Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy
Abstract
:1. Introduction
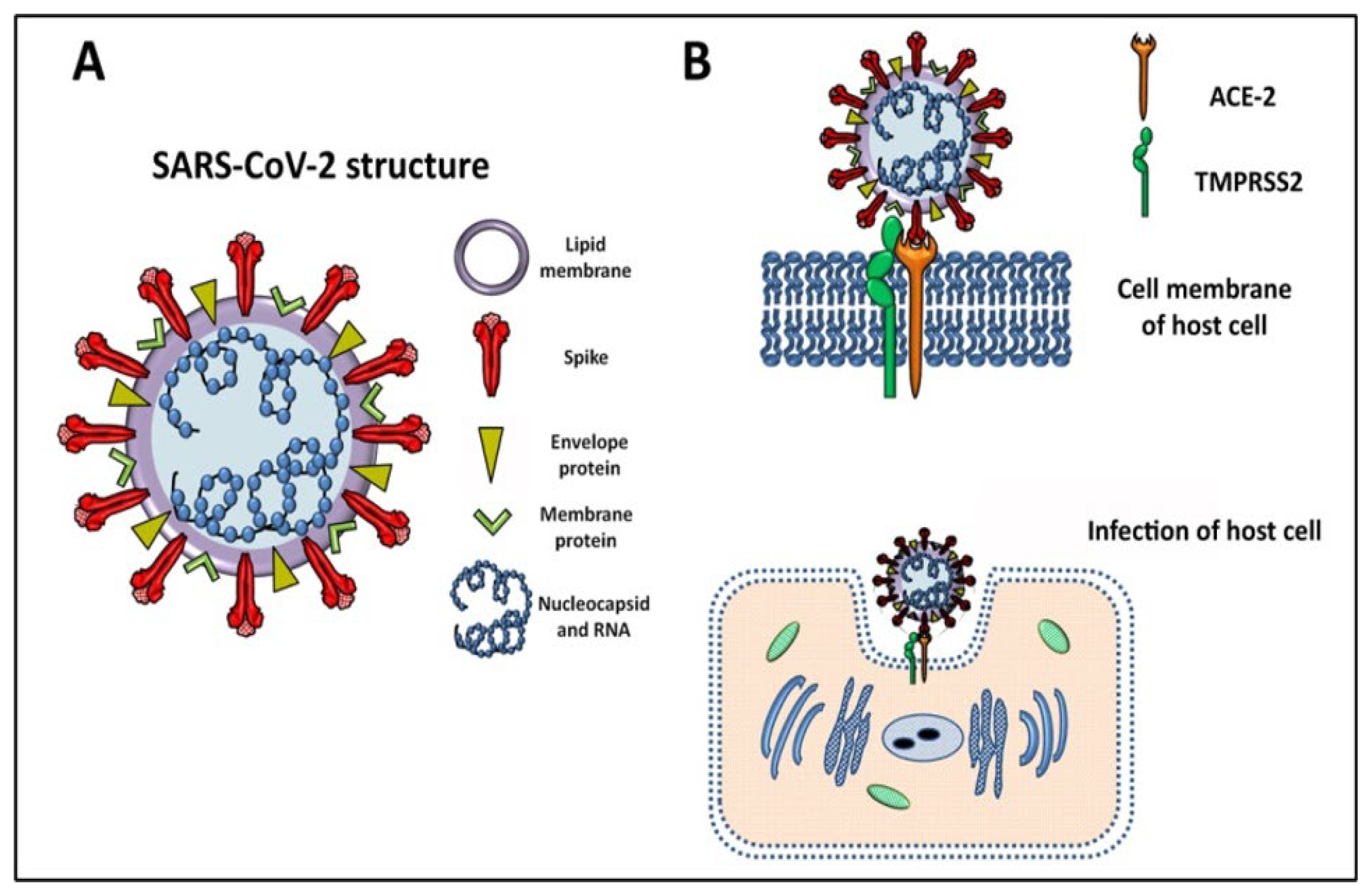
2. SARS-CoV-2 General Features and Mechanism of Infection
2.1. Genome, Structure, and the Variants of Concern behind the High Transmissibility
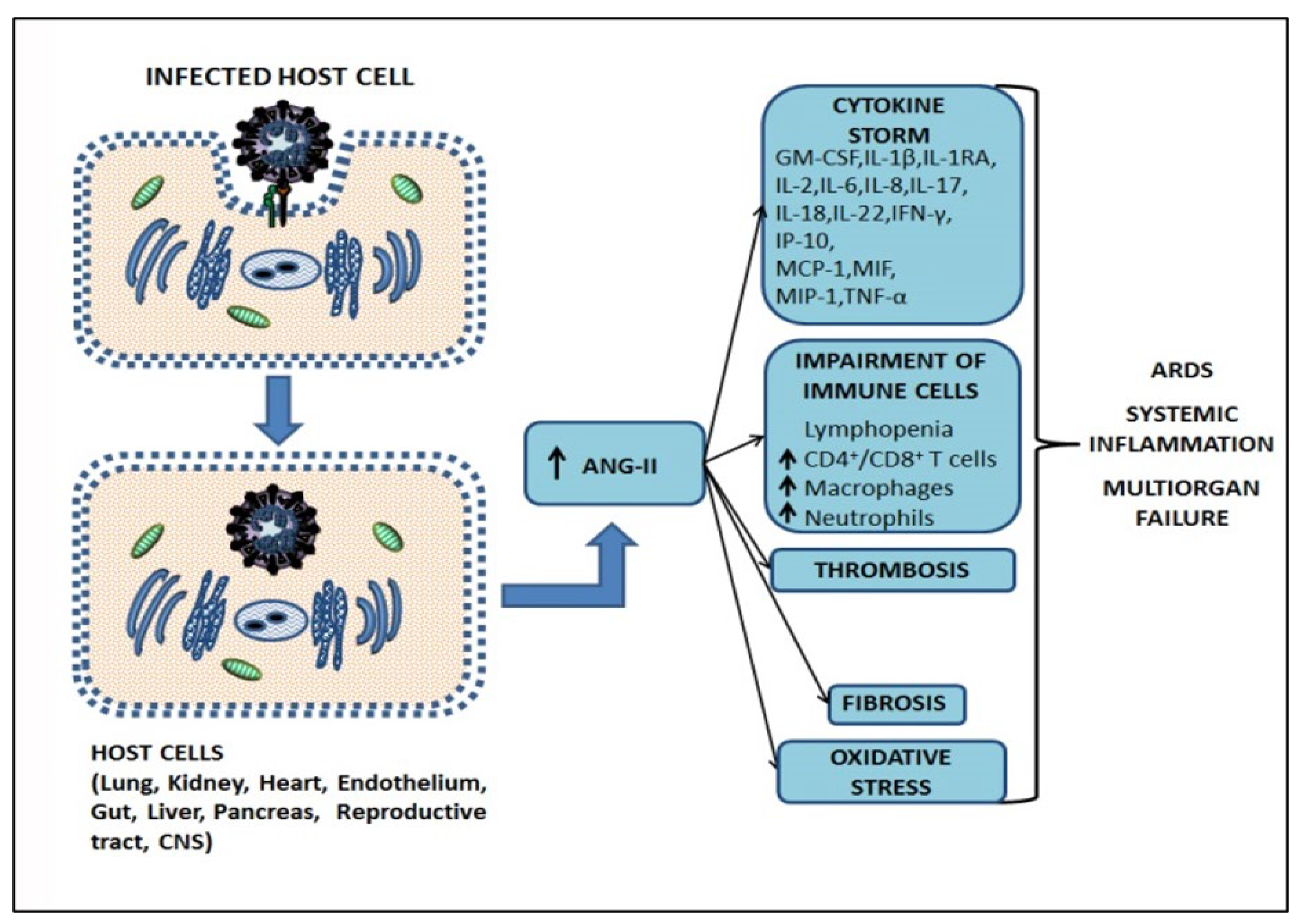
2.2. Mechanism of Infection SARS-CoV-2, the Cytokine Storm, and Pathogenesis of COVID-19
2.3. The Role of Angiotensin II in Tissue Homeostasis Disruption and Multiorgan Failure
3. From the First State of Emergency to the Current Standard Treatments of COVID-19 Patients
3.1. Social Distancing, Face Mask Wearing, and Convalescent Plasma
3.2. IL-6 Receptor Blockers, Monoclonal Antibodies, and Antiviral Agents: The Recommendation of the WHO
3.3. Vital Support: Prone Positioning, Mechanical Ventilation, and Extracorporeal Membrane Oxygenation (ECMO)
4. The Anti-SARS-CoV-2 Vaccines
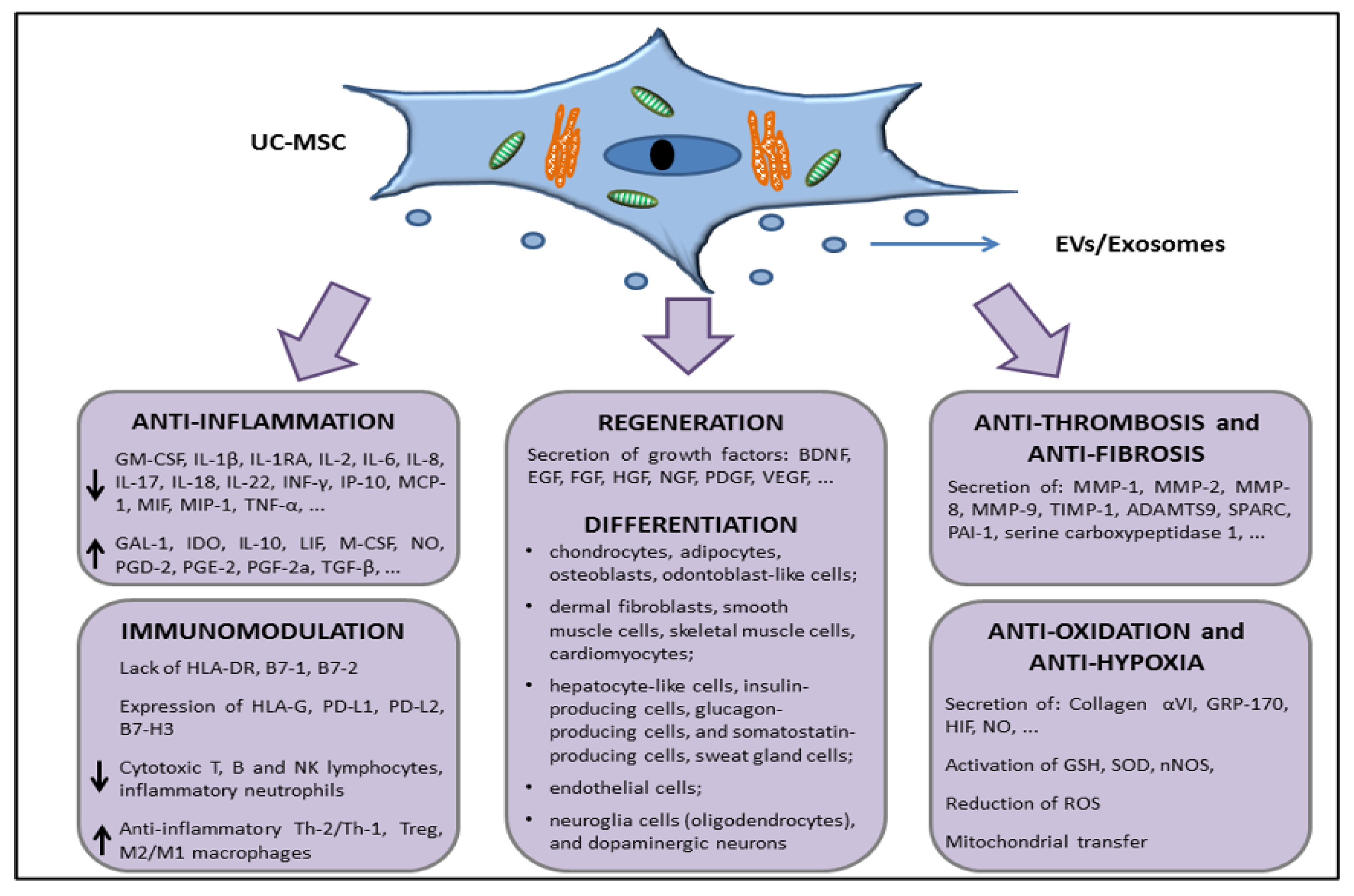
5. Characteristics of UC-MSCs in In Vitro and Preclinical Experimental Evidence Supporting Anti-Inflammatory, Immunomodulation, and Therapeutic Potential
5.1. Adult and Perinatal MSCs: General Features
5.2. UC-MSCs Properties: Multilineage Differentiation, Immune Tolerance, Angiogenesis/Wound Healing, Matrix Remodeling, and Resistance to Hypoxia
5.3. In Vivo Preclinical Data Supporting the Use of UC-MSCs to Treat Organ Dysfunctions
6. MSCs in COVID-19 Patients: Are UC-MSCs Better than the “Gold-Standard” BM-MSCs?
7. Clinical Trials for the Treatment of COVID-19 Patients with UC-MSCs
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef]
- She, J.; Jiang, J.; Ye, L.; Hu, L.; Bai, C.; Song, Y. 2019 novel coronavirus of pneumonia in Wuhan, China: Emerging attack and management strategies. Clin. Transl. Med. 2020, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- WHO. Novel Coronavirus (2019-nCoV): Situation Report, 1. Available online: https://apps.who.int/iris/handle/10665/330760 (accessed on 21 December 2022).
- WHO. Novel Coronavirus (2019-nCoV) Report-1. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 (accessed on 21 December 2022).
- WHO. Statement on the Second Meeting of the International Health Regulations. Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). 2005. Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed on 21 December 2022).
- Nie, J.; Li, Q.; Zhang, L.; Cao, Y.; Zhang, Y.; Li, T.; Wu, J.; Liu, S.; Zhang, M.; Zhao, C.; et al. Functional comparison of SARS-CoV-2 with closely related pangolin and bat coronaviruses. Cell Discov. 2021, 7, 21. [Google Scholar] [CrossRef]
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 21 December 2022).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 December 2022).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- WHO. Living Guidance for Clinical Management of COVID-19. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf (accessed on 22 December 2022).
- Samanipour, R.; Tabatabaee, S.; Delyanee, M.; Tavakoli, A. The promising approach of MSCs therapy for COVID-19 treatment. Cell Tissue Bank. 2022, 16, 1–16. [Google Scholar] [CrossRef]
- Cozene, B.M.; Russo, E.; Anzalone, R.; Rocca, G.; Borlongan, C.V. Mitochondrial activity of human umbilical cord mesenchymal stem cells. Brain. Circ. 2021, 7, 33–36. [Google Scholar] [CrossRef]
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Saadeldin, I.M.; Ahmad, A.; Kumar, D.; Azhar, E.I.; Siddiqui, A.J.; Kurdi, B.; Sajini, A.; Alrefaei, A.F.; Jahan, S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020, 2020, 8835986. [Google Scholar] [CrossRef]
- Russo, E.; Caprnda, M.; Kruzliak, P.; Conaldi, P.G.; Borlongan, C.V.; La Rocca, G. Umbilical Cord Mesenchymal Stromal Cells for Cartilage Regeneration Applications. Stem Cells Int. 2022, 2022, 2454168. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Holmes, E.C. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell 2020, 181, 223–227. [Google Scholar] [CrossRef]
- Peiris, J.S.; Guan, Y.; Yuen, K.Y. Severe acute respiratory syndrome. Nat. Med. 2004, 10, S88–S97. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.B.; Edwards, C.E.; Shaffer, K.M.; Araba, K.C.; Wykoff, J.A.; Williams, D.R.; Asakura, T.; Dang, H.; Morton, L.C.; Gilmore, R.C.; et al. SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13. Proc. Natl. Acad. Sci. USA 2022, 119, e2119680119. [Google Scholar] [CrossRef]
- Mishra, D.; Suri, G.S.; Kaur, G.; Tiwari, M. A comparative insight into genomic landscape of SARS-CoV-2 and identification of mutations associated with origin of infection and diversity. J. Med. Virol. 2020, 93, 2406–2419. [Google Scholar] [CrossRef]
- Forster, P.; Forster, L.; Renfrew, C.; Forster, M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. USA 2020, 117, 9241–9243. [Google Scholar] [CrossRef] [Green Version]
