Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Mice
2.1.2. Primary Antibodies
Antibodies against Rabconnectin3α/DMXL2
2.1.3. Secondary Antibodies
2.1.4. Additional Materials
2.1.5. Plasmids
2.2. Methods
2.2.1. Embedding of Mouse Retinas and Immunohistochemistry on 0.5 μm Thin Resin Sections
2.2.2. Confocal Microscopy of Immunolabelled Sections
2.2.3. Preparation and Immunolabelling of Retinal Cryostat Sections
2.2.4. Embedding of Retinas in LR Gold for Post-Embedding Immunogold Electron Microscopy
2.2.5. Post-Embedding Immunogold Labelling with Ultrasmall Immunogold Particles and Subsequent Silver Intensification
2.2.6. Peptide Arrays for Antibody Epitope Mapping
2.3. Miscellaneous Methods
2.3.1. SDS-PAGE and Western Blotting
2.3.2. Expression and Purification of GST-Tagged Fusion Proteins
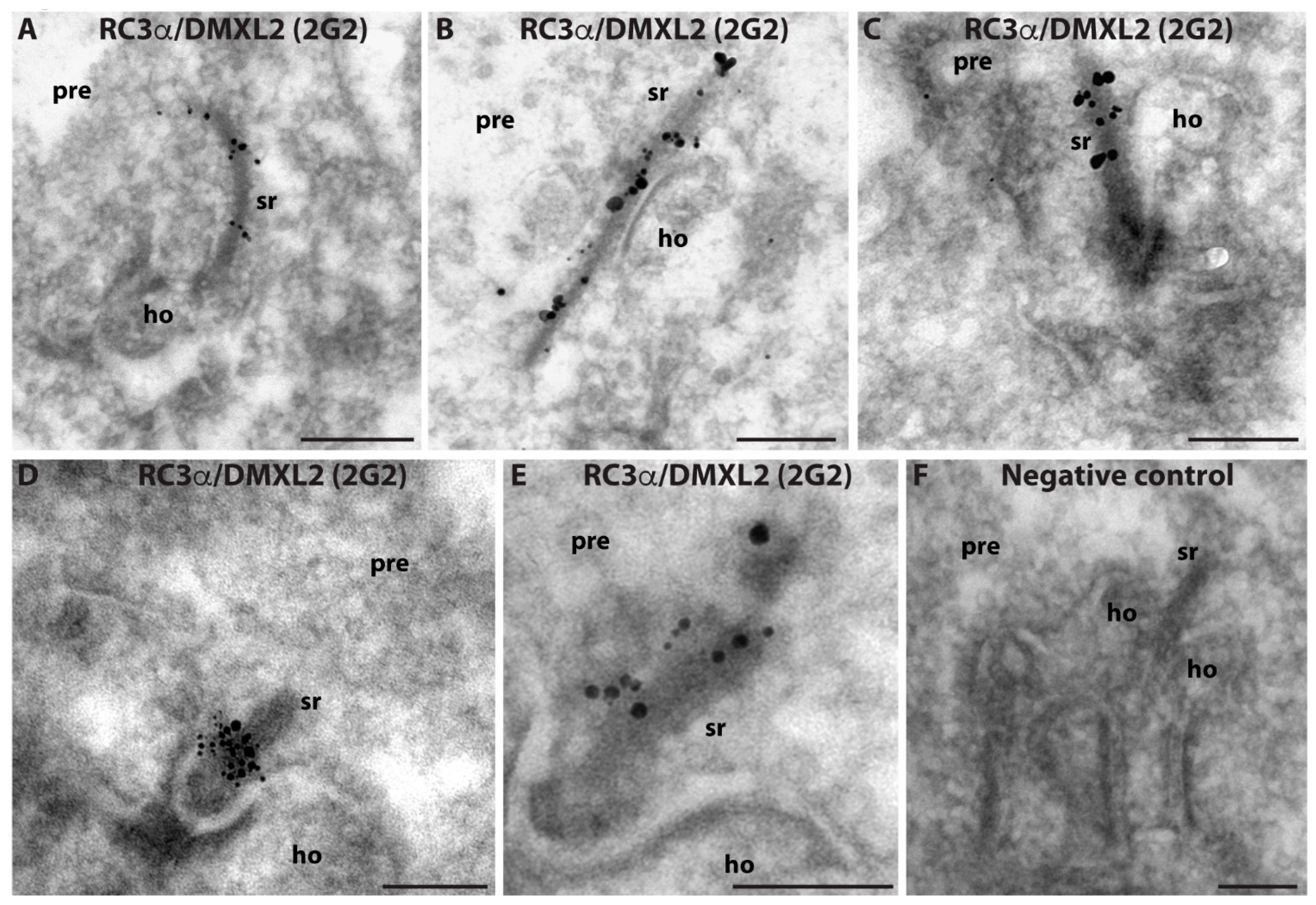
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sjöstrand, F.S. Ultrastructure of retinal rod synapses of the guinea pig eye as revealed by three-dimensional reconstructions from serial sections. J. Ultrastruct. Res. 1958, 2, 122–170. [Google Scholar] [CrossRef]
- Vollrath, L.; Huss, H. The synaptic ribbons of the guinea-pig pineal gland under normal and experimental conditions. Z. Zellforsch. Mikrosk. Anat. 1973, 139, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Wang, D.W. Morphology of afferent and efferent synapses in hearing organ of goldfish. J. Comp. Neurol. 1974, 156, 403–416. [Google Scholar] [CrossRef]
- Krstic, R. Ultracytochemistry of the synaptic ribbons in the rat pineal organ. Cell Tissue Res. 1976, 166, 135–143. [Google Scholar] [CrossRef]
- McNulty, J.A. Ultrastructural observations on synaptic ribbons in the pineal organ of the goldfish. Cell Tissue Res. 1980, 210, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.; Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Grabner, C.P.; Schmitz, F. Sensory processing at ribbon synapses in the retina and the cochlea. Physiol. Rev. 2020, 100, 103–144. [Google Scholar] [CrossRef]
- Hecht, S.; Shlaer, S.; Pirenne, M.H. Energy, quanta, and vision. J. Gen. Physiol. 1942, 25, 819–840. [Google Scholar] [CrossRef] [Green Version]
- Bulmer, M.G.; Howarth, C.I. Noise and the visual threshold. Nature 1957, 180, 1403–1404. [Google Scholar] [CrossRef]
- Sakitt, B. Counting every quantum. J. Physiol. 1972, 223, 131–150. [Google Scholar] [CrossRef] [Green Version]
- Baylor, D.A.; Nunn, B.J.; Schanpf, J.L. The photocurrent, noise and spectral sensitivity of rods of the monkey macaca fascicularis. J. Physiol. 1984, 357, 575–607. [Google Scholar] [CrossRef]
- Schneeweis, D.M.; Schnapf, J.L. Photovoltage of rods and cones in the macaque retina. Science 1995, 268, 1053–1056. [Google Scholar] [CrossRef]
- Rieke, F.; Baylor, D.A. Origin of reproducibility in the responses of retinal rods to single photons. Biophys. J. 1998, 75, 1836–1857. [Google Scholar] [CrossRef] [Green Version]
