Peptide Lv Promotes Trafficking and Membrane Insertion of KCa3.1 through the MEK1–ERK and PI3K–Akt Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mice
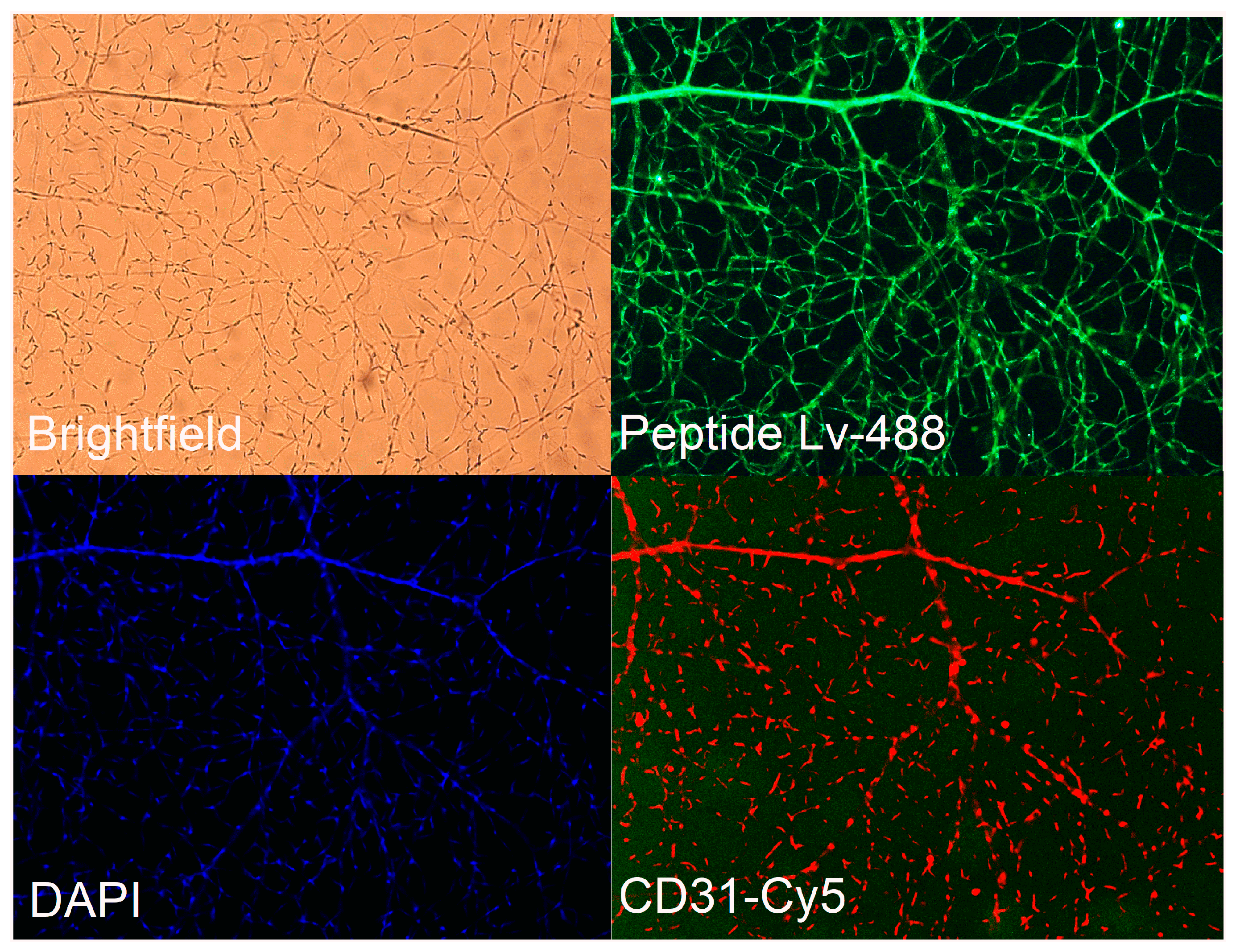
2.3. Retinal Vasculature Immunofluorescent Staining
2.4. Cell Culture
2.5. Patch-Clamp Electrophysiology
2.6. Immunoblotting
2.7. Cell-Surface Biotinylation Assay
2.8. Statistical Analysis
3. Results
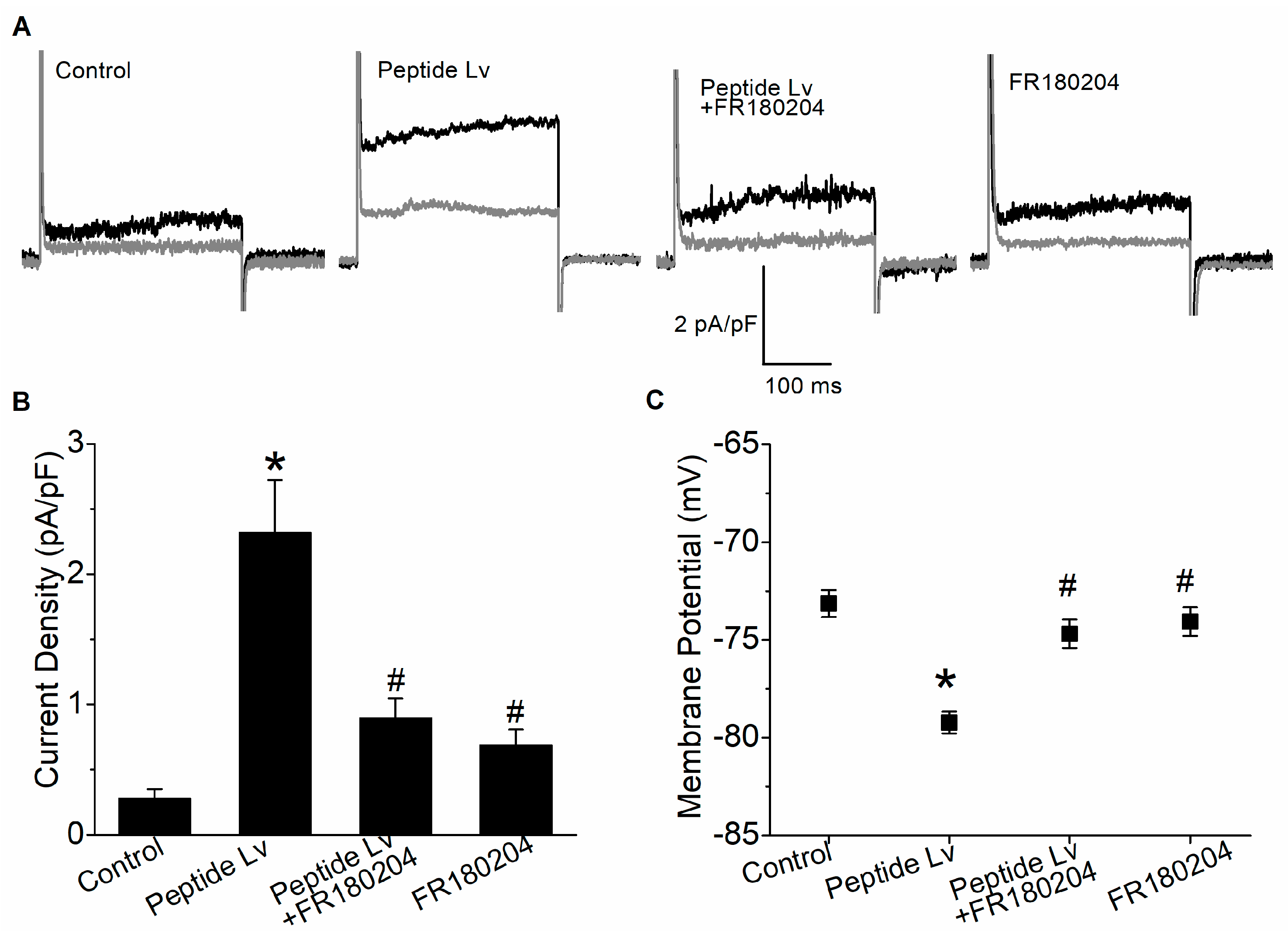
3.1. Peptide Lv Augments KCa3.1 Current Density and Endothelial Hyperpolarization through ERK Activation
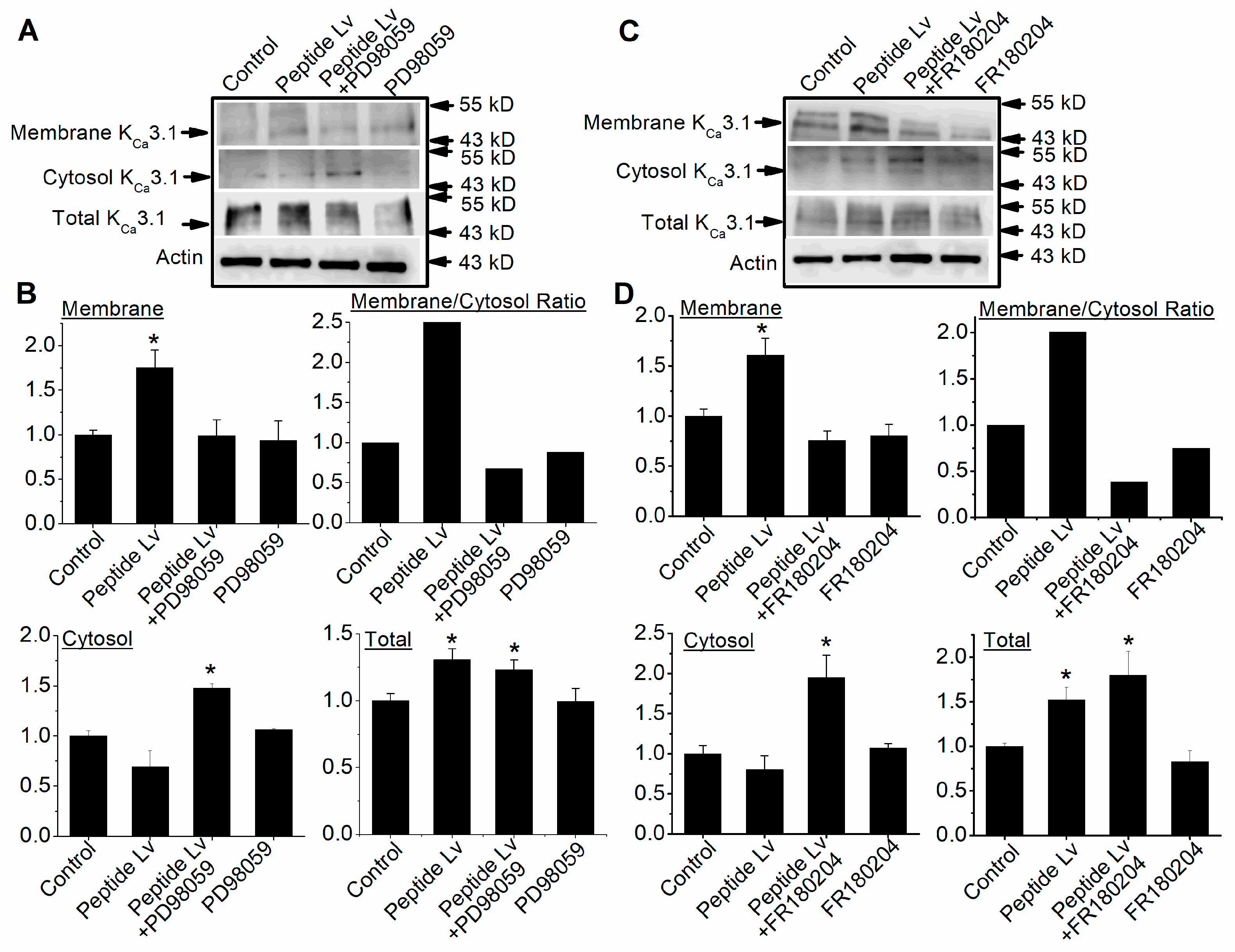
3.2. Peptide Lv Promotes KCa3.1 Channel Trafficking and Membrane Insertion through the MEK1–ERK Signaling Pathway
3.3. Peptide Lv Augments KCa3.1 Current Density and Endothelial Hyperpolarization through Akt Activation
3.4. Peptide Lv Promotes KCa3.1 Channel Trafficking and Membrane Insertion through the PI3K–Akt Signaling Pathway
3.5. Peptide Lv Concurrently Activates the MEK1–ERK and PI3K–Akt Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, L.; Ko, M.L.; Abbott, L.C.; Ko, G.Y. Identification of Peptide lv, a novel putative neuropeptide that regulates the expression of L-type voltage-gated calcium channels in photoreceptors. PLoS ONE 2012, 7, e43091. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ko, S.; Ko, M.L.; Kim, A.J.; Ko, G.Y. Peptide Lv augments L-type voltage-gated calcium channels through vascular endothelial growth factor receptor 2 (VEGFR2) signaling. Biochim. Biophys. Acta 2015, 1853, 1154–1164. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, M.; Abbey, C.A.; Tsai, S.H.; Xie, W.; Pham, D.; Chapman, S.; Bayless, K.J.; Hein, T.W.; Rosa, R.H., Jr.; et al. Newly Identified Peptide, Peptide Lv, Promotes Pathological Angiogenesis. J. Am. Heart Assoc. 2019, 8, e013673. [Google Scholar] [CrossRef]