- Wise, J. Covid-19: New coronavirus variant is identified in UK. BMJ 2020, 371, m4857. [Google Scholar] [CrossRef]
- Scovino, A.; Dahab, E.; Vieira, G.; Freire-de-Lima, L.; Freire-de-Lima, C.; Morrot, A. SARS-CoV-2’s Variants of Concern: A Brief Characterization. Front. Immunol. 2022, 13, 834098. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Trentini, F.; Guzzetta, G.; Marziano, V.; Mammone, A.; Sane Schepisi, M.; Poletti, P.; Molina Grane, C.; Manica, M.; Del Manso, M.; et al. Co-circulation of SARS-CoV-2 Alpha and Gamma variants in Italy, February and March 2021. Euro. Surveill. 2022, 27, 2100429. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Trentini, F.; Petrone, D.; Mammone, A.; Ambrosio, L.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Zardini, A.; et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021 to January 2022. Euro. Surveill. 2022, 27, 2200125. [Google Scholar] [CrossRef]
- Di Domenico, M.; De Rosa, A.; Di Gaudio, F.; Internicola, P.; Bettini, C.; Salzano, N.; Castrianni, D.; Marotta, A.; Boccellino, M. Diagnostic Accuracy of a New Antigen Test for SARS-CoV-2 Detection. Int. J. Env. Res. Public Health 2021, 18, 6310. [Google Scholar] [CrossRef]
- Di Gaudio, F.; Brunacci, G.; Contino, F.; Gallo, A.; Centineo, F. Technical and health governance aspects of the External Quality Assessment Scheme for the SARS-CoV-2 molecular tests: Institutional experience performed in all clinical laboratories of a Regional Health Service. Clin. Chem. Lab. Med. 2023, 61, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, A.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Abotaleb, M.; Nosal, V.; Kajo, K.; Ashrafizadeh, M.; et al. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S. SARS-CoV-2 Cell Entry Depends on ACE2 aTMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Hussman, J.P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020, 11, 1169. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef]
- Albini, A.; Di Guardo, G.; McClain Noonan, D.; Lombardo, M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor-and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020, 15, 759–766. [Google Scholar] [CrossRef]
- Abobaker, A.; Raba, A.A.; Alzwi, A. Extrapulmonary and atypical clinical presentations of COVID-19. J. Med. Virol. 2020, 92, 2458–2464. [Google Scholar] [CrossRef]
- Zirpe, K.G.; Dixit, S.; Kulkarni, A.P.; Sapra, H.; Kakkar, G.; Gupta, R.; Bansal, A.R. Pathophysiological Mechanisms and Neurological Manifestations in COVID-19. Indian J. Crit. Care Med. 2020, 24, 975–980. [Google Scholar] [PubMed]
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Cuschieria, S.; Grech, S. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020, 34, 107637. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Amraei, R.; Yin, W.; Napoleon, M.A.; Suder, E.L.; Berrigan, J.; Zhao, Q.; Olejnik, J.; Chandler, K.B.; Xia, C.; Feldman, J.; et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. bioRxiv 2021, 7, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zeng, J.; Jia, N.; Stavenhagen, K.; Matsumoto, Y.; Zhang, H.; Li, J. SARS-CoV-2 Spike Protein Interacts with Multiple Innate Immune Receptors. BioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, A.; Graham, B.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Wang, T.; Lee, E.; Cremer, P.; Carey, B.; Rajendram, P. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front. Immunol. 2020, 11, 1625. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Affandi, A.J.; Olesek, K.; Grabowska, J.; Nijen Twilhaar, M.K.; Rodriguez, E.; Saris, A.; Zwart, E.S.; Nossent, E.J.; Kalay, H.; de Kok, M.; et al. CD169 Defines Activated CD14(+) Monocytes With Enhanced CD8(+) T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840. [Google Scholar] [CrossRef]
- Abassi, Z.; Knaney, Y.; Karram, T.; Heyman, S. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front. Immunol. 2020, 11, 1312. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Rostami, M.; Mansouritorghabeh, H. D-dimer level in COVID-19 infection: A systematic review. Expert Rev. Hematol. 2020, 13, 1265–1275. [Google Scholar] [CrossRef]
- Gelzo, M.; Cacciapuoti, S.; Pinchera, B.; De Rosa, A.; Cernera, G.; Scialo, F.; Comegna, M.; Mormile, M.; Fabbrocini, G.; Parrella, R.; et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci. Rep. 2022, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Fong, S.W.; Young, B.E.; Chan, Y.H.; Lee, B.; Amrun, S.N.; Chee, R.S.; Yeo, N.K.; Tambyah, P.; Pada, S.; et al. Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients. Open Forum Infect. Dis. 2021, 8, ofab156. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, L.; Pink, I.; Kuhne, J.F.; Beushausen, K.; Keil, J.; Christoph, S.; Sauer, A.; Boblitz, L.; Schmidt, J.; David, S.; et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 2021, 6, 418. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Mentzer, S.J.; Kolb, M.; Jonigk, D. Inflammation and intussusceptive angiogenesis in COVID-19: Everything in and out of flow. Eur. Respir. J. 2020, 56, 2003147. [Google Scholar] [CrossRef]
