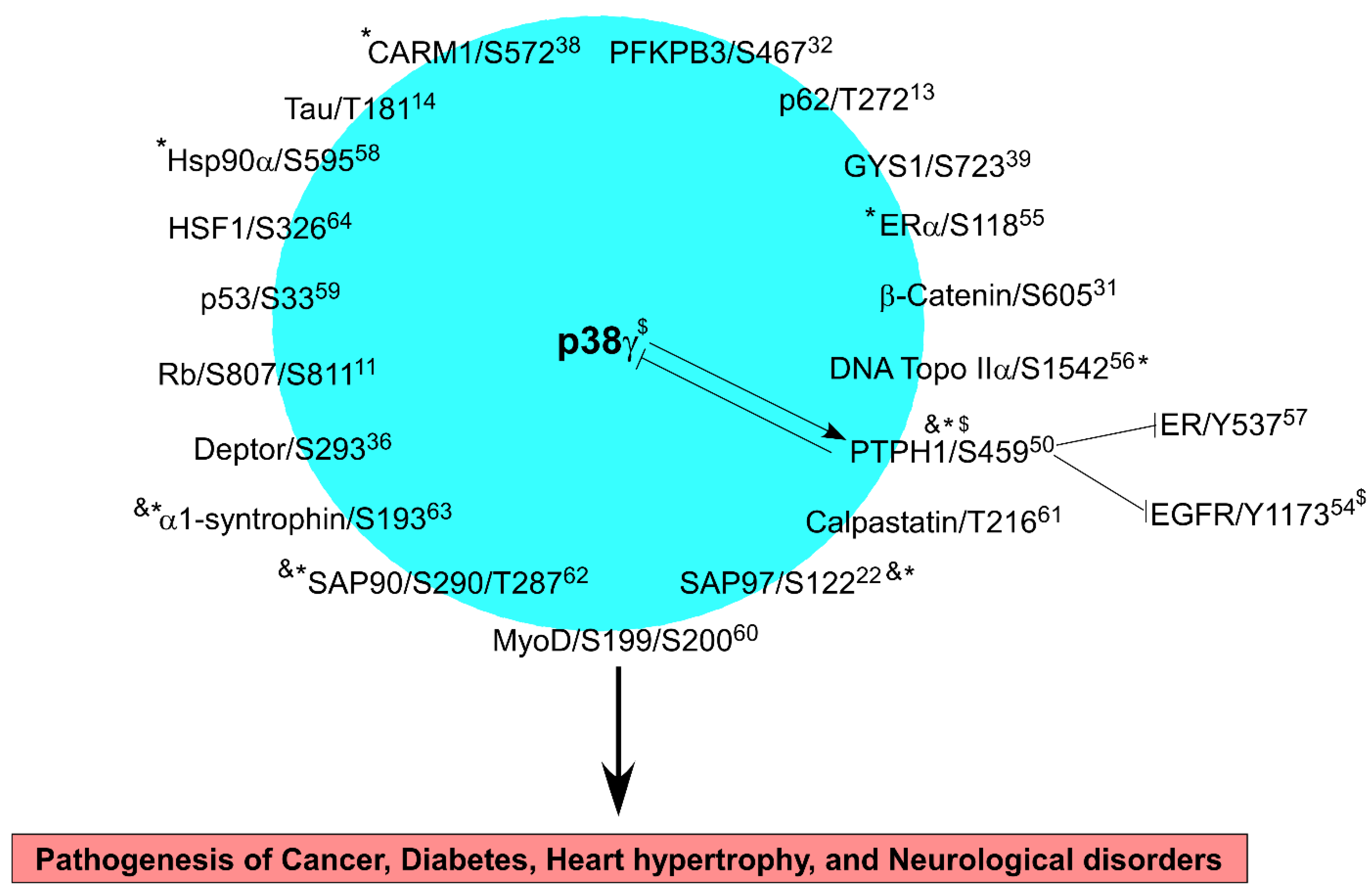
4. p38γ Signaling to Its Substrates in Physiology and Diseases
5. p38γ Specific Inhibitors
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ono, K.; Han, J. The p38 signal transduction pathway. Activation and function. Cell. Sign. 2000, 12, 1–13. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 1–21. [Google Scholar] [CrossRef]
- Qi, X.M.; Wang, F.; Chen, G. p38 Gamma MAPK Encyclopedia of Signaling Molecules, 2nd ed.; Choi, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3718–3727. [Google Scholar]
- Cuenda, A.; Sanz-Ezquerro, J. p38g and p38d: From spectators to key physiological players. Trends Biochem. Sci. 2017, 42, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, R.; Qi, X.; Borowicz, S.; Choubey, D.; Schultz, R.M.; Han, J.; Chen, G. p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun: The determinant role of the isoforms in the p38 MAPK signal specificity. J. Biol. Chem. 2003, 278, 4831–4839. [Google Scholar] [CrossRef]
- Loesch, M.; Chen, G. The p38 MAPK stress pathway as a tumor suppressor or more? Front. Biosci. 2008, 13, 3581–3593. [Google Scholar] [CrossRef]
- Qin, J.; Xin, H.; Qi, X.; Chen, G. Isoform-specific and cell/tissue-dependent effects of p38 MAPKs in regulating inflammation and inflammation-associated oncogenesis. Front. Biosci. Landmark 2022, 27, 031. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Ulevitch, R.J.; Han, J. The primary structure of p38g: A new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 1996, 228, 334–340. [Google Scholar] [CrossRef]
- Lechner, C.; Zahalka, M.A.; Giot, J.; Moller, N.P.; Ullrich, A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc. Natl. Acad. Sci. USA 1996, 93, 4355–4359. [Google Scholar] [CrossRef]
- Mertens, S.; Craxton, M.; Goedert, M. SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Lett. 1996, 383, 273–276. [Google Scholar] [CrossRef]
- Tomas-Loba, A.; Manieri, E.; Gonzalez-Teran, B.; Mora, A.; Leiva-Vega, L.; Santamans, A.M. p38g is essential for cell cycle progression and liver tumorigenesis. Nature 2019, 568, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, F.; Mortensen, M.; Wertz, R.; Chen, G. Targeting an oncogenic kinase/phosphatase signaling network for cancer therapy. Acta Pharm. Sin. B 2018, 8, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Molinaro, A.; Stahlman, M.; Khan, M.T.; Schmidt, C.; Manneras-Holm, L. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018, 175, 947–961. [Google Scholar] [CrossRef]
- Ittner, A.; Chua, S.W.; Bertz, J.; Volkerling, A.; van der Hoven, J.; Gladbach, A. Site-specific phosphorylation of tau inhibits amyloid-b toxicity in alzheimer’s mice. Science 2016, 354, 904–908. [Google Scholar] [CrossRef]