- Field, G.D.; Rieke, F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron 2002, 35, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Schein, S.; Ahmad, K.M. A clockwork hypothesis: Synaptic release by rod photoreceptors must be regular. Biophys. J. 2005, 89, 3931–3949. [Google Scholar] [CrossRef] [Green Version]
- Gross, O.P.; Pugh, E.N.; Burns, M.E. cGMP in mouse rods: The spatiotemporal dynamics underlying single photon responses. Front. Mol. Neurosci. 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reingruber, J.; Holcman, D.; Fain, G.L. How rods respond to single photons: Key adaptations of a G-protein cascade that enable vision at the physical limit of perception. BioEssays 2015, 37, 1243–1252. [Google Scholar] [CrossRef] [Green Version]
- Hays, C.L.; Sladek, A.L.; Field, G.D.; Thoreson, W.B. Properties of multivesicular release from mouse rod photoreceptors support transmission of single-photon responses. eLife 2021, 10, e67446. [Google Scholar] [CrossRef] [PubMed]
- Field, G.D.; Sampath, A.P.; Rieke, F. Retinal processing near absolute threshold: From behavior to mechanism. Annu. Rev. Physiol. 2005, 67, 491–514. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, F.; Königstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons. A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 852–872. [Google Scholar]
- Maxeiner, S.; Luo, F.; Tan, A.; Schmitz, F.; Südhof, T.C. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016, 35, 1098–1114. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Stewart, W.J.; Akanyeti, O.; Frederick, C.; Zhu, J.; Santos-Sacchi, J.; Sheets, L.; Liao, J.C.; Zenisek, D. Synaptic ribbons require Ribeye for electron density, proper synaptic localization, and recruitment of calcium channels. Cell Rep. 2016, 15, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, P.; de la Marina, D.L.; Michanski, S.; Tobón, L.M.J.; Chakrabarti, R.; Picher, M.M.; Neef, J.; Jung, S.; Gültas, M.; Maxeiner, S.; et al. The synaptic ribbon is critical for sound encoding at high rates with temporal precision. eLife 2018, 7, e29275. [Google Scholar] [CrossRef]
- Becker, L.; Schnee, M.E.; Niwa, M.; Sun, W.; Maxeiner, S.; Talaei, S.; Kachar, B.; Rutherford, M.A.; Ricci, A.J. The presynaptic ribbon maintains vesicle populations at the hair cell afferent fiber synapse. eLife 2018, 7, e30241. [Google Scholar] [CrossRef] [PubMed]
- Shankhwar, S.; Schwarz, K.; Katiyar, R.; Jung, M.; Maxeiner, S.; Südhof, T.C.; Schmitz, F. RIBEYE B-domain is essential for RIBEYE A-domain stability and assembly of synaptic ribbons. Front. Mol. Neurosci. 2022, 15, 838311. [Google Scholar] [CrossRef]
- Mesnard, C.S.; Barta, C.L.; Sladek, A.L.; Zenisek, D.; Thoreson, W.B. Eliminating synaptic ribbons from rods and cones halves the releasable vesicle pools and slows down vesicle replenishment. Int. J. Mol. Sci. 2022, 23, 6429. [Google Scholar] [CrossRef]
- Goldberg, J.D.; Yoshida, T.; Brick, P. Crystal structure of a NAD-dependent D-glycerate dehydrogenase at 2.4A resolution. J. Mol. Biol. 1994, 236, 1123–1140. [Google Scholar] [CrossRef]