- Pham, D.L.; Niemi, A.; Ko, M.L.; Ko, G.Y.P. Peptide Lv augments intermediate-conductance calcium-dependent potassium channels (KCa3.1) in endothelial cells to promote angiogenesis. PLoS ONE 2022, 17, e0276744. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.W.; Rosa, R.H., Jr.; Ren, Y.; Xu, W.; Kuo, L. VEGF Receptor-2-Linked PI3K/Calpain/SIRT1 Activation Mediates Retinal Arteriolar Dilations to VEGF and Shear Stress. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Liles, J.T.; Taylor, J.T.; Sears, B.W.; Saenz, R.; Kadowitz, P.J. Role of potassium channels in the nitric oxide-independent vasodilator response to acetylcholine. Pharmacol. Res. 2004, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Dora, K.A.; Gardener, M.J.; Garland, C.J.; Weston, A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Kaistha, B.P.; Hoyer, J.; Kohler, R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery. Br. J. Pharmacol. 2009, 157, 509–526. [Google Scholar] [CrossRef]
- Hein, T.W.; Kuo, L. cAMP-independent dilation of coronary arterioles to adenosine: Role of nitric oxide, G proteins, and K(ATP) channels. Circ. Res. 1999, 85, 634–642. [Google Scholar] [CrossRef]
- Michaelis, U.R.; Fleming, I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol. Ther. 2006, 111, 584–595. [Google Scholar] [CrossRef]
- Dora, K.A.; Gallagher, N.T.; McNeish, A.; Garland, C.J. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008, 102, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A.; Koller, A. Increases in endothelial Ca2+ activate K(Ca) channels and elicit EDHF-type arteriolar dilation via gap junctions. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1760–H1767. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.M.; Hampl, V.; Nelson, D.P.; Shultz, P.J.; Weir, E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 7583–7587. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Eichler, I.; Heinau, P.; Si, H.; Brakemeier, S.; Hoyer, J.; Kohler, R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef]