- Hale, K.K.; Trollinger, D.; Rihanek, M.; Manthey, C.L. Differential expression and activation of p38 mitogen-activated protein kinase a, b, g, and g in inflammatory cell lineages. J. Immun. 1999, 162, 4246–4252. [Google Scholar] [CrossRef]
- Korb, A.; Tohidast-Akrad, M.; Cetin, E.; Axmann, R.; Smolen, J.; Schett, G. Differential tissue expression and activation of p38 MAPK a, b, g, and d isoforms in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Cohen, P.; Buee-Scherrer, V.; Goedert, M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38). EMBO J. 1997, 16, 295–305. [Google Scholar] [CrossRef]
- Long, D.L.; Loeser, R.F. p38g mitogen-activated protein kinase suppresses chondrocyte production of MMP-13 in response to catabolic stimulation. Osteoarthr. Cartil. 2010, 18, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yuan, W.; Fujita, N.; Wang, J.; Wang, H.; Shapiro, I.M.; Risbud, M.V. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kB. Am. J. Pathol. 2013, 182, 2310–2321. [Google Scholar] [CrossRef]
- Zhang, J.; Macartney, T.; Peggie, M.; Cohen, P. Interleukin-1 and TRCF6-dependent activation of TAK1 in the absence of TAB2 and TAB3. Biochem. J. 2017, 474, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Criado, G.; Risco, A.; Alsina-Beauchamp, D.; Perez-Lorenzo, M.; Escos, A.; Cuenda, A. Alternative p38 MAPKs Are Essential for Collagen-Induced Arthritis. Arthritis Rheumatol. 2013, 66, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Simon, J.; Arthur, C.; Kuma, Y.; Peggie, M.; Carr, J.; Murray-Tait, V.; Centeno, F.; Goebeler, M.; Morrice, N.; et al. p38g regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005, 24, 1134–1145. [Google Scholar] [CrossRef]
- Risco, A.; Fresno, C.; Mambol, A.; Alsina-Beauchamp, D.; MacKenzie, K.F.; Yang, H.A.C. p38g and p38d kinases regulate the toll-like receptor 4 (TLR4)-induced cytokine production by controlling ERK1/2 protein kinase pathway activation. Proc. Natl. Acad. Sci. USA 2012, 109, 11200–11205. [Google Scholar] [CrossRef]
- Gonzalez-Teran, B.; Cortes, J.R.; Manieri, E.; Matesanz, N.; Verdygo, A.; Rodriguez, M. Eukaryotic elongation factor 2 controls TNF-a translation in LPS-induced hepatitis. J. Clin. Investig. 2013, 123, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Escos, A.; Martin-Gomez, J.; Gonzalez-Romero, D.; Diaz-Mora, E.; Francisco-Velilla, R.; Santiago, C. TPL2 kinase expression is regulated by the p38γ/p38δ-dependent association of aconitase-1 with TPL2 mRNA. Proc. Natl. Acad. Sci. USA 2022, 119, e2204752119. [Google Scholar] [CrossRef]
- Risco, A.; Martin-Serrano, M.; Barber, D.F.; Cuenda, A. p38γ and p38δ Are Involved in T Lymphocyte Development. Front. Immunol. 2018, 9, 65. [Google Scholar] [CrossRef]