- Eslamifar, Z.; Behzadifard, M.; Soleimani, M.; Behzadifard, S. Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor. Thromb. J. 2020, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Miesbach, W. Pathological Role of Angiotensin II in Severe COVID-19. TH Open 2020, 4, e138–e144. [Google Scholar] [CrossRef]
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension 2018, 71, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Garcia, J.; Osca-Verdegal, R.; Pallardo, F.V.; Ferreres, J.; Rodriguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; Garcia-Gimenez, J.L. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Montiel, V.; Lobysheva, I.; Gerard, L.; Vermeersch, M.; Perez-Morga, D.; Castelein, T.; Mesland, J.B.; Hantson, P.; Collienne, C.; Gruson, D.; et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. EBioMedicine 2022, 77, 103893. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef] [Green Version]
- Rupérez, M.; Lorenzo, O.; Blanco-Colio, L.M.; Esteban, V.; Egido, J.; Ruiz-Ortega, M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 2003, 108, 1499–1505. [Google Scholar] [CrossRef] [Green Version]
- Mauviel, A. Transforming growth factor-beta: A key mediator of fibrosis. Methods Mol. Med. 2005, 117, 69–80. [Google Scholar]
- Hirano, T.; Murakami, M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 2020, 52, 731–733. [Google Scholar] [CrossRef]
- Hrenak, J.; Simko, F. Renin–Angiotensin System: An Important Player in the Pathogenesis of Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 8038. [Google Scholar] [CrossRef]
- Murphy, A.M.; Wong, A.L.; Bezuhlly, M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair. 2015, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J. Med. Virol. 2022, 94, 1886–1892. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schunemann, H.J.; COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Kwon, S.; Joshi, A.D.; Lo, C.H.; Drew, D.A.; Nguyen, L.H.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Astley, C.M.; et al. Association of social distancing and masking with risk of COVID-19. medRxiv 2020, 12, 3737. [Google Scholar] [CrossRef]
- Piechotta, V.; Iannizzi, C.; Chai, K.L.; Valk, S.J.; Kimber, C.; Dorando, E.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 5, CD013600. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, Z.; Ma, H.; Shao, S.; Kang, H.; Tong, Z. The efficiency of convalescent plasma in COVID-19 patients: A systematic review and meta-analysis of randomized controlled clinical trials. Front. Immunol. 2022, 13, 964398. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Y.; Lv, H.; Guan, Z.; Gu, J. Caution against corticosteroid-based COVID-19 treatment. Lancet 2020, 395, 1759–1760. [Google Scholar] [CrossRef]
- Shuto, H.; Komiya, K.; Yamasue, M.; Uchida, S.; Ogura, T.; Mukae, H.; Tateda, K.; Hiramatsu, K.; Kadota, J.I. A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19. Sci. Rep. 2020, 10, 20935. [Google Scholar] [CrossRef] [PubMed]
- Minkoff, J.M.; tenOever, B. Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Rubbert-Roth, A.; Furst, D.E.; Nebesky, J.M.; Jin, A.; Berber, E. A Review of Recent Advances Using Tocilizumab in the Treatment of Rheumatic Diseases. Rheumatol. Ther. 2018, 5, 21–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Prequalifies First Monoclonal Antibody—Tocilizumab—To Treat COVID-19. Available online: https://www.who.int/news/item/11-02-2022-who-prequalifies-first-monoclonal-antibody---tocilizumab-to-treat-covid-19 (accessed on 21 December 2022).
- Brown, M.J.; Alazawi, W.; Kanoni, S. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 1147. [Google Scholar] [CrossRef]
- Rosas, I.O.; Brau, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.P.T.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- Campochiaro, C.; Tomelleri, A.; Matucci-Cerinic, M.; Dagna, L. One year later: The case of tocilizumab in COVID-19. Eur. J. Intern. Med. 2022, 95, 5–6. [Google Scholar] [CrossRef]
- WHO. Therapeutics and COVID-19: Living Guideline, 16 September 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5 (accessed on 21 December 2022).
- Focosi, D.; McConnell, S.; Casadevall, A. The Omicron variant of concern: Diversification and convergent evolution in spike protein, and escape from anti-Spike monoclonal antibodies. Drug. Resist. Updat. 2022, 65, 100882. [Google Scholar] [CrossRef] [PubMed]
- WHO. TAG-VE Statement on Omicron Sublineages BQ.1 and XBB. Available online: https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sublineages-bq.1-and-xbb (accessed on 28 December 2022).