- Piatigorski, J. Dual use of the transcriptional repressor (CtBP2)/ribbon synapse (RIBEYE) gene: How prevalent are multifunctional genes? Trends Neurosci. 2001, 24, 555–557. [Google Scholar] [CrossRef]
- Chinnadurai, G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell 2002, 9, 213–224. [Google Scholar] [CrossRef]
- Chinnadurai, G. CtBP family proteins: More than transcriptional repressors. Bioessays 2003, 25, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, R.; Thoreson, W.B.; Witkovsky, P. Synaptic transmission at retinal ribbon synapses. Prog. Ret. Eye Res. 2005, 24, 682–720. [Google Scholar] [CrossRef] [Green Version]
- Thoreson, W.B. Transmission at rod and cone ribbon synapses in the retina. Europ. J. Physiol. 2021, 473, 1469–1491. [Google Scholar] [CrossRef] [PubMed]
- Zenisek, D.; Steyer, J.A.; Almers, W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature 2000, 406, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xu, S.; Montpetit, R.; Kramer, R.H. Rab3A mediates vesicle delivery at photoreceptor ribbon synapses. J. Neurosci. 2012, 32, 6931–6936. [Google Scholar] [CrossRef] [Green Version]
- Van Hook, M.J.; Parmelee, C.M.; Chen, M.; Cork, K.M.; Curto, C.; Thoreson, W.B. Calmodulin enhances ribbon replenishment and shapes filtering of synaptic transmission by cone photoreceptors. J. Gen. Physiol. 2014, 144, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Vaithianathan, T.; Wollmuth, L.P.; Henry, D.; Zenisek, D.; Matthews, G. Tracking newly released synaptic vesicle proteins at ribbon active zones. iScience 2019, 17, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Joselevitch, C.; Zenisek, D. Direct observation of vesicle transport on the synaptic ribbon provides evidence that vesicles are mobilized and prepared rapidly for release. J. Neurosci. 2020, 40, 7390–7404. [Google Scholar] [CrossRef]
- Matsui, Y.; Kikuchi, A.; Kondo, J.; Hishida, T.; Teranishi, Y.; Takai, Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J. Biol. Chem. 1988, 263, 11071–11074. [Google Scholar] [CrossRef]
- Fischer von Mollard, G.; Mignery, G.A.; Baumert, M.; Perin, M.S.; Hanson, T.J.; Burger, P.M.; Jahn, R.; Südhof, T.C. Rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc. Natl. Acad. Sci. USA 1990, 87, 1988–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, Y.; Sasaki, T.; Shirataki, H.; Nakanishi, H. Rab3 small GTP binding protein in Ca2+-dependent exocytosis. Genes Cells 1996, 1, 615–632. [Google Scholar] [CrossRef]
- Geppert, M.; Bolshakov, V.Y.; Siegelbaum, S.A.; Takei, K.; De Camilli, P.; Hammer, R.E.; Südhof, T.C. The role of Rab3A in neurotransmitter release. Nature 1994, 369, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Geppert, M.; Goda, Y.; Stevens, C.F.; Südhof, T.C. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 1997, 387, 810–814. [Google Scholar] [CrossRef]
