Increased Prevalence of Alpha-1-Antitrypsin Deficiency in Patients with Biliary Tract Cancer and Its Associated Clinicopathological Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genotyping and qPCR
2.2. Cohort
2.3. Public Datasets
2.4. Statistical Analysis
3. Results
3.1. Increased Prevalence of Alpha-1-Antitrypsin Deficiency in Patients with Biliary Tract Cancer
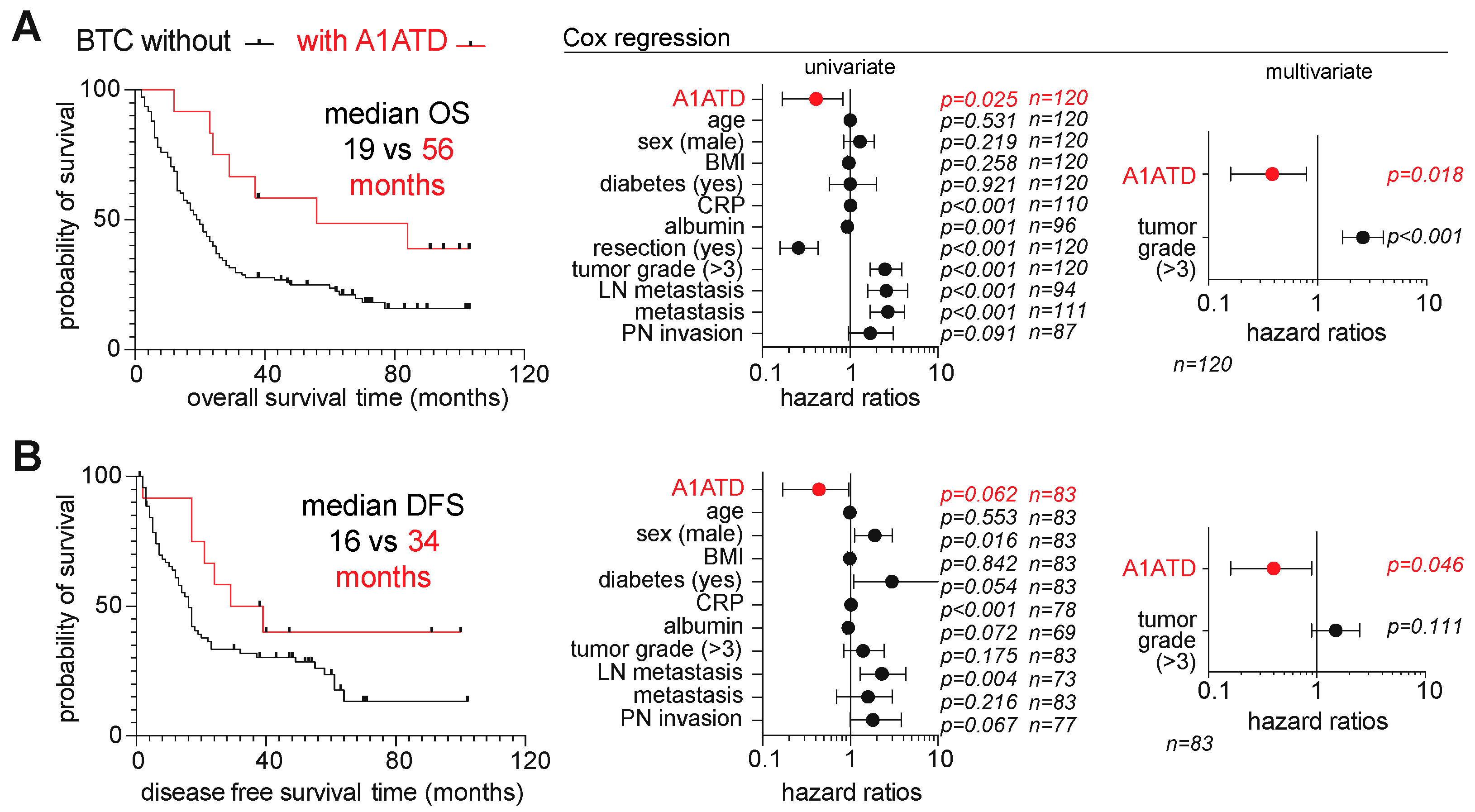
3.2. Comparative Clinicopathological and Survival Features of BTC Patients with and without A1ATD
3.3. Increased Expression of A1AT in Intrahepatic Cholangiocarcinoma and Associated Biological Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vuurberg, N.E.; Boom, A.L.V.; den Heuvel, M.C.V.; den Klaase, J.M. Intrahepatic cholangiocarcinoma in a non-cirrhotic liver in a pa-tient with homozygous ZZ alpha-1 antitrypsin deficiency. BMJ Case Rep. 2021, 14, e240077. [Google Scholar] [CrossRef]
- Mihalache, F.; Hoblinger, A.; Grunhage, F.; Krawczyk, M.; Gartner, B.C.; Acalovschi, M.; Sauerbruch, T.; Lammert, F.; Zimmer, V. Heterozygosity for the al-pha1-antitrypsin Z allele may confer genetic risk of cholangiocarcinoma. Aliment. Pharmacol. Ther. 2011, 33, 389–394. [Google Scholar] [CrossRef]
- Chan, E.; Berlin, J. Biliary tract cancers: Understudied and poorly understood. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Marciniak, S.J.; Teckman, J.; Ferrarotti, I.; Brantly, M.L.; Lomas, D.A.; Stoller, J.K. Antitrypsin deficiency. Nat. Rev. Dis. Prim. 2016, 2, 16052. [Google Scholar]
- Fromme, M.; Schneider, C.V.; Trautwein, C.; Brunetti-Pierri, N.; Strnad, P. Alpha-1 antitrypsin deficiency: A re-surfacing adult liver disorder. J. Hepatol. 2021, 76, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, H.; Onuma, A.E.; Tsung, A. Tumor Microenvironment, State of the Science. Adv. Exp. Med. Biol. 2020, 1263, 13–23. [Google Scholar]
- Greulich, T.; Vogelmeier, C.F. Alpha-1-antitrypsin deficiency: Increasing awareness and improving diagnosis. Ther. Adv. Respir. Dis. 2015, 10, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Carpino, G.; Cardinale, V.; Di Giamberardino, A.; Overi, D.; Donsante, S.; Colasanti, T.; Amato, G.; Mennini, G.; Franchitto, M.; Conti, F.; et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J. Hepatol. 2021, 75, 1377–1386. [Google Scholar] [CrossRef]
- Dong, L.; Lu, D.; Chen, R.; Lin, Y.; Zhu, H.; Zhang, Z.; Cai, S.; Cui, P.; Song, G.; Rao, D.; et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell 2021, 40, 70–87. [Google Scholar] [CrossRef]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.-J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef]
- Ciardiello, D.; Maiorano, B.A.; Parente, P.; Rodriquenz, M.G.; Latiano, T.P.; Chiarazzo, C.; Pazienza, V.; Guerrera, L.P.; Amoruso, B.; Normanno, N.; et al. Immunotherapy for Biliary Tract Cancer in the Era of Precision Medicine: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 820. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guide-line for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef]