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023, 388, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, P.; Maggiore, S.M.; Mercat, A. Fifty Years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 964–984. [Google Scholar] [CrossRef] [PubMed]
- Touchon, F.; Trigui, Y.; Prud’homme, E.; Lefebvre, L.; Giraud, A.; Dols, A.M.; Martinez, S.; Bernardi, M.; Begne, C.; Granier, P.; et al. Awake prone positioning for hypoxaemic respiratory failure: Past, COVID-19 and perspectives. Eur. Respir. Rev. 2021, 30, 210022. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Jubran, A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care 2020, 10, 78. [Google Scholar] [CrossRef]
- Cabrera-Benitez, N.E.; Laffey, J.G.; Parotto, M.; Spieth, P.M.; Villar, J.; Zhang, H.; Slutsky, A.S. Mechanical Ventilation–associated Lung Fibrosis in Acute Respiratory Distress Syndrome A Significant Contributor to Poor Outcome. Anesthesiology 2014, 121, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Pierrakos, C.; Karanikolas, M.; Scolletta, S.; Karamouzos, V.; Velissaris, D. Acute Respiratory Distress Syndrome: Pathophysiology and Therapeutic Options. J. Clin. Med. Res. 2012, 4, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Hajage, D.; Combes, A.; Guervilly, C.; Lebreton, G.; Mercat, A.; Pavot, A.; Nseir, S.; Mekontso-Dessap, A.; Mongardon, N.; Mira, J.P.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome Associated with COVID-19: An Emulated Target Trial Analysis. Am. J. Respir. Crit. Care Med. 2022, 206, 281–294. [Google Scholar] [CrossRef]
- Bertini, P.; Guarracino, F.; Falcone, M.; Nardelli, P.; Landoni, G.; Nocci, M.; Paternoster, G. ECMO in COVID-19 Patients: A Systematic Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2700–2706. [Google Scholar] [CrossRef]
- Fanelli, V.; Giani, M.; Grasselli, G.; Mojoli, F.; Martucci, G.; Grazioli, L.; Alessandri, F.; Mongodi, S.; Sales, G.; Montrucchio, G.; et al. Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: A multicenter retrospective cohort study. Crit. Care 2022, 26, 34. [Google Scholar] [CrossRef]
- WHO. The COVID-19 Vaccine Tracker and Landscape Compiles Detailed Information of Each COVID-19 Vaccine Candidate in Development by Closely Monitoring Their Progress through the Pipeline. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 5 January 2023).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020; online ahead of print. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 920–1931. [Google Scholar] [CrossRef]
- Grana, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Busa, R.; Miele, M.; Sorrentino, M.C.; Amico, G.; Timoneri, F.; Miceli, V.; Di Bella, M.; Russelli, G.; Gallo, A.; Zito, G.; et al. Long-Term Effectiveness of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine on B Cell Compartment: Efficient Recall of SARS-CoV-2-Specific Memory B Cells. Int. J. Mol. Sci. 2022, 23, 15046. [Google Scholar] [CrossRef] [PubMed]
- Ailsworth, S.M.; Keshavarz, B.; Richards, N.E.; Workman, L.J.; Murphy, D.D.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster vaccination and comparison of BNT162b2 with mRNA-1273. Ann. Allergy Asthma Immunol. 2023, 130, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Russelli, G.; Miele, M.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Di Mento, G.; Mularoni, A.; Vitulo, P.; Conaldi, P.G.; et al. Immune Response after the Fourth Dose of SARS-CoV-2 mRNA Vaccine Compared to Natural Infection in Three Doses’ Vaccinated Solid Organ Transplant Recipients. Viruses 2022, 14, 2299. [Google Scholar] [CrossRef]
- Emary, K.; Golubchik, T.; Aley, P.; Ariani, C.; Angus, B.; Bibi, S.; Blane, B. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Krause, P.; Fleming, T.; Peto, R.; Longini, I.; Figueroa, J.; Sterne, J.; Cravioto, A. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Lenero, C.; Bowles, A.C.; Correa, D.; Kouroupis, D. Characterization and response to inflammatory stimulation of human endometrial-derived mesenchymal stem/stromal cells. Cytotherapy 2022, 24, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Q.M.; Yang, R.; Zhang, M.; Zou, W.H.; Qian, N.N.; Xu, Q.J.; Chen, H.; Peng, J.C.; Luo, X.P.; Zhang, Q.; et al. Peripheral Blood-Derived Mesenchymal Stem Cells Modulate Macrophage Plasticity through the IL-10/STAT3 Pathway. Stem Cells Int. 2022, 2022, 5181241. [Google Scholar] [CrossRef]
- Rotter, N.; Oder, J.; Schlenke, P.; Lindner, U.; Bohrnsen, F.; Kramer, J.; Rohwedel, J.; Huss, R.; Brandau, S.; Wollenberg, B.; et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008, 17, 509–518. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, H.R.; Lim, J.Y.; Lim, Y.C. Single clonal glandular stem cells derived from human parotid glands do not attain malignant phenotype during long-term in vitro culture. Neoplasma 2021, 68, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Tappenbeck, N.; Schroder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546–560. [Google Scholar] [CrossRef]
- Vander Beken, S.; de Vries, J.C.; Meier-Schiesser, B.; Meyer, P.; Jiang, D.; Sindrilaru, A.; Ferreira, F.F.; Hainzl, A.; Schatz, S.; Muschhammer, J.; et al. Newly Defined ATP-Binding Cassette Subfamily B Member 5 Positive Dermal Mesenchymal Stem Cells Promote Healing of Chronic Iron-Overload Wounds via Secretion of Interleukin-1 Receptor Antagonist. Stem Cells 2019, 37, 1057–1074. [Google Scholar] [CrossRef] [Green Version]
- Najar, M.; Lagneaux, L. Foreskin as a source of immunotherapeutic mesenchymal stromal cells. Immunotherapy 2017, 9, 207–217. [Google Scholar] [CrossRef]
- Najar, M.; Merimi, M.; Faour, W.H.; Lombard, C.A.; Moussa Agha, D.; Ouhaddi, Y.; Sokal, E.M.; Lagneaux, L.; Fahmi, H. In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics 2021, 13, 1736. [Google Scholar] [CrossRef]
- Neybecker, P.; Henrionnet, C.; Pape, E.; Mainard, D.; Galois, L.; Loeuille, D.; Gillet, P.; Pinzano, A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Amemiya, M.; Tsuji, K.; Katagiri, H.; Miyatake, K.; Nakagawa, Y.; Sekiya, I.; Muneta, T.; Koga, H. Synovial fluid-derived mesenchymal cells have non-inferior chondrogenic potential and can be utilized for regenerative therapy as substitute for synovium-derived cells. BioChem. Biophys. Res. Commun. 2020, 523, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Komaki, M.; Rudnicki, M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001, 68, 245–253. [Google Scholar] [CrossRef]
- Kang, J.S.; Krauss, R.S. Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Elashry, M.I.; Kinde, M.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S. The effect of hypoxia on myogenic differentiation and multipotency of the skeletal muscle-derived stem cells in mice. Stem Cell Res. Ther. 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L.; Funderburgh, M.L.; Du, Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016, 14, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5507–5517. [Google Scholar] [CrossRef]
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Anzalone, R.; Corrao, S.; Lo Iacono, M.; Loria, T.; Corsello, T.; Cappello, F.; Di Stefano, A.; Giannuzzi, P.; Zummo, G.; Farina, F.; et al. Isolation and characterization of CD276+/HLA-E+ human subendocardial mesenchymal stem cells from chronic heart failure patients: Analysis of differentiative potential and immunomodulatory markers expression. Stem Cells Dev. 2013, 22, 1–17. [Google Scholar] [CrossRef]
- Rolandsson Enes, S.; Andersson Sjöland, A.; Skog, I.; Hansson, L.; Larsson, H.; Le Blanc, K.; Eriksson, L. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci. Rep. 2016, 6, 29160. [Google Scholar] [CrossRef] [Green Version]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal 2011, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef]
- Pampalone, M.; Corrao, S.; Amico, G.; Vitale, G.; Alduino, R.; Conaldi, P.G.; Pietrosi, G. Human Amnion-Derived Mesenchymal Stromal Cells in Cirrhotic Patients with Refractory Ascites: A Possible Anti-Inflammatory Therapy for Preventing Spontaneous Bacterial Peritonitis. Stem Cell Rev. Rep. 2021, 17, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. BioChem. Biophys. Res. Commun. 2020, 522, 171–176. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Corrao, S.; La Rocca, G.; Lo Iacono, M.; Corsello, T.; Farina, F.; Anzalone, R. Umbilical cord revisited: From Wharton’s jelly myofibroblasts to mesenchymal stem cells. Histol. Histopathol. 2013, 28, 1235–1244. [Google Scholar] [CrossRef]
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. BioSci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Zeddou, M.; Briquet, A.; Relic, B.; Josse, C.; Malaise, M.G.; Gothot, A.; Lechanteur, C.; Beguin, Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol. Int. 2010, 34, 693–701. [Google Scholar] [CrossRef]
- Vangsness, C.T., Jr.; Sternberg, H.; Harris, L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy 2015, 31, 1836–1843. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef] [Green Version]
- Facchin, F.; Bianconi, E.; Romano, M.; Impellizzeri, A.; Alviano, F.; Maioli, M.; Canaider, S.; Ventura, C. Comparison of Oxidative Stress Effects on Senescence Patterning of Human Adult and Perinatal Tissue-Derived Stem Cells in Short and Long-term Cultures. Int. J. Med. Sci. 2018, 15, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Watson, N.; Divers, R.; Kedar, R.; Mehindru, A.; Mehindru, A.; Borlongan, M.C.; Borlongan, C.V. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy 2015, 17, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef]
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Loria, T.; Lo Iacono, M.; Di Stefano, A.; Giannuzzi, P.; Marasà, L.; Cappello, F.; et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: Differentiation potential and detection of new markers. Histochem. Cell Biol. 2009, 131, 267–282. [Google Scholar] [CrossRef]