- Barrio, L.; Roman-Garcia, S.; Diaz-Mora, E.; Risco, A.; Jimenz-Saiz, R.; Carrasco, Y. B Cell Development and T-Dependent Antibody Response Are Regulated by p38g and p38d. Front. Cell Dev. Biol. 2020, 8, 189. [Google Scholar] [CrossRef]
- Alsina-Beauchamp, D.; Escos, A.; Fajardo, P.; Gonzalez-Romero, D.; Diaz-Mora, E.; Risco, A. Myeloid cell deficiency of p38g/p38d protects against candidiasis and regulates antifungal immunity. EMBO Mol. Med. 2018, e8485, 1–15. [Google Scholar]
- Del Reino, P.; Alsina-Beauchamp, D.; Escos, A.; Cerezo-Guisado, M.I.; Risco, A.; Aparicio, N. Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38g and p38d, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res. 2014, 74, 6150–6160. [Google Scholar] [CrossRef] [PubMed]
- Zur, R.; Garcia-Ibanez, L.; Nunez-Buiza, A.; Aparicio, N.; Liappas, G.; Escos, A. Combined deletion of p38g and p38d reduces skin inflammation and protects from carcinogenesis. Oncotarget 2015, 6, 12920–12935. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Qi, X.; Tsai, S.; Lu, Y.; Basir, Z.; Oshima, K. p38g MAPK is required for inflammation-associated colon tumorigenesis. Oncogene 2016, 35, 1039–1048. [Google Scholar] [CrossRef]
- Wang, F.; Qi, X.; Wertz, R.; Mortensen, M.; Hagen, C.; Evans, J. p38g MAPK is essential for aerobic glycolysis and pancreatic tumorigenesis. Cancer Res. 2020, 80, 3251–3264. [Google Scholar] [CrossRef] [PubMed]
- Lluis, F.; Ballestar, E.; Suelves, M.; Esteller, M.; Munoz-Canoves, P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005, 24, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Q.; Xiao, F.; Jiang, Y.; Wu, Z. Involvement of the p38 mitogen-activated protein kinase a, b, and g isoforms in myogenic differentiation. Mol. Biol. Cell 2008, 19, 1519–1528. [Google Scholar] [CrossRef]
- Pogozelski, A.; Geng, T.; Li, P.; Lira, V.; Zhang, M.; Chi, J.T.; Yan, Z. p38g mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE 2009, 4, e7934. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Teran, B.; Lopez, J.A.; Rodriguez, E.; Leiva, L.; Martinez-Martinez, S.; Bernal, J.A. p38g and d promote heart hypertrophy by targeting the mTOR-inhibitory protein DEPTOR for degradation. Nat. Commun. 2016, 7, 10477. [Google Scholar] [CrossRef]
- Gonzalez-Teran, B.; Matesanz, N.; Nikolic, I.; Verdugo, M.A.; Sreeramkumar, V.; Hernandez-Cosido, L. p38g and p38d reprogram liver metabolism by modulating neutrophil infiltration. EMBO J. 2016, 35, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Sincennes, M.; Chevalier, F.P.; Brun, C.E.; Lacaria, M.; Segales, J. The dystrophin glycoprotein complex regulates the epigenetic activation of muscle stem cell commitment. Cell Metab. 2019, 22, 755–768. [Google Scholar] [CrossRef]