- Leenders, A.G.M.; Lopes da Silva, F.H.; Ghijsen, W.E.J.M.; Verhage, M. Rab3A is involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals. Mol. Biol. Cell 2001, 12, 3095–3102. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, O.M.; Schmitz, F.; Jahn, R.; Südhof, T.C. A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 2004, 24, 6629–6637. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, O.M.; Baus, J.; Südhof, T.C.; Rosenmund, C. Rab3 superprimes synaptic vesicles for release: Implications for short-term synaptic plasticity. J. Neurosci. 2006, 26, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Südhof, T.C. Function of Rab3 GDP-GTP exchange. Neuron 1997, 18, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Okamoto, M.; Schmitz, F.; Hofmann, K.; Südhof, T.C. RIM is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 1997, 388, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Uthaiah, R.C.; Hudspeth, A.J. Molecular anatomy of the hair cell’s ribbon synapse. J. Neurosci. 2010, 30, 12387–12399. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, C.; Weil, B.; Christmann, M.; Schmidt, E.R. The new gene DmX from Drosophila melanogaster encodes a novel WD-repeat protein. Gene 1998, 216, 267–276. [Google Scholar] [CrossRef]
- Nagano, F.; Kawabe, H.; Nakanishi, H.; Shinohara, M.; Deguchi-Tawarada, M.; Takeuchi, M.; Sasaki, T.; Takai, Y. Rabconnectin-3, a novel protein that binds both GD/GTP exchange protein and GTPase-activating protein for Rab3 small G protein family. J. Biol. Chem. 2002, 277, 9629–9632. [Google Scholar] [CrossRef] [Green Version]
- Kawabe, H.; Sakisaka, T.; Yasumi, M.; Shingai, T.; Izumi, G.; Nagano, F.; Deguchi-Tawarada, M.; Takeuchi, M.; Nakanishi, H.; Takai, Y. A novel Rabconnectin-3-binding protein that directly binds a GDP/GTP exchange protein for Rab3A small G protein implicated in Ca2+-dependent exocytosis of neurotransmitter. Genes Cells 2003, 8, 537–546. [Google Scholar] [CrossRef]
- Dembla, M.; Kesharwani, A.; Natarajan, S.; Fecher-Trost, C.; Fairless, R.; Williams, S.K.; Flockerzi, V.; Diem, R.; Schwarz, K.; Schmitz, F. Early auto-immune targeting of photoreceptor ribbon synapses in mouse models of multiple sclerosis. EMBO Mol. Med. 2018, 10, e8926. [Google Scholar] [CrossRef]
- Irie, M.; Hata, Y.; Takeuchi, M.; Ichtchenko, K.; Toyoda, A.; Hirao, K.; Takai, Y.; Rosahl, T.W.; Südhof, T.C. Binding of neuroligins to PSD-95. Science 1997, 277, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Katiyar, R.; Dembla, E.; Dembla, E.; Kumar, P.; Belkacemi, A.; Jung, M.; Beck, A.; Flockerzi, V.; Schwarz, K.; et al. Disturbed presynaptic Ca2+ signaling in photoreceptors in the EAE mouse model of multiple sclerosis. iScience 2020, 23, 101830. [Google Scholar] [CrossRef]
- Dembla, M.; Wahl, S.; Katiyar, R.; Schmitz, F. ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms an NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis. J. Neurosci. 2014, 34, 5245–5260. [Google Scholar] [CrossRef] [Green Version]