- Lo Iacono, M.; Russo, E.; Anzalone, R.; Baiamonte, E.; Alberti, G.; Gerbino, A.; Maggio, A. Wharton’s Jelly Mesenchymal Stromal Cells Support the Expansion of Cord Blood–derived CD34+ Cells Mimicking a Hematopoietic Niche in a Direct Cell–cell Contact Culture System. Cell Transpl. 2018, 27, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 610544. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Abumaree, M.H.; Silini, A.R.; Anzalone, R.; Saieva, S.; Russo, E.; Trapani, M.E.; La Rocca, G.; Parolini, O. Chapter 6. The Immunomodulatory Features of Mesenchymal Stromal Cells Derived from Wharton’s Jelly, Amniotic Membrane, and Chorionic Villi: In Vitro and In Vivo Data. In Placenta: The Tree of Life; Parolini, O., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 91–128. [Google Scholar]
- Anzalone, R.; Lo Iacono, M.; Loria, T.; Di Stefano, A.; Giannuzzi, P.; Farina, F.; La Rocca, G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: Extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011, 7, 342–363. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.; Shivakumar, S.B.; Park, J.K.; Ullah, I.; Subbarao, R.B.; Park, J.S.; Lee, S.L.; Park, B.W.; Rho, G.J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rocca, G.; Lo Iacono, M.; Corsello, T.; Corrao, S.; Farina, F.; Anzalone, R. Human Wharton’s jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: New perspectives for cellular therapy. Curr. Stem Cell Res. Ther. 2013, 8, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Corrao, S.; La Rocca, G.; Lo Iacono, M.; Zummo, G.; Gerbino, A.; Farina, F.; Anzalone, R. New frontiers in regenerative medicine in cardiology: The potential of Wharton’s jelly mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2013, 8, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.C.; Park, I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016, 6, 23544. [Google Scholar] [CrossRef] [Green Version]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Chai, J.; Sun, T.; Li, D.; Tao, R. Differentiation of human umbilical cord mesenchymal stem cells into dermal fibroblasts in vitro. BioChem. Biophys. Res. Commun. 2011, 413, 561–565. [Google Scholar] [CrossRef]
- Conconi, M.T.; Burra, P.; Di Liddo, R.; Calore, C.; Turetta, M.; Bellini, S.; Bo, P.; Nussdorfer, G.G.; Parnigotto, P.P. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006, 18, 1089–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.H.; Mo, X.M.; Zhou, B.; Lu, S.H.; Yang, S.G.; Liu, Y.L.; Han, Z.C. Cardiac potential of stem cells from whole human umbilical cord tissue. J. Cell Biochem. 2009, 107, 926–932. [Google Scholar] [CrossRef]
- Campard, D.; Lysy, P.A.; Najimi, M.; Sokal, E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 2008, 134, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, R.; Iacono, M.L.; Corrao, S.; Magno, F.; Loria, T.; Cappello, F.; Zummo, G.; Farina, F.; La Rocca, G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010, 19, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Belame Shivakumar, S.; Bharti, D.; Baregundi Subbarao, R.; Park, J.M.; Son, Y.B.; Ullah, I.; Choe, Y.H.; Lee, H.J.; Park, B.W.; Lee, S.L.; et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J. Cell Physiol. 2019, 234, 3933–3947. [Google Scholar] [CrossRef]
- Sarang, S.; Viswanathan, C. Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production—Another Opportunity in Cell Therapy. Int. J. Stem Cells 2016, 9, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, K.E.; Weiss, M.L.; Mitchell, B.M.; Martin, P.; Davis, D.; Morales, L.; Helwig, B.; Beerenstrauch, M.; Abou-Easa, K.; Hildreth, T.; et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 2003, 21, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsello, T.; Amico, G.; Corrao, S.; Anzalone, R.; Timoneri, F.; Lo Iacono, M.; Russo, E. Wharton’s Jelly Mesenchymal Stromal Cells from Human Umbilical Cord: A Close-up on Immunomodulatory Molecules Featured In Situ and In Vitro. Stem Cell Rev. Rep. 2019, 15, 900–918. [Google Scholar] [CrossRef]
- Tesarova, L.; Jaresova, K.; Simara, P.; Koutna, I. Umbilical Cord-Derived Mesenchymal Stem Cells Are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. Int. J. Mol. Sci. 2020, 21, 5366. [Google Scholar] [CrossRef]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Weng, J.; Li, H.; Ma, Y.; Liu, L.; Ma, W. Advances in the study of HLA class Ib in maternal-fetal immune tolerance. Front. Immunol. 2022, 13, 976289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal-Fetal Immunity. Front. Immunol. 2020, 11, 458. [Google Scholar] [CrossRef] [Green Version]
- Sawai, K.; Matsuzaki, N.; Kameda, T.; Hashimoto, K.; Okada, T.; Shimoya, K.; Nobunaga, T.; Taga, T.; Kishimoto, T.; Saji, F. Leukemia inhibitory factor produced at the fetomaternal interface stimulates chorionic gonadotropin production: Its possible implication during pregnancy, including implantation period. J. Clin. Endocrinol. Metab. 1995, 80, 1449–1456. [Google Scholar] [CrossRef]
- Hamelin-Morrissette, J.; Dallagi, A.; Girouard, J.; Ravelojaona, M.; Oufqir, Y.; Vaillancourt, C.; Van Themsche, C.; Carrier, C.; Reyes-Moreno, C. Leukemia inhibitory factor regulates the activation of inflammatory signals in macrophages and trophoblast cells. Mol. Immunol. 2020, 120, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Koh, I.; Sugimoto, J. Localization of Indoleamine 2,3-Dioxygenase-1 and Indoleamine 2,3-Dioxygenase-2 at the Human Maternal-Fetal Interface. Int. J. Tryptophan. Res. 2020, 13, 1178646920984163. [Google Scholar] [CrossRef]
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Rouas-Freiss, N.; Goncalves, R.M.; Menier, C.; Dausset, J.; Carosella, E.D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 11520–11525. [Google Scholar] [CrossRef] [Green Version]
- LeMaoult, J.; Zafaranloo, K.; Le Danff, C.; Carosella, E.D. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005, 19, 662–664. [Google Scholar] [CrossRef]
- Gieseke, F.; Bohringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Muller, I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- McGuirk, J.P.; Smith, J.R.; Divine, C.L.; Zuniga, M.; Weiss, M.L. Wharton’s Jelly-Derived Mesenchymal Stromal Cells as a Promising Cellular Therapeutic Strategy for the Management of Graft-versus-Host Disease. Pharmaceuticals 2015, 8, 196–220. [Google Scholar] [CrossRef] [Green Version]
- Dunavin, N.; Dias, A.; Li, M.; McGuirk, J. Mesenchymal Stromal Cells: What Is the Mechanism in Acute Graft-Versus-Host Disease? Biomedicines 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.G.; Chen, J.R.; Hernandez, L.; Alvord, W.G.; Stewart, C.L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. USA 2001, 98, 8680–8685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, K.; Van den Haute, C.; Baekelandt, V.; Lucas, S.; van Horssen, J.; Somers, V.; Van Wijmeersch, B. Leukemia inhibitory factor tips the immune balance towards regulatory T cells in multiple sclerosis. Brain. Behav. Immun. 2015, 45, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.A.; Zheng, T.; Whiting, N.L.; Marcovici, A.; Trow, T.K. Cytokine-cytokine synergy and protein kinase C in the regulation of lung fibroblast leukemia inhibitory factor. Am. J. Physiol. 1994, 266, L426–L435. [Google Scholar] [CrossRef]
- Corrao, S.; Campanella, C.; Anzalone, R.; Farina, F.; Zummo, G.; Conway de Macario, E.; Macario, A.J.; Cappello, F.; La Rocca, G. Human Hsp10 and Early Pregnancy Factor (EPF) and their relationship and involvement in cancer and immunity: Current knowledge and perspectives. Life Sci. 2010, 86, 145–152. [Google Scholar] [CrossRef]
- Corrao, S.; Anzalone, R.; Lo Iacono, M.; Corsello, T.; Di Stefano, A.; D’Anna, S.E.; Balbi, B.; Carone, M.; Sala, A.; Corona, D.; et al. Hsp10 nuclear localization and changes in lung cells response to cigarette smoke suggest novel roles for this chaperonin. Open Biol. 2014, 4, 140125. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.J.; Wang, H.S.; Lin, G.J.; Chou, S.C.; Chu, T.H.; Chuan, W.T.; Lu, Y.J. Undifferentiated Wharton’s Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transpl. 2015, 24, 1555–1570. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.Y.; Wang, H.W.; Chang, S.J.; Liao, K.H.; Lee, I.H.; Lin, W.S.; Wu, C.H.; Lin, W.Y.; Cheng, S.M. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE 2013, 8, e72604. [Google Scholar] [CrossRef] [Green Version]
- Arutyunyan, I.; Fatkhudinov, T.; Kananykhina, E.; Usman, N.; Elchaninov, A.; Makarov, A.; Bolshakova, G.; Goldshtein, D.; Sukhikh, G. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: In vitro study. Stem Cell Res. Ther. 2016, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound. Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Edwards, S.S.; Zavala, G.; Prieto, C.P.; Elliott, M.; Martinez, S.; Egana, J.T.; Bono, M.R.; Palma, V. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis 2014, 17, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.; Lee, J.Y.; Nguyen, H.; Corrao, S.; Anzalone, R.; La Rocca, G.; Borlongan, C.V. Energy Metabolism Analysis of Three Different Mesenchymal Stem Cell Populations of Umbilical Cord Under Normal and Pathologic Conditions. Stem Cell Rev. Rep. 2020, 16, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Napoli, E.; Borlongan, C.V. Healthy mitochondria for stroke cells. Brain Circ. 2018, 4, 95–98. [Google Scholar] [PubMed]
- Lin, H.Y.; Liou, C.W.; Chen, S.D.; Hsu, T.Y.; Chuang, J.H.; Wang, P.W.; Huang, S.T. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 2015, 22, 31–44. [Google Scholar] [CrossRef]
- Alberti, G.; Russo, E.; Corrao, S.; Anzalone, R.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines 2022, 10, 2822. [Google Scholar] [CrossRef]
- Alberti, G.; Sánchez-López, C.M.; Andres, A.; Santonocito, R.; Campanella, C.; Cappello, F.; Marcilla, A. Molecular Profile Study of Extracellular Vesicles for the Identification of Useful Small “Hit” in Cancer Diagnosis. Appl. Sci. 2021, 11, 10787. [Google Scholar] [CrossRef]
- Stefano, F.; Mariantonia, L.; Giusi, A.; Claudia, C. Exosomal Hsp60: A tumor biomarker? In Heat Shock Protein 60 in Human Diseases and Disorders; Asea, A., Kaur, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 107–116. [Google Scholar]