- Santamans, A.M.; Montalvo-Romeral, V.; Mora, A.; Lopez, J.A.; Gonzalez-Romero, F.; Jimenez-Blasco, D. p38γ and p38δ regulate postnatal cardiac metabolism through glycogen synthase 1. PLoS Biol. 2021, 19, e3001447. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, Z.; Li, H.; Wang, S.; Wu, Y.; Hu, Y. P38γ modulates the lipid metabolism in non-alcoholic fatty liver disease by regulating the JAK–STAT signaling pathway. FASEB J. 2023, 37, e22716. [Google Scholar] [CrossRef]
- Kerk, S.A.; Papagiannakopouls, T.; Shah, Y.M.; Lyssiotis, C.A. Metabolic networks in mutant KRAS-driven tumours: Tissue specificities and the microenvironment. Nat. Rev. Cancer 2021, 21, 510–525. [Google Scholar] [CrossRef]
- Ho, R.C.; Alcazar, O.; Fujii, N.; Hirshman, M.F.; Goodyear, L.J. p38g MAPK regulation of glucose transporter expression and glucose uptake in Ly myotubes and mouse skeletal muscle. Am. J. Psyhol. Regul. Integr. Comp. Physiol. 2003, 286, R342–R349. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Guo, F.; Li, P.; Peng, D.; He, J. Impact of p38γ mitogen-activated protein kinase (MAPK) on MDA-MB-231 breast cancer cells using metabolomic approach. Int. J. Biochem. Cell Biol. 2019, 107, 6–13. [Google Scholar] [CrossRef]
- Tang, J.; Qi, X.; Mercola, D.; Han, J.; Chen, G. Essential role of p38g in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 2005, 280, 23910–23917. [Google Scholar] [CrossRef] [PubMed]
- Pohl, N.M.; Qi, X.; Loesch, M.; Tang, J.; Li, Q.; Chen, G. Tissue-specific roles of p38g MAPK in Ras transformation. AACR Proc. 2006, 47, 2534. [Google Scholar]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.W.; Zhi, H.Y.; Pohl, N.; Loesch, M.; Qi, X.M.; Li, R.S. PTPH1 Dephosphorylates and Cooperates with p38γ MAPK to Increase Ras Oncogenesis through PDZ-Mediated Interaction. Cancer Res. 2010, 70, 2910. [Google Scholar] [CrossRef]
- Xu, M.; Ren, Z.; Wang, X.; Comer, A.; Frank, J.A.; Ke, Z. ErB2 and p38g MAPK mediate alcohol-induced increase in breast cancer stem cells. Mol. Cancer 2016, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Lepp, A.; Chen, G. p38 gamma MAP kinase. UCSD-Nat. Mol. Pages 2010. [Google Scholar] [CrossRef]
- Hou, S.W.; Suresh, P.S.; Qi, X.; Lepp, A.; Mirza, S.; Chen, G. p38g Mitogen-activated Protein Kinase Signals through Phosphorylating Its Phosphatase PTPH1 in Regulating Ras Protein Oncogenesis and Stress Response. J. Biol. Chem. 2012, 287, 27895–27905. [Google Scholar] [CrossRef]
- Zhi, H.; Hou, S.W.; Li, R.; Basir, Z.; Xiang, A.; Szabo, A. PTPH1 cooperates with vitamin D receptor to stimulate breast cancer growth through their mutual stabilization. Oncogene 2011, 30, 1706–1715. [Google Scholar] [CrossRef]
- Ma, S.; Yin, N.; Qi, X.; Pfister, S.L.; Zhang, M.; Ma, R.; Chen, G. Tyrosine dephosphorylation enhances the therapeutic target activity of epidermal growth factor receptor (EGFR) by disrupting its interaction with estrogen receptor (ER). Oncotarget 2015, 6, 13320–13333. [Google Scholar] [CrossRef]