- Wahl, S.; Katiyar, R.; Schmitz, F. A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons. J. Neurosci. 2013, 33, 10278–10300. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Magupalli, V.G.; Dembla, M.; Katiyar, R.; Schwarz, K.; Köblitz, L.; Alpadi, K.; Krause, E.; Rettig, J.; Sung, C.H.; et al. The Disease Protein Tulp1 Is Essential for Periactive Zone Endocytosis in Photoreceptor Ribbon Synapses. J. Neurosci. 2016, 36, 2473–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembla, E.; Dembla, M.; Maxeiner, S.; Schmitz, F. Synaptic ribbons foster active zone stability and illumination-dependent active zone enrichment of RIM2 and Cav1.4. Sci. Rep. 2020, 10, 5957. [Google Scholar] [CrossRef] [Green Version]
- Kesharwani, A.; Schwarz, K.; Dembla, E.; Dembla, M.; Schmitz, F. Early changes in exo- and endocytosis in the EAE mouse model of multiple sclerosis correlate with decreased synaptic ribbon size and reduced ribbon-associated vesicle pools in rod photoreceptor synapses. Int. J. Mol. Sci. 2021, 221, 789. [Google Scholar] [CrossRef]
- Punge, A.; Rizzoli, S.O.; Jahn, R.; Wildanger, J.D.; Meyer, L.; Schönle, A.; Kastrup, L.; Hell, S.W. 3D reconstruction of high-resolution STED microscope images. Microsc. Res. Tech. 2008, 71, 644–650. [Google Scholar] [CrossRef]
- Eich, M.L.; Dembla, E.; Wahl, S.; Schwarz, K.; Schmitz, F. The calcineurin binding, activity-dependent splice variant dynamin1xb is highly enriched in synapses in various regions of the central nervous system. Front. Mol. Neurosci. 2017, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, F.; Bechmann, M.; Drenckhahn, D. Purification of synaptic ribbons, structural components of the active zone complex of photoreceptor synapses. J. Neurosci. 1996, 16, 7109–7116. [Google Scholar] [CrossRef] [Green Version]
- Ronald, F. The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports-principles and applications. J. Immunol. Meth. 2002, 267, 13–26. [Google Scholar]
- Hilpert, K.; Winkler, D.F.H.; Hancock, R.E.W. Peptide arrays on cellulose support: SPOT synthesis, a time and cost-efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat. Protocols 2007, 2, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Harsman, A.; Kopp, A.; Wagner, R.; Zimmermann, R.; Jung, M. Calmodulin regulation of the calcium-leak channel Sec61 is unique to vertebrates. Channels 2011, 5, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Suiwal, S.; Dembla, M.; Schwarz, K.; Katiyar, R.; Jung, M.; Carius, Y.; Maxeiner, S.; Lauterbach, M.A.; Lancaster, C.R.D.; Schmitz, F. Ciliary proteins repurposed by the synaptic ribbon: Trafficking myristoylated proteins at rod photoreceptor synapses. Int. J. Mol. Sci. 2022, 23, 7135. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann-Schuppert, A.; Schnittler, H.J. A simple assay for quantification of protein in tissue sections, cell cultures and cell homogenates and of protein immobilized on solid surfaces. Cell Tissue Res. 1997, 288, 119–126. [Google Scholar] [CrossRef]
- Magupalli, V.G.; Schwarz, K.; Alpadi, K.; Natarajan, S.; Seigel, G.M.; Schmitz, F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of the synaptic ribbon. J. Neurosci. 2008, 28, 7954–7967. [Google Scholar] [CrossRef] [Green Version]