- Miceli, V.; Bertani, A.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Carcione, C.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 510. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826. [Google Scholar] [CrossRef]
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transpl. 2011, 20, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donders, R.M.; Vanheusden, R.M.; Bogie, J.F.; Ravanidis, S.; Thewissen, K.; Stinissen, P.; Gyselaers, W. Human Wharton’s jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell Transpl. 2015, 24, 2077–2098. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qiu, X.; Ni, P.; Qiu, X.; Lin, X.; Wu, W.; Xie, L. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int. J. Mol. Med. 2014, 33, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos Nascimento, D.; Mosqueira, D.; Sousa, L.M.; Teixeira, M.; Filipe, M.; Resende, T.P.; Araujo, A.F.; Valente, M.; Almeida, J.; Martins, J.P.; et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, S.; Masterson, C.; Brady, J.; Loftus, P.; Horan, E.; O’Flynn, L.; Elliman, S. Umbilical cord-derived CD362+ mesenchymal stromal cells for E. coli pneumonia: Impact of dose regimen, passage, cryopreservation, and antibiotic therapy. Stem Cell Res. Ther. 2020, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R. Human Umbilical Cord Mesenchymal Stem Cells Reduce Fibrosis of Bleomycin-Induced Lung Injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol Sci. 2020, 21, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Fischer, U.; Harting, M.; Jimenez, F.; Monzon-Posadas, W.; Xue, H.; Savitz, S.; Laine, G.; Cox, C.J. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Yao, W.; Shi, L.; Zhang, Y.; Dong, H.; Zhang, Y. Mesenchymal stem/stromal cell therapy for COVID-19 pneumonia: Potential mechanisms, current clinical evidence, and future perspectives. Stem Cell Res. Ther. 2022, 13, 124. [Google Scholar] [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef]
- Grégoire, C.; Ritacco, C.; Hannon, M.; Seidel, L.; Delens, L.; Belle, L.; Dubois, S.; Vériter, S.; Lechanteur, C.; Briquet, A.; et al. Comparison of Mesenchymal Stromal Cells from Different Origins for the Treatment of Graft-vs.-Host-Disease in a Humanized Mouse Model. Front. Immunol. 2019, 10, 619. [Google Scholar] [CrossRef]
- Ji, L.; Zhan, Y.; Hua, F.; Li, F.; Zou, S.; Wang, W.; Song, D.; Min, Z.; Chen, H.; Cheng, Y. The ratio of Treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PLoS ONE 2012, 7, e50909. [Google Scholar] [CrossRef] [Green Version]
- Lapietra, G.; Ferretti, A.; Baldacci, E.; Chistolini, A.; Santoro, C. Immune thrombocytopenia management during COVID-19 pandemic: An Italian monocentric experience. EJHaem 2022, 3, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Zhu, X.L.; Xue, J.; Liu, X.; Long Zheng, X.; Chang, Y.J.; Liu, K.Y.; Huang, X.J.; Zhang, X.H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genom. 2018, 18, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, Z.; Wang, Y.; Xu, R.; Sun, Y.; Zhang, M.; Yu, X.; Wang, H.; Meng, L.; Su, H.; et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl. Med. 2017, 6, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Ganguly, A.; Swaminathan, G.; Garcia-Marques, F.; Regmi, S.; Yarani, R.; Primavera, R.; Chetty, S.; Bermudez, A.; Pitteri, S.J.; Thakor, A.S. Integrated transcriptome-proteome analyses of human stem cells reveal source-dependent differences in their regenerative signature. Stem Cell Rep. 2023, 18, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [Green Version]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Wang, H.; Bian, L.; Huang, J.; Wu, D.; Zhang, R.; Fei, F. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev. Rep. 2022, 18, 2152–2163. [Google Scholar] [CrossRef]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target. Ther. 2020, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.; Aditianingsih, D.; Sugiarto, A.; Burhan, E.; Damayanti, T.; Sitompul, P.; Mariana, N. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 361. [Google Scholar] [CrossRef]
- Feng, G.; Shi, L.; Huang, T.; Ji, N.; Zheng, Y.; Lin, H.; Niu, C. Human Umbilical Cord Mesenchymal Stromal Cell Treatment of Severe COVID-19 Patients: A 3-Month Follow-Up Study Following Hospital Discharge. Stem Cells Dev. 2021, 30, 773–781. [Google Scholar] [CrossRef]
- Saleh, M.; Vaezi, A.; Aliannejad, R.; Sohrabpour, A.; Kiaei, S.; Shadnoush, M.; Siavashi, V. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: A phase 1 clinical trial. Stem Cell Res. Ther. 2021, 12, 410. [Google Scholar] [CrossRef]
- Kaffash Farkhad, N.; Sedaghat, A.; Reihani, H.; Adhami Moghadam, A.; Bagheri Moghadam, A.; Khadem Ghaebi, N.; Khodadoust, M. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: A successful phase 1, control-placebo group, clinical trial. Stem Cell Res. Ther. 2022, 13, 283. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, J.; Wu, J.; Xu, Y.; Chen, B.; Jiang, L.; Xiang, H. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020, 53, e12947. [Google Scholar] [CrossRef]
- Zhu, R.; Yan, T.; Feng, Y.; Liu, Y.; Cao, H.; Peng, G.; Yang, Y. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021, 31, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S. Treatment with human umbilical cord-1 derived mesenchymal stem cells for COVID-19 patients with lung damage: A randomised, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yuan, X.; Yao, W.; Wang, S.; Zhang, C.; Zhang, B.; Song, J. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine 2022, 75, 103789. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Meaux, J.; Tchoumba, N.; Diehl, J. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: A multicenter randomized double-blind trial. Crit. Care 2022, 26, 48. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.; Kouroupis, D.; Alvarez Gil, A. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021; online ahead of print. [Google Scholar]
- Kouroupis, D.; Lanzoni, G.; Linetsky, E.; Messinger Cayetano, S.; Wishnek Metalonis, S.; Leñero, C.; Stone, L. Umbilical Cord-derived Mesenchymal Stem Cells modulate TNF and soluble TNF Receptor 2 (sTNFR2) in COVID-19 ARDS patients. Eur. Rev. Med. Pharm. Sci. 2021, 25, 4435–4438. [Google Scholar]
- O Ercelen, N.; Pekkoc-Uyanik, K.; Alpaydin, N.; Gulay, G.; Simsek, M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev. Rep. 2021, 17, 1917–1925. [Google Scholar] [CrossRef]
- Adas, G.; Cukurova, Z.; Yasar, K.; Yilmaz, R.; Isiksacan, N.; Kasapoglu, P.; Yesilbag, Z. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled Trial. Cell Transpl. 2021, 30, 9636897211024942. [Google Scholar] [CrossRef]
- Rebelatto, C.; Senegaglia, A.; Franck, C.; Daga, D.; Shigunov, P.; Stimamiglio, M.; Marsaro, D. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: A randomized clinical trial. Stem Cell Res. Ther. 2022, 13, 122. [Google Scholar] [CrossRef]
- Hashemian, S.; Aliannejad, R.; Zarrabi, M.; Soleimani, M.; Vosough, M.; Hosseini, S.; Hossieni, H. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Res. Ther. 2021, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- da Silva, K.; Pinheiro, P.; Gobatto, A.; Passos, R.; Paredes, B.; França, L.; Nonaka, C. Immunomodulatory and Anti-fibrotic Effects Following the Infusion of Umbilical Cord Mesenchymal Stromal Cells in a Critically Ill Patient With COVID-19 Presenting Lung Fibrosis: A Case Report. Front. Med. 2021, 8, 767291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, R.; Liu, K.; Li, X.; Chen, D.; Bai, D.; Luo, J. Human Umbilical Cord Mesenchymal Stem Cells for Adjuvant Treatment of a Critically Ill COVID-19 Patient: A Case Report. Infect. Drug. Resist. 2020, 13, 3295–3300. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Ren, S.; Wang, W.; Yang, Y.; Li, S.; Meng, M. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020, 11, 207. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, K.; Lv, J.; Fang, X.; He, J.; Lv, A.; Sun, X. Case Report: Human Umbilical Cord Mesenchymal Stem Cells as a Therapeutic Intervention for a Critically Ill COVID-19 Patient. Front. Med. 2021, 8, 691329. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine 2020, 99, e21429. [Google Scholar] [CrossRef]
- Senegaglia, A.; Rebelatto, C.; Franck, C.; Lima, J.; Boldrini-Leite, L.; Daga, D.; Leitão, C. Combined Use of Tocilizumab and Mesenchymal Stromal Cells in the Treatment of Severe Covid-19: Case Report. Cell Transpl. 2021, 30, 9636897211021008. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, S.; Xu, X.; Li, J.; Liu, A.; Han, J.; Liu, S.; Liu, L.; Qiu, H. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediat. Inflamm. 2016, 2016, 2347938. [Google Scholar] [CrossRef] [Green Version]
- Madureira, G.; Soares, R. The misunderstood link between SARS-CoV-2 and angiogenesis. A narrative review. Pulmonology 2021, in press. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Cheng, T.Y.; Yang, L.; Huang, Y.H.; Li, C.; Han, J.Z.; Li, X.H.; Fang, L.J.; Feng, D.D.; Tang, Y.T.; et al. G-CSF Inhibits Pulmonary Fibrosis by Promoting BMSC Homing to the Lungs via SDF-1/CXCR4 Chemotaxis. Sci. Rep. 2020, 10, 10515. [Google Scholar] [CrossRef]
- Macri, C.; Pang, E.S.; Patton, T.; O’Keeffe, M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018, 84, 11–21. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, J.; Chen, B.G.; Zheng, D.; Zhang, J.G.; Lin, R.H.; Zhou, Y.P. Dynamics of peripheral immune cells and their HLA-G and receptor expressions in a patient suffering from critical COVID-19 pneumonia to convalescence. Clin. Transl. Immunol. 2020, 9, e1128. [Google Scholar] [CrossRef]
- Metcalfe, S. COVID-19 lockdown: De-risking exit by protecting the lung with leukaemia inhibitory factor (LIF). Med. Drug. Discov. 2020, 6, 100043. [Google Scholar] [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- FDA. Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 16 March 2023).