- Chen, K.; Lin, S.; Wu, M.; Ho, M.; Santhanam, A.; Chou, C.; Meng, T.; Wang, A. Reciprocal allosteric regulation of p38g and PTPN3 involves a PDZ domain-modulated complex formation. Sci. Signal. 2014, 7, ra98. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Lepp, A.; Ji, Y.; Mortensen, M.; Hou, S.; Qi, X. The K-Ras effector p38g MAPK confers intrinsic resistance to tyrosine kinase inhibitors by stimulating EGFR transcription and EGFR dephosphorylation. J. Biol. Chem. 2017, 292, 15070–15079. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhi, H.; Lepp, A.; Wang, P.; Huang, J.; Basir, Z. p38g mitogen-activated protein kinase (MAPK) confers breast cancer hormone sensitivity by switching estrogen receptor (ER) signaling from classical to nonclassical pathway via stimulating ER phosphorylation and c-Jun transcription. J. Biol. Chem. 2012, 287, 14681–14691. [Google Scholar] [CrossRef]
- Qi, X.; Hou, S.; Lepp, A.; Li, R.; Basir, Z.; Lou, Z.; Chen, G. Phosphorylation and stabilization of topoisomerase IIa protein by p38g mitogen-activated protein kinase sensitize breast cancer cells to its poisons. J. Biol. Chem. 2011, 286, 35883–35890. [Google Scholar] [CrossRef]
- Suresh, P.S.; Ma, S.; Migliaccio, A.; Chen, G. Protein-tyrosine phosphatase H1 increases breast cancer sensitivity to antiestrogens by dephosphorylating estrogen receptor at tyr537. Mol. Cancer Ther. 2014, 13, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.M.; Xie, C.; Hou, S.; Li, G.; Yin, N.; Dong, L.; Lepp, A.; Chesnik, M.A.; Mirza, S.P.; Szabo, A.; et al. Identification of a ternary protein-complex as a therapeutic target for K-Ras-dependent colon cancer. Oncotarget 2014, 5, 4269–4282. [Google Scholar] [CrossRef]
- Kwong, J.; Hong, L.; Liao, R.; Deng, Q.; Han, J.; Sun, P. p38a and p38g mediates oncogenic ras-induced senescence through different mechanisms. J. Biol. Chem. 2009, 284, 11237–11246. [Google Scholar] [CrossRef]
- Gillespie, M.A.; Grand, F.L.; Scime, A.; Kuang, S.; von Maltzahn, J.; Seale, V.; Cuenda, A.; Ranish, J.A.; Rudnicki, M.A. p38g-dependent gene silencing restricts entry into the myogenic differentiation program. J. Cell Biol. 2009, 187, 991–1005. [Google Scholar] [CrossRef]
- Loonat, A.A.; Martin, E.D.; Sarafraz-Shekary, N.; Tilgner, K.; Hertz, N.T.; Levin, R. p38g MAPK contributes to left ventricular remodeling after pathologic stress and disinhibits calpain through phosphorylation of calpastatin. FASEB J. 2019, 33, 13131–13144. [Google Scholar] [CrossRef]
- Sabio, G.; Reuver, S.; Feijoo, C.; Hasegawa, M.; Thomas, G.M.; Centeno, F.; Kuhlendahl, S.; Leal-Ortiz, S.; Goedert, M.; Garner, C.; et al. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38g and ERK1/ERK2. Biochem. J. 2004, 380, 19–30. [Google Scholar] [CrossRef]