- Alpadi, K.; Magupalli, V.G.; Käppel, S.; Köblitz, L.; Schwarz, K.; Seigel, G.M.; Sung, C.H.; Schmitz, F. RIBEYE recruits Munc118, the mammalian ortholog of the Caenorhabditis elegans protein unc119 to synaptic ribbons of photoreceptor synapses. J. Biol. Chem. 2008, 283, 26461–26467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederick, C.E.; Zenisek, D. Ribbon synapses and retinal disease: Review. Int. J. Mol. Sci. 2023, 24, 5090. [Google Scholar] [CrossRef]
- Holt, M.; Cooke, A.; Neef, A.; Lagnado, L. High mobility of vesicles supports continuous exocytosis at a ribbon synapse. Curr. Biol. 2004, 14, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Tabares, L.; Khimich, D.; Strenzke, N.; de la Villa-Polo, P.; Castellano-Munoz, M.; Bulankina, A.; Moser, T.; Fernandez-Chacon, R.; Südhof, T.C. CSPalpha-deficiency causes massive and rapid photoreceptor degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 2926–2931. [Google Scholar] [CrossRef] [Green Version]
- Koulen, P.; Fletcher, E.L.; Craven, S.E.; Wässle, H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J. Neurosci. 1998, 18, 10136–101349. [Google Scholar] [CrossRef] [Green Version]
- tom Dieck, S.; Altrock, W.D.; Kessels, M.M.; Qualmann, B.; Regus, H.; Brauner, D.; Fejtova, A.; Bracko, O.; Gundelfinger, E.D.; Brandstätter, J.H. Molecular dissection of the photoreceptor ribbon synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the synaptic ribbon complex. J. Cell Biol. 2005, 168, 825–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Kriegstein, K.; Schmitz, F.; Link, E.; Südhof, T.C. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur. J. Neurosci. 1999, 11, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Usukura, J.; Yamada, E. Ultrastructure of the synaptic ribbons in photoreceptor cells of Rana catesbaiana revealed by freeze-etching and freeze-substitution. Cell Tissue Res. 1987, 247, 483–488. [Google Scholar] [CrossRef]
- Grabner, C.P.; Gandini, M.A.; Rehak, R.; Le, Y.; Zamponi, G.W.; Schmitz, F. RIM1/2-mediated facilitation of Cav1.4 channels opening is required for Ca2+-stimulated release in mouse rod photoreceptors. J. Neurosci. 2015, 35, 13133–13147. [Google Scholar] [CrossRef] [Green Version]
- Löhner, M.; Babai, N.; Müller, T.; Gierke, K.; Atorf, J.; Joachimsthaler, A.; Peukert, A.; Martens, H.; Feigenspan, A.; Kremers, J.; et al. Analysis of RIM expression and function at mouse photoreceptor ribbon synapses. J. Neurosci. 2015, 37, 7848–7863. [Google Scholar] [CrossRef]
- Sakisata, T.; Takai, Y. Purification and properties of Rabconnectin-3. Methods Enzymol. 2005, 403, 401–407. [Google Scholar]
- Yan, Y.; Denef, N.; Schupbach, T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev. Cell 2009, 17, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Li, K.W.; Chen, N.; Klemmer, P.; Koopmans, F.; Karupothula, R.; Smit, A.B. Identifying true protein complex constituents in interaction proteomics: The example of the DMXL2 protein complex. Proteomics 2012, 12, 2428–2432. [Google Scholar]