- EMA. First Stem-Cell Therapy Recommended for Approval in EU. Available online: https://www.ema.europa.eu/en/news/first-stem-cell-therapy-recommended-approval-eu (accessed on 22 March 2023).
- EMA. EU/3/17/1852: Orphan Designation for the Treatment in Haematopoietic Stem Cell Transplantation. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-17-1852 (accessed on 25 March 2023).
- Mebarki, M.; Iglicki, N.; Marigny, C.; Abadie, C.; Nicolet, C.; Churlaud, G.; Maheux, C.; Boucher, H.; Monsel, A.; Menasche, P.; et al. Development of a human umbilical cord-derived mesenchymal stromal cell-based advanced therapy medicinal product to treat immune and/or inflammatory diseases. Stem Cell Res. Ther. 2021, 12, 571. [Google Scholar] [CrossRef] [PubMed]
| N° | Trial ID | Recruitment Status | Study Status | Treatment | Phase | Country |
|---|---|---|---|---|---|---|
| 1 | NCT04573270 | Completed | Completed | UC-MSCs | 1 | USA |
| 2 | NCT04288102 | Completed | Completed | UC-MSCs | 2 | China |
| 3 | NCT04625738 | Completed | Completed | WJ-MSCs | 2 | France |
| 4 | NCT04355728 | Completed | Completed | UC-MSCs | 1–2 | USA |
| 5 | NCT04392778 | Completed | Completed | UC-MSCs | 1–2 | Turkey |
| 6 | NCT04400032 | Completed | Completed | UC-MSCs | 1–2 | Canada |
| 7 | NCT04333368 | Completed | Completed | WJ-MSCs | 1–2 | France |
| 8 | NCT04252118 | Completed | Completed | UC-MSCs | 1 | China |
| 9 | NCT04457609 | Completed | Completed | UC-MSCs | 1 | Indonesia |
| 10 | NCT05286255 | Recruiting | Ongoing | UC-MSCs | 1 | USA |
| 11 | NCT04896853 | Recruiting | Ongoing | WJ-MSCs | 1 | Sweden |
| 12 | NCT05387278 | Recruiting | Ongoing | UC-MSCs and PL-derived exosomes | 1 | USA |
| 13 | NCT04869397 | Recruiting | Ongoing | WJ-MSCs | 2 | Canada |
| 14 | NCT04865107 | Recruiting | Ongoing | UC-MSCs | 2 | Canada |
| 15 | NCT04390139 | Recruiting | Ongoing | WJ-MSCs | 1–2 | Spain |
| 16 | NCT04390152 | Recruiting | Ongoing | WJ-MSCs | 1–2 | Colombia |
| 17 | NCT04494386 | Recruiting | Ongoing | CL-MSCs | 1–2 | USA |
| 18 | NCT04399889 | Recruiting | Ongoing | UC-MSCs | 1–2 | USA |
| 19 | NCT03042143 | Recruiting | Ongoing | CD362 enriched UC-MSCs | 1–2 | UK |
| 20 | NCT05132972 | Recruiting | Ongoing | UC-MSCs | 2–3 | Indonesia |
| 21 | NCT05240430 | Recruiting | Ongoing | UC-MSCs | N/A | Turkey |
| 22 | NCT04313322 | Recruiting | Unknown | WJ-MSCs | 1 | Jordan |
| 23 | NCT04437823 | Recruiting | Unknown | UC-MSCs | 2 | Pakistan |
| 24 | NCT04269525 | Recruiting | Unknown | UC-MSCs | 2 | China |
| 25 | NCT04339660 | Recruiting | Unknown | UC-MSCs | 1–2 | China |
| 26 | NCT04371601 | Not yet recruiting | Active, not recruiting | UC-MSCs | Early 1 | China |
| 27 | NCT04456361 | Not yet recruiting | Active, not recruiting | WJ-MSCs | Early 1 | Mexico |
| 28 | NCT04452097 | Not yet recruiting | Active, not recruiting | UC-MSCs | 1–2 | USA |
| 29 | NCT05501418 | Not yet recruiting | Active, not recruiting | UC-MSCs | 1–2 | Taiwan |
| 30 | NCT04398303 | Not yet recruiting | Unknown | UC-MSCs | 1–2 | USA |
| 31 | NCT04429763 | Not yet recruiting | Unknown | UC-MSCs | 2 | Colombia |
| 32 | NCT04273646 | Not yet recruiting | Unknown | UC-MSCs | N/A | China |
| 33 | EUCTR2020-002772-12 | Completed | Completed | WJ-MSCs | 2 | France |
| 34 | EUCTR2020-001505-22 | Recruiting | Ongoing | WJ-MSCs | 1–2 | Spain |
| 35 | EUCTR2020-001577-70 | Recruiting | Ongoing | UC-MSCs and others MSCs | 1–2 | Italy |
| 36 | ChiCTR2000030173 | Completed | Completed | UC-MSCs | Early 1 | China |
| 37 | ChiCTR2000030088 | Completed | Completed | WJ-MSCs | Early 1 | China |
| 38 | ChiCTR2000030866 | Completed | Completed | UC-MSCs | Early 1 | China |
| 39 | ChiCTR2000030261 | Completed | Completed | WJ-MSCs-derived exosomes | Early 1 | China |
| 40 | ChiCTR2000030944 | Completed | Completed | UC-MSCs | 1 | China |
| 41 | ChiCTR2000030138 | Completed | Completed | UC-MSCs | 2 | China |
| 42 | ChiCTR2000031430 | Completed | Completed | UC-MSCs | 2 | China |
| 43 | ChiCTR2000030116 | Completed | Completed | UC-MSCs | N/A | China |
| 44 | ChiCTR2000030835 | Completed | Completed | UC-MSCs | N/A | China |
| 45 | ChiCTR2000030484 | Not yet recruiting | Active, not recruiting | UC-MSCs and exosomes | N/A | China |
| 46 | ChiCTR2000031494 | Recruiting | Ongoing | UC-MSCs | 1 | China |
| 47 | IRCT20190717044241N2 | Completed | Completed | WJ-MSCs | 1 | Iran |
| 48 | IRCT20200217046526N2 | Completed | Completed | UC-MSCs | 2–3 | Iran |
| 49 | IRCT20190101042197N2 | Completed | Unknown | UC-MSCs-derived exosomes | 1–3 | Iran |
| 50 | IRCT20201202049568N3 | Completed | Unknown | UC-MSCs-derived exosomes | 1–2 | Iran |
| 51 | IRCT20160809029275N1 | Completed | Unknown | UC-MSCs | 2–3 | Iran |
| 52 | IRCT20200421047150N1 | Completed | Unknown | WJ-MSCs | 2–3 | Iran |
| 53 | IRCT20200426047206N2 | Completed | Unknown | UC-MSCs | 3 | Iran |
| 54 | IRCT20140528017891N8 | Completed | Unknown | UC-MSCs | 3 | Iran |
| 55 | IRCT20211012052743N1 | Recruiting | Ongoing | UC-MSCs | 3 | Iran |
| 56 | JPRN-JapicCTI-205465 | Recruiting | Ongoing | UC-MSCs | 1 | Japan |
| 57 | CTRI/2020/08/027043 | Not yet recruiting | Unknown | UC-MSCs | 1 | India |
| 58 | CTRI/2021/09/036645 | Recruiting | Ongoing | UC-MSCs | 1–2 | India |
| 59 | RBR-3fz9yr | Completed | Ongoing | UC-MSCs | N/A | Brazil |
| 60 | RBR-4jh63b | Not yet recruiting | Unknown | UC-MSCs | 1–2 | Brazil |
| 61 | RBR-8zg5rg7 | Recruiting | Ongoing | UC-MSCs | 1–2 | Brazil |
| Type of Study | Phase | Number of Patients | COVID Symptoms | Treatment | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Pilot trial | Early 1 | 7 | Mild 5 Severe 2 | Nebulization ranged from 7.66 × 100.8 to 7.00 × 100.7 WJ-MSCs-derived exosomes/mL, twice a day, up to discharge. | Reduction of pulmonary lesions and period of hospitalization in mild cases and reduction in cellular residue in severe cases. No adverse events were observed. | Chu et al., 2022 [228] |
| Parallel assigned controlled, nonrandomized trial | 1 | 18 | Moderate 10 Severe 8 | Moderate = 5; Severe = 4; Infusion of 3 × 107 UC-MSCs for 3 times on days 0, 3, and 6. | Reduced trend in plasma levels of inflammatory cytokines IFN-γ, TNF-α, MCP-1, IP-10, IL-1RA, IL-6, IL-8, IL-18, IL-22 and MIP-1. No serious adverse events were observed. | Meng et al., 2020 [229] |
| Double-blind, multicenter, randomized controlled trial | 1 | 40 | Critical | N = 20 patients; Infusion of 1 × 106 UC-MSCs/kg in single dose. | Increased survival rate. Decrease trend in IL-6 levels and increase trend in IL-10, LIF and VEGF levels in plasma. No adverse events were observed. | Dilogo et al., 2021 [230] |
| Single-center open-label, individually randomized, standard treatment-controlled trial | 1 | 41 | Severe | N = 12 patients; Infusion of 2 × 106 UC-MSCs/kg in single dose. | No progression from severe to critical illness. Reduction of weakness, fatigue, shortness of breath, and low oxygen saturation. Significant decreased in CRP and IL-6 plasma levels. Faster normalization in lymphocyte count and reduction of lung inflammation. No adverse events were observed. A 3-month follow-up of 28 patients (treated = 8, control = 20) revealed reduction of partial pulmonary function recovery time, ameliorated HRQL, and no adverse events were observed after 3 months. | Shu et al., 2020 [231] and Feng et al., 2021 [232] |
| Open-label, single-center trial | 1 | 5 | Severe | Injection of 150 × 106 WJ-MSCs for 3 times on days 0, 3, and 6. | Increase in IL-10 and SDF-1 and decrease of VEGF, TGF-β, IFN-γ, IL-6, and TNF-α plasma levels. Improvement in hematology, myocardial enzyme, inflammation, and biochemical tests. No adverse events were observed. | Saleh et al., 2021 [233] |