- Hasegawa, M.; Cuenda, A.; Spillantini, M.G.; Thomas, G.M.; Buee-Scherrer, V.; Cohen, P.; Goedert, M. Stress-activated protein kinase-3 interacts with the PDZ domain of a1-syntrophin: A mechanism for specific substrate recognition. J. Biol. Chem. 1999, 274, 12626–12631. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.D.; Sutherland, C.; Zhang, Y.; Risco, A.; Vega, L.D.L.; Caunt, C.J. Heat shock factor 1 is a substrate for p38 mitogen-activated protein kinases. Mol. Cell. Biol. 2016, 36, 2403–2417. [Google Scholar] [CrossRef]
- Ozes, O.; Blatt, L.M.; Seiwert, S.D. Use of Pirfenidone in Therapeutic Regimens. United States Patent-US 7,407,973 B2, 5 August 2008. pp. 1–46. [Google Scholar]
- King, T.E.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Kuma, Y.; Sabio, G.; Bain, J.; Shpiro, N.; Marquesz, R.; Cuenda, A. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J. Biol. Chem. 2005, 280, 19472–19479. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Mo, Q.; Zhang, Y.; Gao, Y.; Wu, Y.; Li, J. The p38 MAPK inhibitor BIRB796 enhances the antitumor effects of VX680in cervical cancer. Cancer Biol. Ther. 2016, 17, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.; Capolino, A.; Cirillo, P.F.; Gilmore, T.; Graham, A.G.; Hickey, E. Structure-Activity Relationships of the p38a MAP Kinase Inhibitor 1-(5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl)-3-[4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl]urea (BIRB 796). J. Med. Chem. 2003, 46, 4676–4686. [Google Scholar] [CrossRef]
- Zhang, X.H.; Nam, S.; Wu, J.; Chen, C.; Liu, X.; Li, H. Multi-kinase inhibitor with anti-p38g activity in cutaeneous T-cell lymphoma. J. Investig. Dermatol. 2018, 138, 2377–2387. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, C.; Li, H.; Hsiang, J.; Wu, X.; Hu, W. Targeting the non-ATP-binding pocket of the MAP kinase p38g mediates a novel mechanism of cytotoxicity in cutaneous T-cell lymphoma (CTCL). FEBS 2021, 595, 2570–2592. [Google Scholar] [CrossRef]
- Yu, J.X.; Craig, A.J.; Duffy, M.E.; Villacorta-Martin, C.; Miguela, V.; de Galarreta, M.R. Phenotype-Based Screens with Conformation-Specific Inhibitors Reveal p38 Gamma and Delta as Targets for HCC Polypharmacology. Mol. Cancer Ther. 2019, 18, 1506–1519. [Google Scholar] [CrossRef]
- Chen, G.; Hitomi, M.; Han, J.; Stacey, D.W. The p38 pathway provides negative feedback to Ras proliferative signaling. J. Biol. Chem. 2000, 275, 38973–38980. [Google Scholar] [CrossRef]
- Qi, X.; Pohl, N.M.; Loesch, M.; Hou, S.; Li, R.; Qin, J.Z.; Cuenda, A.; Chen, G. p38a antagonizes p38g activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J. Biol. Chem. 2007, 282, 31398–31408. [Google Scholar] [CrossRef]