- Strnad, P.; Mandorfer, M.; Choudhury, G.; Griffiths, W.; Trautwein, C.; Loomba, R.; Schluep, T.; Chang, T.; Yi, M.; Given, B.D.; et al. Fazirsiran for Liver Disease Associated with Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2022, 387, 514–524. [Google Scholar] [CrossRef]
- Angkathunyakul, N.; Rosini, F.; Heaton, N.; Foskett, P.; Quaglia, A. BRAF V600E mutation in biliary proliferations associated with α1-antitrypsin deficiency. Histopathology 2017, 70, 485–491. [Google Scholar] [CrossRef]
- Norton, B.; Denson, J.; Briggs, C.; Bowles, M.; Stell, D.; Aroori, S. Delayed diagnosis of alpha-1-antitrypsin deficiency following post-hepatectomy liver failure: A case report. World J. Gastroenterol. 2016, 22, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Hempel, M.; Schmitz, A.; Winkler, S.; Kucukoglu, O.; Brückner, S.; Niessen, C.; Christ, B. Pathological implications of cadherin zonation in mouse liver. Cell. Mol. Life Sci. 2015, 72, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Habibzadeh, S.; Shahi, J.M.; Ghobadi, H. The first report of two cases of fatal liver injury due to anti-tuberculosis drugs in the presence of alpha-1 antitrypsin deficiency. Int. J. Mycobacteriol. 2017, 6, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Reilkoff, R.; Stephenson, L. Fulminant hepatic failure in the setting of progressive ANCA-associated vasculitis associated with a rare alpha-1 antitrypsin phenotype, ‘PiEE’. BMJ Case Rep. 2018, 2018, bcr2017222036. [Google Scholar] [CrossRef] [PubMed]
- Jedicke, N.; Struever, N.; Aggrawal, N.; Welte, T.; Manns, M.P.; Malek, N.P.; Zender, L.; Janciauskiene, S.; Wuestefeld, T. Alpha-1-antitrypsin inhibits acute liver failure in mice. Hepatology 2014, 59, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- El-Akawi, Z.J.; Al-Hindawi, F.K.; A Bashir, N. Alpha-1 antitrypsin (alpha1-AT) plasma levels in lung, prostate and breast cancer patients. Neuro Endocrinol. Lett. 2008, 29, 482–484. [Google Scholar]
- Tzonou, A.; Sparos, L.; Kalapothaki, V.; Zavitsanos, X.; Rebelakos, A.; Trichopoulos, D. Alpha 1-antitrypsin and survival in hepato-cellular carcinoma. Br. J. Cancer. 1990, 61, 72–73. [Google Scholar] [CrossRef] [Green Version]
- Pirisi, M.; Fabris, C.; Soardo, G.; Toniutto, P.; Vitulli, D.; Bartoli, E. Prognostic value of serum alpha-1-antitrypsin in hepatocellular carcinoma. Eur. J. Cancer 1996, 32, 221–225. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Hu, K.; Wang, D.; Wang, Z.; Huang, Y. Perineural invasion as a prognostic factor for intrahepatic cholangiocar-cinoma after curative resection and a potential indication for postoperative chemotherapy: A retrospective cohort study. BMC Cancer. 2020, 20, 270. [Google Scholar]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2020, 18, 9–34. [Google Scholar] [CrossRef]