- Merkulova, M.; Paunescu, T.G.; Azroyan, A.; Marshansky, V.; Breton, S.; Brown, D. Mapping the H+ (V)-ATPase interactome: Identification of proteins involved in the trafficking, folding, assembly and phosphorylation. Sci. Rep. 2015, 5, 14827. [Google Scholar] [CrossRef] [Green Version]
- Jaskolka, M.C.; Kane, P.C. Interaction between the yeast RAVE complex and Vph1-containing Vo sectors is a central glucose-sensitive interaction required for v-ATPase reassembly. J. Biol. Chem. 2020, 295, 2259–2269. [Google Scholar] [CrossRef]
- Smardon, A.M.; Tarsio, M.; Kane, P.M. The RAVE complex is essential for stable assembly of the yeast V-ATPase. J. Biol. Chem. 2002, 277, 13831–13839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipos, G.; Brickner, J.H.; Brace, E.J.; Chen, L.; Rambourg, A.; Kepes, F.; Fuller, R.S. Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of trans-Golgi network transmembrane proteins. Mol. Biol. Cell 2004, 15, 3196–3209. [Google Scholar] [CrossRef] [Green Version]
- Seol, J.H.; Shevchenko, A.; Deshaies, R.J. Skp1 forms multiple protein complexes, including RAVE, a regulator of V-ATPase assembly. Nat. Cell Biol. 2001, 3, 384–391. [Google Scholar] [CrossRef]
- Gowrisankaran, S.; Milosevic, I. Regulation of synaptic vesicle acidification at the neuronal synapse. IUBMB Life 2020, 7, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Einhorn, Z.; Trapani, J.G.; Liu, Q.; Nicolson, T. Rabconnectin3a promotes stable activity of the H+-pump on synaptic vesicles in hair cells. J. Neurosci. 2012, 32, 11144–11156. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, J.E.; Li, F.; Edwards, R.H. The mechanism and regulation of vesicular glutamate transport: Coordination with the synaptic vesicle cycle. Biochem. Biophys. Acta Biomembr. 2020, 1862, 183259. [Google Scholar] [CrossRef]
- Pietrancosta, N.; Djibo, M.; Daumas, S.; Mestikawy, S.E.; Erickson, J.D. Molecular, structural, functional, and pharmacological sites for vesicular glutamate transporter regulation. Mol. Neurobiol. 2020, 57, 3118–3142. [Google Scholar] [CrossRef]
- Bodzeta, A.; Kahms, M.; Klingauf, J. The presynaptic v-ATPase reversibly disassembles and thereby modulates exocytosis but is not part of the fusion machinery. Cell Rep. 2017, 20, 1348–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, R.H. The neurotransmitter cycle and quantal size. Neuron 2007, 55, 835–857. [Google Scholar] [CrossRef] [Green Version]
- Takamori, S. Presynaptic molecular determinants of quantal size. Front. Syn. Neurosci. 2016, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Crummy, E.; Mani, M.; Thellman, J.C.; Martin, T.F. The priming factor CAPS1 regulates dense core vesicle acidification by interacting with rabconnectin3b/WDR7 in neuroendocrine cells. J. Biol. Chem. 2019, 294, 9402–9415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, M.A.; Souza, I.A.; Fan, J.; Li, K.; Wang, D.; Zamponi, G.W. Interactions of Rabconnectin-3 with Cav2 calcium-channels. Mol. Brain 2019, 12, 62. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Liu, X.-F.; Lin, X.-J.; Zhang, D.; Chai, Y.-C.; Yu, D.-H.; Sun, C.-L.; Wang, X.-L.; Zhu, W.-D.; Chen, Y.; et al. A dominant variant in DMXL2 is linked to nonsyndromic hearing loss. Genet. Med. 2017, 19, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, A.; Falace, A.; Wagner, M.; Gal, M.; Mei, D.; Conti, V.; Pisano, T.; Aprile, D.; Cerullo, M.S.; De Fusco, A.; et al. Biallelic DMXL2 mutations impair autophagy and cause Ohtahara syndrome with progressive course. Brain 2019, 142, 3876–3891. [Google Scholar] [CrossRef]