| Single-center, open-label, placebo-controlled trial | 1 | 20 | Mild-to-moderate | N = 10 patients; Infusion of 1 × 106 UC-MSCs/kg for 3 times on days 1, 3 and 5. | Significant improvement in SpO2/FiO2 ratio. Significant reduction in cytokine IL-6, IFN-γ, TNF-α, IL-17 A, and CRP levels and increase in cytokine levels of TGF-β, IL-1B, and IL-10. No serious adverse events were observed. | Kaffash Farkhad et al., 2022 [234] |
| Single-arm, pilot trial | 2 | 16 | Severe 9 Critical 7 | Infusion of 1 × 108 UC-MSCs for 4 rounds of transplantation. | Amelioration of oxygenation index. Increase of CD4+ T, CD8+ T, and NK lymphocytes. IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and CRP have returned in the normal range. No adverse events were observed. | Feng et al., 2020 [235] |
| Single-blind, randomized, placebo-controlled trial | 2 | 58 | Mild 31 Severe 21 Critical 6 | N = 29 patients; Mild 15 Severe 11 Critical 3 Infusion of 1 × 106 UC-MSCs/kg in single dose. | Shorter hospital stay and symptoms remission. Improved CT scans. Reduction of CD14+ monocytes, CRP, NETs and proinflammatory cytokines IL-1RA, IL-18, IL-27, IL-17E/IL-25, IL-17F, GRO-alpha (CXCL-1), and IL-5. High expression of genes involved in chemotaxis, telomerase assembly and maturation, angiopoiesis, HSCs mobilization, and fetal extramedullary hematopoiesis, including VNN2. Stimulation of SARS-CoV-2 specific antibodies production. No adverse events were observed. | Zhu et al., 2021 [236] |
| Double-blind, randomized, placebo-controlled trial | 2 | 100 | Severe | N = 65 patients; Infusion of 4 × 107 UC-MSCs for 3 times on days 0, 3, and 6. | Improvement in lung lesion volume, improved restoration of the integrated reserve capability, and normal CT scans after 1 year. Similar incidence of adverse events and tumor markers to placebo group after 1 year. | Shi et al., 2021 [237] and Shi et al., 2022 [238] |
| Multicenter, double-blind, randomized, placebo-controlled trial | 2 | 45 | Mild 31.1%; Moderate 48.9%; Severe 20% | N = 21 patients; Infusion of 0.9 ± 0.1 × 106 UC-MSCs/kg per dose over 5 days (on day 1, day 3 ± 1, and day 5 ± 1) N = 17 − 3 doses N = 2 − 2 doses N = 2 − 1 dose. | UC-MSC-treated patients’ greater PaO2/FiO2-ratio increased between D0 and D7, with lack of statistically significant differences, compared to controls. No adverse events were observed. | Monsel et al., 2022 [239] |
| Double-blind, randomized, controlled trial | 1–2 | 24 | Mild-to-moderate 6; Moderate-to-severe 18 | N = 12 patients; Mild-to-moderate 3 Moderate-to-severe 9 Infusion of 100 ± 20 × 106 UC-MSCs for 2 times on days 0 and 3. | Significantly improved SAE-free survival and time to recovery. Significant reduction in plasma levels of inflammatory cytokines, chemokines, and growth factors GM-CSF, IFN-γ, IL-5, IL-6, IL-7, TNFα, TNF-β, PDGF-BB, RANTES, and sTNFR2. No difference in adverse events among both groups. | Lanzoni et al., 2021 [240] and Kouroupis et al., 2021 [241] |
| Randomized trial | 1–2 | 210 | Severe 111; Critical 99 | Infusion of 1–2 × 106 UC-MSCs/kg in single dose. | After 2–3 weeks after transplantation, improvement oxygen saturation and high survival rate, especially before intubation. No adverse events observed. | O Ercelen et al., 2021 [242] |
| Prospective double controlled trial | 1–2 | 30 | Moderate 10; Critical 20 | N = 10 critical patients; Infusion of 3 × 106 WJ-MSCs/kg for 3 times on days 0, 3, and 6. | Decrease in proinflammatory and profibrotic factors IL-6, CRP, IFNγ, IL-2, IL-12, IL-17A, MMP-9, and MMP-3 plasma levels. Increase in anti-inflammatory and angiogenesis promoting factors IL-10, TGF-β, VEGF, KGF, and NGF plasma levels. Reduction of the mechanical ventilation period and high survival rate. No adverse events were observed. | Adas et al., 2021 [243] |
| Prospective, single-center, randomized, double-blind, placebo-controlled trial | 1–2 | 17 | Critical | N = 11 patients; Infusion of 5 × 105 UC-MSCs/kg every 48 h for 3 times. | Decrease in ferritin, IL-6 and MCP-1-CCL2, CRP, D-dimer, and neutrophils levels and reduction of lung damage. Increase in the numbers of lymphocytes T CD3+, CD4+ and NK. All these values are maintained until 4 months. No serious adverse events were observed. | Rebelatto et al., 2022 [244] |
| Primary report of a two-center, open-label, single-arm trial | 2–3 | 11 | Critical | Infusion of 200 × 106 UC-MSCs (N = 6) and PL-MSCs (N = 5) every other day for 3 times. | Increased of SpO2. Significant reductions in serum levels of TNF-α, IL-8, and CRP. No adverse events were observed. | Hashemian et al., 2021 [245] |
| Case report | N/A | 1 | Severe | Infusion of 5 × 107 UC-MSCs for 2 times on days 30 and 32. | Increased PaO2/FiO2 ratio. Decrease of inflammatory monocytes and increase of patrolling monocytes, CD4+ T lymphocytes, and cDC2. Reduction of lung infiltrates and fibrosis. No adverse events were observed. | da Silva et al., 2021 [246] |
| Case report | N/A | 1 | Critical | Infusion of 1 × 106 UC-MSCs/kg in single dose. | Increase of SpO2 and absolute number of the lymphocytes. Reduction of GGO, lung infiltration, and plasma levels of CRP and D-dimer. No adverse events were observed. | Zhu et al., 2020 [247] |
| Case report | N/A | 1 | Severe | Infusion of 1 × 106 WJ-MSCs/kg in single dose. | Reduction of GGO, lung infiltration and plasma levels of CRP, IL-6 and TNF-α. Increase of CD3+, CD4+, and CD8+ T lymphocytes. No adverse events were observed. | Zhang Y. et al., 2020 [248] |
| Case report | N/A | 1 | Critical | Infusion of 1 × 106 UC-MSCs/kg for 8 times divided in 3 rounds. | Reduction of fiber strands and GGO. Reduction in IL-6, IL-10, WBCs, CRP, and D-dimer levels. Increase of CD4+ T lymphocytes and decrease of NK cells. No adverse events were observed. | Zhang Q. et al., 2021 [249] |
| Case report | N/A | 1 | Critical | Infusion of 5 × 107 UC-MSCs for 3 times on days 13, 16, and 19. | Reduction of GGO, D-dimer, WBCs, neutrophils, and lymphocytes/neutrophils ratio. Increase of CD3+, CD4+, and CD8+ T lymphocytes. No adverse events were observed. | Liang et al., 2020 [250] |
| Case report | N/A | 1 | Severe | Infusion of 0.5 × 106 UC-MSCs/kg for 3 times. | Reduction of GGO, plasmablasts, creatinine, GOT, ferritin, D-dimer, and CRP levels. Increase in the absolute number of total lymphocytes, CD4+ T, and Treg lymphocytes. No adverse events were observed. | Senegaglia et al., 2021 [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, E.; Corrao, S.; Di Gaudio, F.; Alberti, G.; Caprnda, M.; Kubatka, P.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Borlongan, C.V.; et al. Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy. Cells 2023, 12, 1664. https://doi.org/10.3390/cells12121664
Russo E, Corrao S, Di Gaudio F, Alberti G, Caprnda M, Kubatka P, Kruzliak P, Miceli V, Conaldi PG, Borlongan CV, et al. Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy. Cells. 2023; 12(12):1664. https://doi.org/10.3390/cells12121664
Chicago/Turabian StyleRusso, Eleonora, Simona Corrao, Francesca Di Gaudio, Giusi Alberti, Martin Caprnda, Peter Kubatka, Peter Kruzliak, Vitale Miceli, Pier Giulio Conaldi, Cesario Venturina Borlongan, and et al. 2023. "Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy" Cells 12, no. 12: 1664. https://doi.org/10.3390/cells12121664
APA StyleRusso, E., Corrao, S., Di Gaudio, F., Alberti, G., Caprnda, M., Kubatka, P., Kruzliak, P., Miceli, V., Conaldi, P. G., Borlongan, C. V., & La Rocca, G. (2023). Facing the Challenges in the COVID-19 Pandemic Era: From Standard Treatments to the Umbilical Cord-Derived Mesenchymal Stromal Cells as a New Therapeutic Strategy. Cells, 12(12), 1664. https://doi.org/10.3390/cells12121664