- Inoue, J.; Inazawa, J. Cancer-associated miRNAs and their therapeutic potential. J. Hum. Genet. 2021, 66, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Duwe, L.; Munoz-Garrido, P.; Lewinska, M.; Lafuente-Barquero, J.; Satriano, L.; Høgdall, D.; Taranta, A.; Nielsen, B.S.; Ghazal, A.; Matter, M.S.; et al. Mi-croRNA-27a-3p targets FoxO signalling to induce tumour-like phenotypes in bile duct cells. J. Hepatol. 2023, 78, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Aroni, K.; Kittas, C.; Papadimitriou, C.S.; Papacharalampous, N.X. An immunocytochemical study of the distribution of lysozyme, a1-antitrypsin and a1-antichymotrypsin in the normal and pathological gall bladder. Virchows Arch. 1984, 403, 281–289. [Google Scholar] [CrossRef]
- Callea, F.; Stuyck, J.M.; Massi, G.; Huyghe, J.D.; Van Gijsegem, D.F.; Jadoul, D.H.; Desmet, V.J. Alpha-1-Antitrypsin (AAT) Deposits in Gall Bladder Adenocarcinoma and Liver in Partial AAT Deficiency (Pi SZ Phenotype). Am. J. Clin. Pathol. 1982, 78, 878–883. [Google Scholar] [CrossRef]
- Callea, F.; Fevery, J.; Massi, G.; Groote, J.; de Desmet, V.J. Storage of alpha-1-antitrypsin in intrahepatic bile duct cells in al-pha-1-antitrypsin deficiency (Pi Z phenotype). Histopathology 1985, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.; Toth, E.; Sahlin, S.; Eriksson, S. Immunochemical and functional properties of biliary alpha-1-antitrypsin. Scand. J. Clin. Lab. Investig. 1996, 56, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Lockett, A.D. Alpha-1 Antitrypsin Transcytosis and Secretion. Methods Mol. Biol. 2017, 1639, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Parham, D.M.; Paterson, J.R.; Gunn, A.; Guthrie, W. Cholangiocarcinoma in two siblings with emphysema and alpha-1-antitrypsin deficiency. Q. J. Med. 1989, 71, 359–367. [Google Scholar]
- Sun, Z.; Yang, P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progres-sion. Lancet. Oncol. 2004, 5, 182–190. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornillet, M.; Zemack, H.; Jansson, H.; Sparrelid, E.; Ellis, E.; Björkström, N.K. Increased Prevalence of Alpha-1-Antitrypsin Deficiency in Patients with Biliary Tract Cancer and Its Associated Clinicopathological Features. Cells 2023, 12, 1663. https://doi.org/10.3390/cells12121663
Cornillet M, Zemack H, Jansson H, Sparrelid E, Ellis E, Björkström NK. Increased Prevalence of Alpha-1-Antitrypsin Deficiency in Patients with Biliary Tract Cancer and Its Associated Clinicopathological Features. Cells. 2023; 12(12):1663. https://doi.org/10.3390/cells12121663
Chicago/Turabian StyleCornillet, Martin, Helen Zemack, Hannes Jansson, Ernesto Sparrelid, Ewa Ellis, and Niklas K. Björkström. 2023. "Increased Prevalence of Alpha-1-Antitrypsin Deficiency in Patients with Biliary Tract Cancer and Its Associated Clinicopathological Features" Cells 12, no. 12: 1663. https://doi.org/10.3390/cells12121663