- Qi, X.; Tang, J.; Pramanik, R.; Schultz, R.M.; Shirasawa, S.; Sasazuki, T.; Han, J.; Chen, G. p38 MAPK activation selectively induces cell death in K-ras mutated human colon cancer cells through regulation of vitamin D receptor. J. Biol. Chem. 2004, 279, 22138–22144. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tang, J.; Loesch, M.; Pohl, N.; Alkan, S.; Chen, G. p38g MAPK integrates signaling cross-talk between Ras and estrogen receptor to increase breast cancer invasion. Cancer Res. 2006, 66, 7540–7547. [Google Scholar] [CrossRef]
- Xu, W.; Liu, R.; Dai, Y.; Hong, S.; Dong, H.; Wang, H. The role of p38g in cancer: From review to outlook. Int. J. Biol. Sci. 2021, 17, 4036–4046. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.M.; Yin, N.; Ma, S.; Lepp, A.; Tang, J.; Jing, W. p38g MAPK is a therapeutic target for triple-negative breast cancer by stimulation of cancer stem-like cell expansion. Stem Cells 2015, 33, 2738–2747. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Mehta, A.; Shim, W. ErbB4 activated p38g MAPK isoform mediates early cardiogenesis through NKx2.5 in human pluripotent stem cells. Stem Cells 2016, 34, 288–298. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Wang, Y.; Wu, H.; Frank, J.A.; Zhang, Z. Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018, 1864, 3605–3617. [Google Scholar] [CrossRef]
- Loesch, M.; Zhi, H.; Hou, S.; Qi, X.; Li, R.; Basir, Z.; Iftner, T.; Cuenda, A.; Chen, G. p38g MAPK cooperates with c-Jun in trans-activating matrix metalloproteinase 9. J. Biol. Chem. 2010, 285, 15149–15158. [Google Scholar] [CrossRef] [PubMed]
| Inhibitor Name | Selectivity | Concentration In Vitro; Dose In Vivo | Refs. |
|---|---|---|---|
| Pirfenidone (PFD) | p38γ > α > β | 100–400 μg/mL (0.54–2.16 mM); 500 mg/kg | [32,65,66] |
| BIRB796 | p38α > β > γ > δ | 0.1–10 μM; 10 mg/kg | [67,68,69] |
| PIK75 | PI3K and p38γ | 0.01–1 μM; 2–10 mg/kg | [70] |
| AD80 | p38γ and p38δ | 0.01–10 μM; 20 mg/kg | [72] |
| CSH71 | p38γ | 0.01–1 μM | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.-M.; Chen, G. p38γ MAPK Inflammatory and Metabolic Signaling in Physiology and Disease. Cells 2023, 12, 1674. https://doi.org/10.3390/cells12131674
Qi X-M, Chen G. p38γ MAPK Inflammatory and Metabolic Signaling in Physiology and Disease. Cells. 2023; 12(13):1674. https://doi.org/10.3390/cells12131674
Chicago/Turabian StyleQi, Xiao-Mei, and Guan Chen. 2023. "p38γ MAPK Inflammatory and Metabolic Signaling in Physiology and Disease" Cells 12, no. 13: 1674. https://doi.org/10.3390/cells12131674
APA StyleQi, X.-M., & Chen, G. (2023). p38γ MAPK Inflammatory and Metabolic Signaling in Physiology and Disease. Cells, 12(13), 1674. https://doi.org/10.3390/cells12131674