- Chae, K.S.; Martin-Caraballo, M.; Anderson, M.; Dryer, S.E. Akt activation is necessary for growth factor-induced trafficking of functional K(Ca) channels in developing parasympathetic neurons. J. Neurophysiol. 2005, 93, 1174–1182. [Google Scholar] [CrossRef]
- Bertuccio, C.A.; Lee, S.L.; Wu, G.; Butterworth, M.B.; Hamilton, K.L.; Devor, D.C. Anterograde trafficking of KCa3.1 in polarized epithelia is Rab1- and Rab8-dependent and recycling endosome-independent. PLoS ONE 2014, 9, e92013. [Google Scholar] [CrossRef]
- Lhuillier, L.; Dryer, S.E. Developmental regulation of neuronal KCa channels by TGFbeta 1: Transcriptional and posttranscriptional effects mediated by Erk MAP kinase. J. Neurosci. 2000, 20, 5616–5622. [Google Scholar] [CrossRef]
- Yu, F.; Chapman, S.; Pham, D.L.; Ko, M.L.; Zhou, B.; Ko, G.Y. Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open. Diabetes Res. Care 2020, 8, e001446. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ko, M.L.; Ko, G.Y. Decreased MicroRNA-150 Exacerbates Neuronal Apoptosis in the Diabetic Retina. Biomedicines 2021, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Yu, F.; Shi, L.; Ko, M.L.; Ko, G.Y. Melatonin Affects Mitochondrial Fission/Fusion Dynamics in the Diabetic Retina. J. Diabetes Res. 2019, 2019, 8463125. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.S.; Palade, P. Perforated patch recording with beta-escin. Pflugers Arch. 1998, 436, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A. An analysis of critical factors for quantitative immunoblotting. Sci. Signal. 2015, 8, rs2. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Ko, M.L.; Ko, G.Y. A new functional role for mechanistic/mammalian target of rapamycin complex 1 (mTORC1) in the circadian regulation of L-type voltage-gated calcium channels in avian cone photoreceptors. PLoS ONE 2013, 8, e73315. [Google Scholar] [CrossRef]
- Wong, R.; Schlichter, L.C. PKA reduces the rat and human KCa3.1 current, CaM binding, and Ca2+ signaling, which requires Ser332/334 in the CaM-binding C terminus. J. Neurosci. 2014, 34, 13371–13383. [Google Scholar] [CrossRef]
- Robertson, B.E.; Schubert, R.; Hescheler, J.; Nelson, M.T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am. J. Physiol. 1993, 265, C299–C303. [Google Scholar] [CrossRef]
- Lee, B.S.; Devor, D.C.; Hamilton, K.L. Modulation of Retrograde Trafficking of KCa3.1 in a Polarized Epithelium. Front. Physiol. 2017, 8, 489. [Google Scholar] [CrossRef]
- Estadella, I.; Pedros-Gamez, O.; Colomer-Molera, M.; Bosch, M.; Sorkin, A.; Felipe, A. Endocytosis: A Turnover Mechanism Controlling Ion Channel Function. Cells 2020, 9, 1833. [Google Scholar] [CrossRef]
- Turnham, R.E.; Scott, J.D. Protein kinase A catalytic subunit isoform PRKACA., History, function and physiology. Gene 2016, 577, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, A.C.; Gangopadhyay, N.N.; Devor, D.C. Kinase-dependent regulation of the intermediate conductance, calcium-dependent potassium channel, hIK1. J. Biol. Chem. 2000, 275, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, A.C.; Syme, C.A.; Giltinan, L.; Adelman, J.P.; Devor, D.C. ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a C-terminal domain. J. Biol. Chem. 2001, 276, 10963–10970. [Google Scholar] [CrossRef] [PubMed]
- Shiojima, I.; Walsh, K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 2002, 90, 1243–1250. [Google Scholar] [CrossRef]