- Wonkam-Tingang, E.; Schrauwen, I.; Esoh, K.K.; Bharadwaj, T.; Nouel-Saied, A.; Acharya, A.; Nasir, A.; Leal, S.M.; Wonkam, A. A novel variant in DMXL2 gene is associated with autosomal dominant non-syndromic hearing impairment (DFNA71) in a cameroonian family. Exptl. Biol. Med. 2021, 246, 1524–1532. [Google Scholar] [CrossRef]
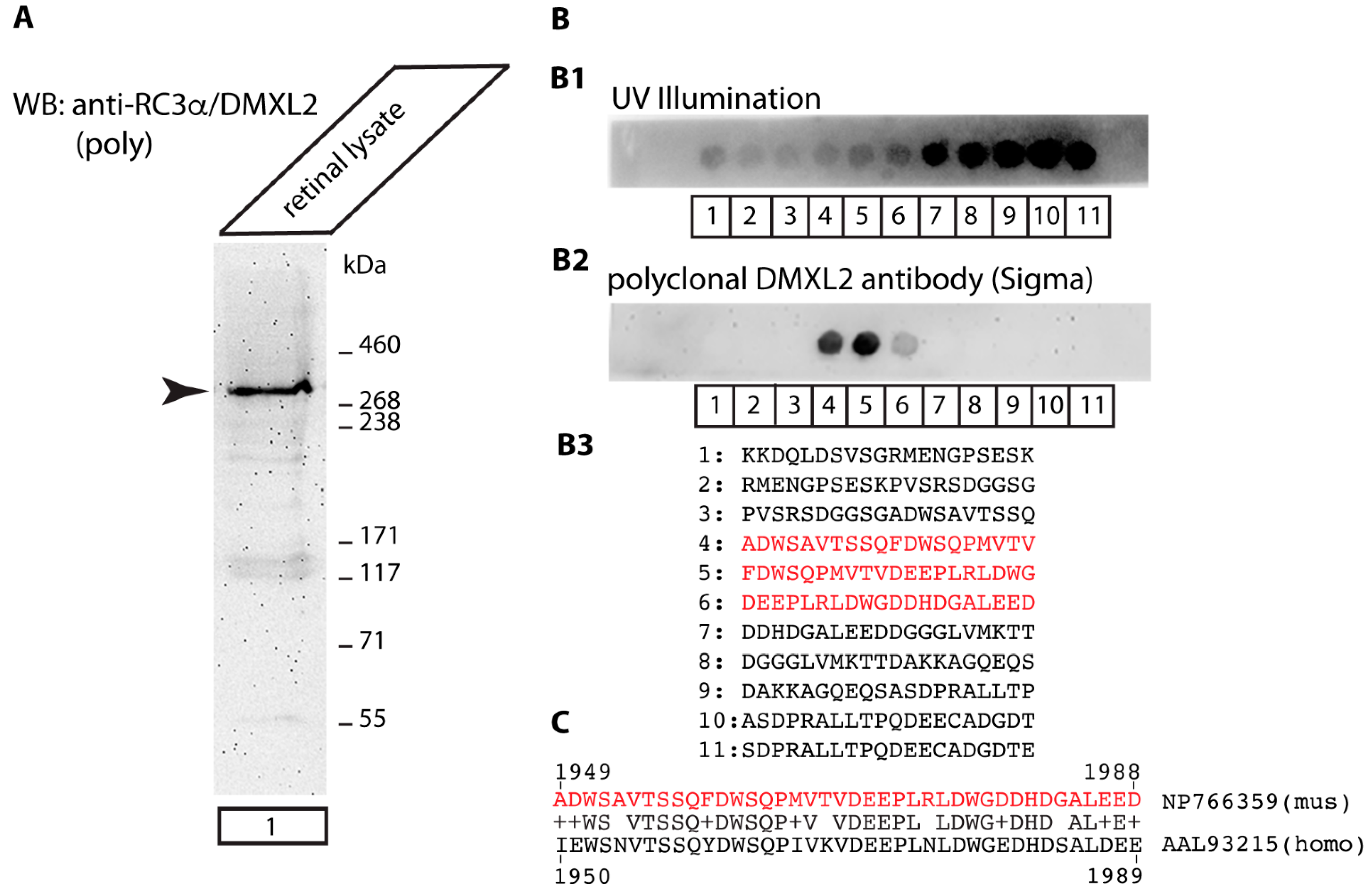
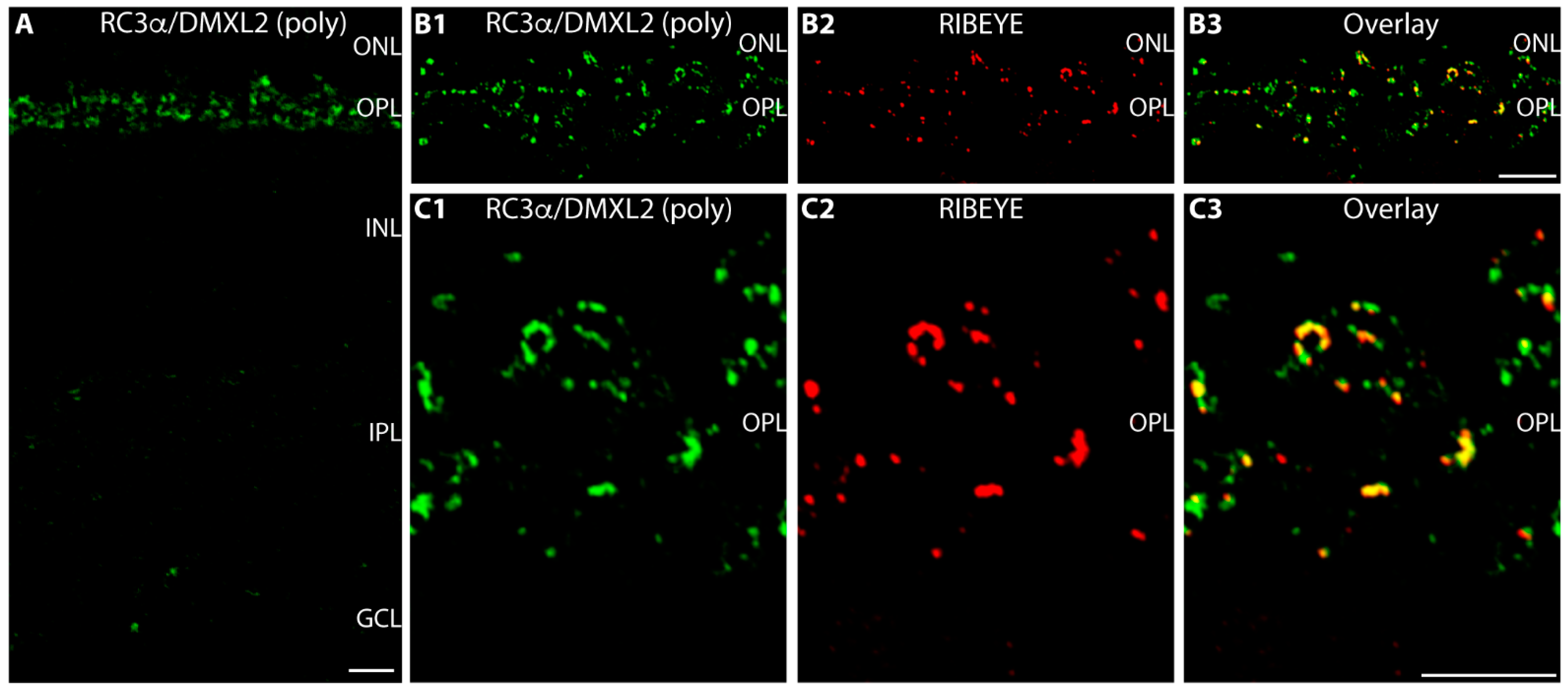
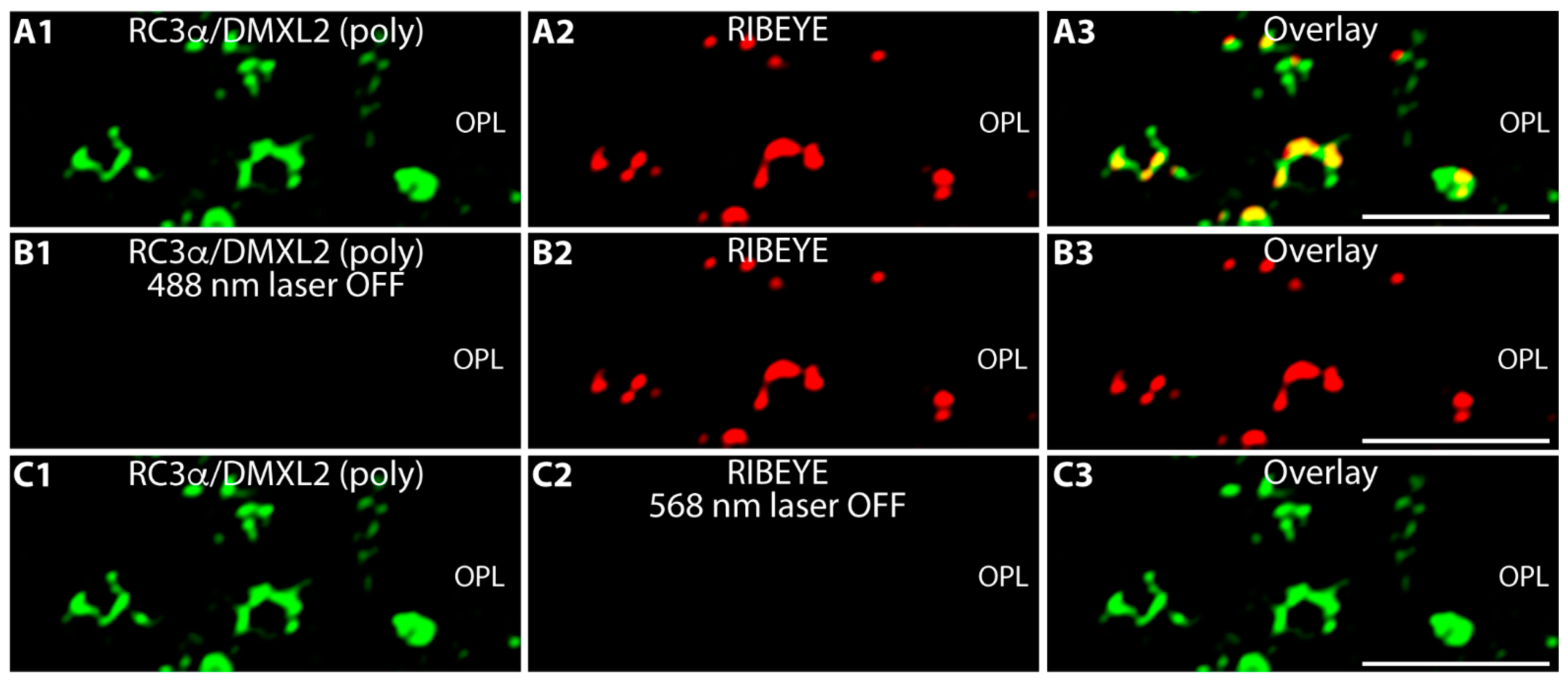

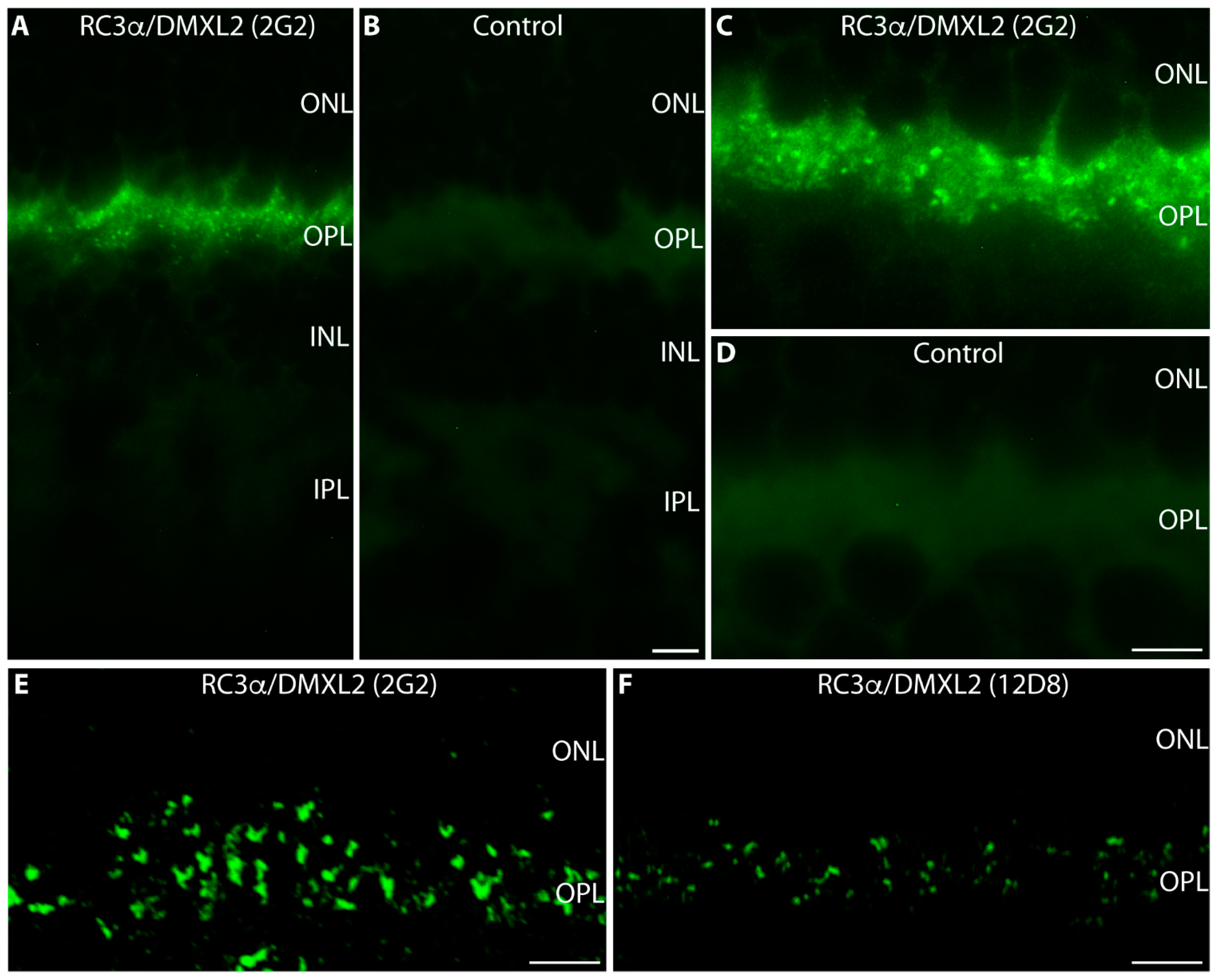

| Antibody | Source | Reference | Dilution |
|---|---|---|---|
| RIBEYE(B) U2656, rabbit polyclonal | Lab-made | [20] | 1:10,000 (IF) |
| RIBEYE(B) (2D9), mouse monoclonal | Lab-made | [52] | 1:200 (IF) 1:400 (EM) |
| PSD-95 (postsynaptic density protein-95), rabbit polyclonal | Gift Dr. T.C. Südhof | [53] | 1:1000 (IF) |
| CSP (cysteine-string protein), rabbit polyclonal | Lab-made | Raised against recombinant full-length mouse CSP | 1:500 (IF) |
| Cav1.4 Nterm, rabbit polyclonal | Lab-made | [54] | 1:500 (IF) |
| GST, mouse monoclonal | Sigma-Aldrich, G1160 | [55] | 1:10,000 (WB) |
| Antibody | Source | Dilution |
|---|---|---|
| Donkey anti-mouse Alexa 488 | Invitrogen; Karlsruhe, Germany; A-21202 | 1:1000 (IF) |
| Chicken anti-mouse DyLight 488 | Jackson ImmunoResearch; 715485150 | 1:1000 (IF) |
| Chicken anti-rabbit Alexa 488 | Invitrogen; Karlsruhe, Germany; A-21441 | 1:1000 (IF) |
| Chicken anti-rabbit Alexa 568 | Invitrogen; Karlsruhe, Germany; A-10042 | 1:1000 (IF) |
| Chicken anti-mouse Alexa 488 | ThermoFisher; Karlsruhe, Germany; 10114192 | 1:1000 (IF) |
| Goat anti-mouse peroxidase-conjugate (POX) | Sigma; Taufkirchen, Germany; A3673 | 1:5000 (WB) |
| Goat anti-mouse conjugated to 1.4 nm Nanogold | Nanoprobes/Biotrend, Cologne, Germany, #N-2001 | 1:100 (EM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittrich, A.; Ramesh, G.; Jung, M.; Schmitz, F. Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses. Cells 2023, 12, 1665. https://doi.org/10.3390/cells12121665
Dittrich A, Ramesh G, Jung M, Schmitz F. Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses. Cells. 2023; 12(12):1665. https://doi.org/10.3390/cells12121665
Chicago/Turabian StyleDittrich, Alina, Girish Ramesh, Martin Jung, and Frank Schmitz. 2023. "Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses" Cells 12, no. 12: 1665. https://doi.org/10.3390/cells12121665
APA StyleDittrich, A., Ramesh, G., Jung, M., & Schmitz, F. (2023). Rabconnectin-3α/DMXL2 Is Locally Enriched at the Synaptic Ribbon of Rod Photoreceptor Synapses. Cells, 12(12), 1665. https://doi.org/10.3390/cells12121665