- Song, M.; Finley, S.D. ERK and Akt exhibit distinct signaling responses following stimulation by pro-angiogenic factors. Cell Commun. Signal. 2020, 18, 114. [Google Scholar] [CrossRef]
- Somanath, P.R.; Razorenova, O.V.; Chen, J.; Byzova, T.V. Akt1 in endothelial cell and angiogenesis. Cell Cycle 2006, 5, 512–518. [Google Scholar] [CrossRef]
- Okuda, K.S.; Keyser, M.S.; Gurevich, D.B.; Sturtzel, C.; Mason, E.A.; Paterson, S.; Chen, H.; Scott, M.; Condon, N.D.; Martin, P.; et al. Live-imaging of endothelial Erk activity reveals dynamic and sequential signalling events during regenerative angiogenesis. eLife 2021, 10, e62196. [Google Scholar] [CrossRef]
- Srinivasan, R.; Zabuawala, T.; Huang, H.; Zhang, J.; Gulati, P.; Fernandez, S.; Karlo, J.C.; Landreth, G.E.; Leone, G.; Ostrowski, M.C. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 2009, 4, e8283. [Google Scholar] [CrossRef]
- Costa, G.; Harrington, K.I.; Lovegrove, H.E.; Page, D.J.; Chakravartula, S.; Bentley, K.; Herbert, S.P. Asymmetric division coordinates collective cell migration in angiogenesis. Nat. Cell Biol. 2016, 18, 1292–1301. [Google Scholar] [CrossRef]
- Morales-Ruiz, M.; Fulton, D.; Sowa, G.; Languino, L.R.; Fujio, Y.; Walsh, K.; Sessa, W.C. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 2000, 86, 892–896. [Google Scholar] [CrossRef]
- Kureishi, Y.; Luo, Z.; Shiojima, I.; Bialik, A.; Fulton, D.; Lefer, D.J.; Sessa, W.C.; Walsh, K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000, 6, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Somanath, P.R.; Razorenova, O.; Chen, W.S.; Hay, N.; Bornstein, P.; Byzova, T.V. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat. Med. 2005, 11, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Ackah, E.; Yu, J.; Zoellner, S.; Iwakiri, Y.; Skurk, C.; Shibata, R.; Ouchi, N.; Easton, R.M.; Galasso, G.; Birnbaum, M.J.; et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J. Clin. Investig. 2005, 115, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Gerber, H.P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamaguchi, S.; Chida, K.; Shibuya, M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001, 20, 2768–2778. [Google Scholar] [CrossRef]
- Mukai, M.; Uchida, K.; Okubo, T.; Takano, S.; Matsumoto, T.; Satoh, M.; Inoue, G.; Takaso, M. Regulation of Tumor Necrosis Factor-alpha by Peptide Lv in Bone Marrow Macrophages and Synovium. Front. Med. 2021, 8, 702126. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, D.L.; Niemi, A.; Blank, R.; Lomenzo, G.; Tham, J.; Ko, M.L.; Ko, G.Y.-P. Peptide Lv Promotes Trafficking and Membrane Insertion of KCa3.1 through the MEK1–ERK and PI3K–Akt Signaling Pathways. Cells 2023, 12, 1651. https://doi.org/10.3390/cells12121651
Pham DL, Niemi A, Blank R, Lomenzo G, Tham J, Ko ML, Ko GY-P. Peptide Lv Promotes Trafficking and Membrane Insertion of KCa3.1 through the MEK1–ERK and PI3K–Akt Signaling Pathways. Cells. 2023; 12(12):1651. https://doi.org/10.3390/cells12121651
Chicago/Turabian StylePham, Dylan L., Autumn Niemi, Ria Blank, Gabriella Lomenzo, Jenivi Tham, Michael L. Ko, and Gladys Y.-P. Ko. 2023. "Peptide Lv Promotes Trafficking and Membrane Insertion of KCa3.1 through the MEK1–ERK and PI3K–Akt Signaling Pathways" Cells 12, no. 12: 1651. https://doi.org/10.3390/cells12121651
APA StylePham, D. L., Niemi, A., Blank, R., Lomenzo, G., Tham, J., Ko, M. L., & Ko, G. Y.-P. (2023). Peptide Lv Promotes Trafficking and Membrane Insertion of KCa3.1 through the MEK1–ERK and PI3K–Akt Signaling Pathways. Cells, 12(12), 1651. https://doi.org/10.3390/cells12121651






