Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders
Abstract
:1. Introduction
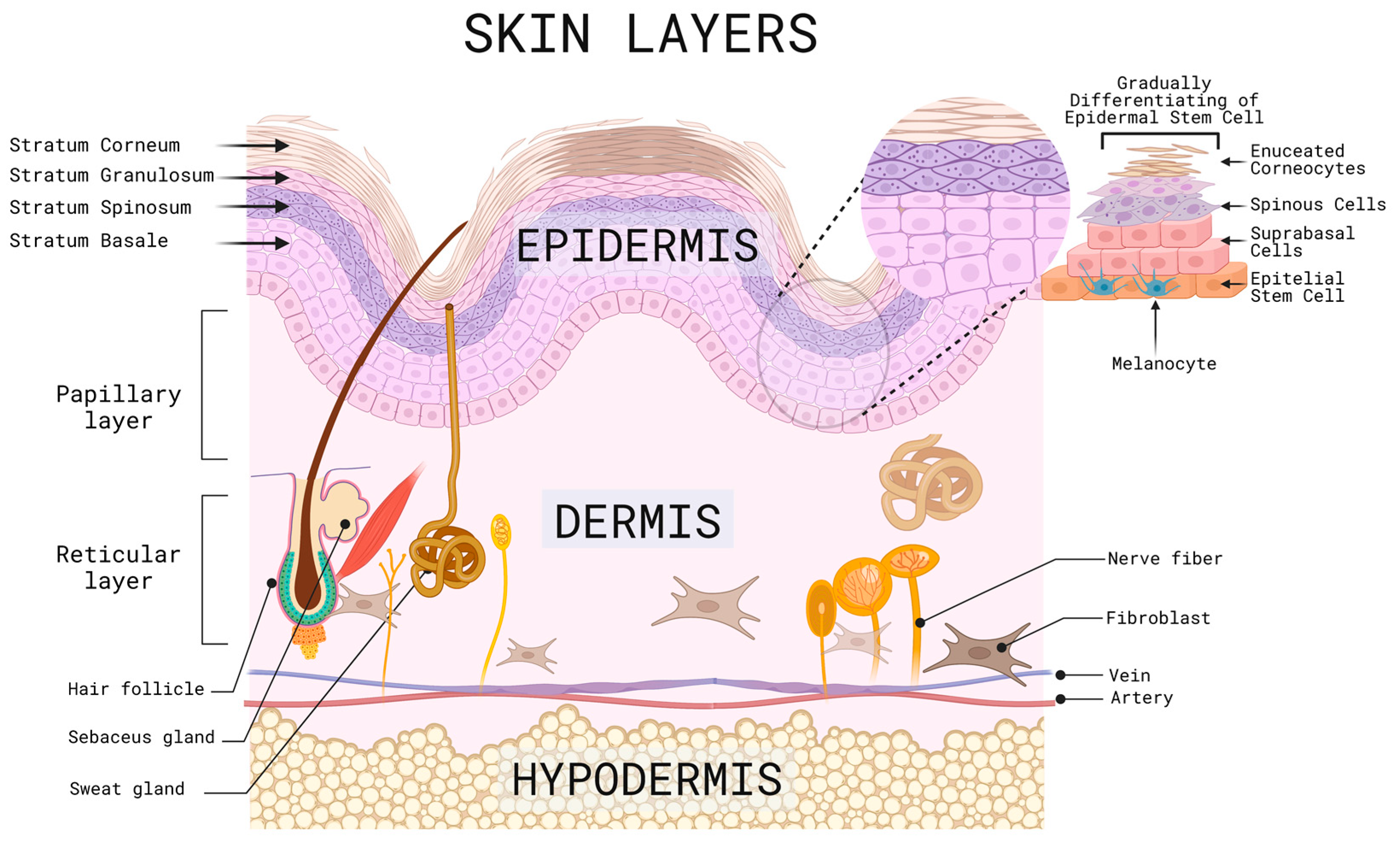
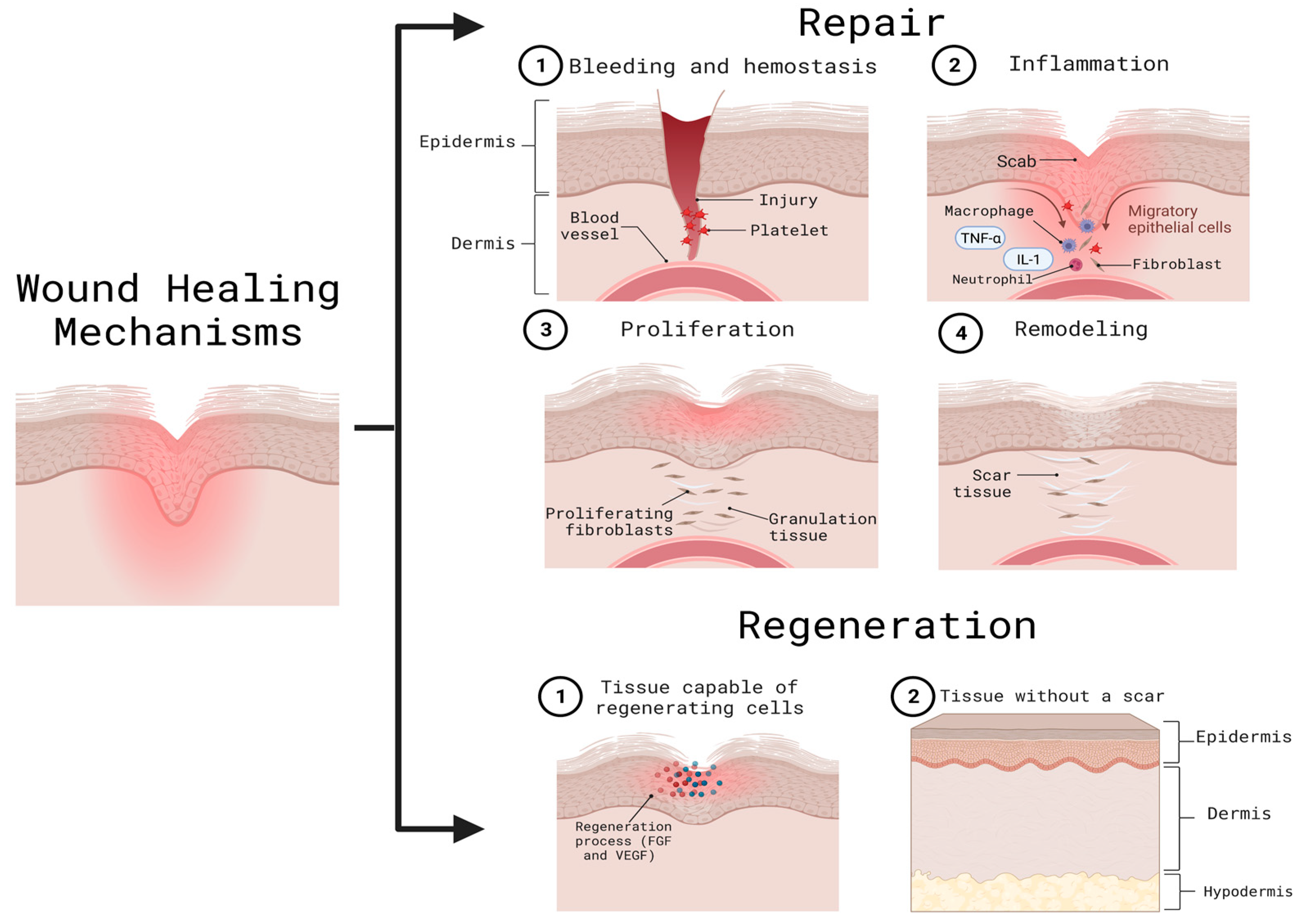
2. Mechanisms of Skin Related Injury and Repair
2.1. Skin Response and Inflammation to Ultraviolet (UV) Light
2.2. Skin Response to Infrared Radiation (IR)
2.3. Skin Response to Diabetic Wounds
2.4. Skin Response to Burn Wounds & Macrophage-Mediated Skin Wound Healing
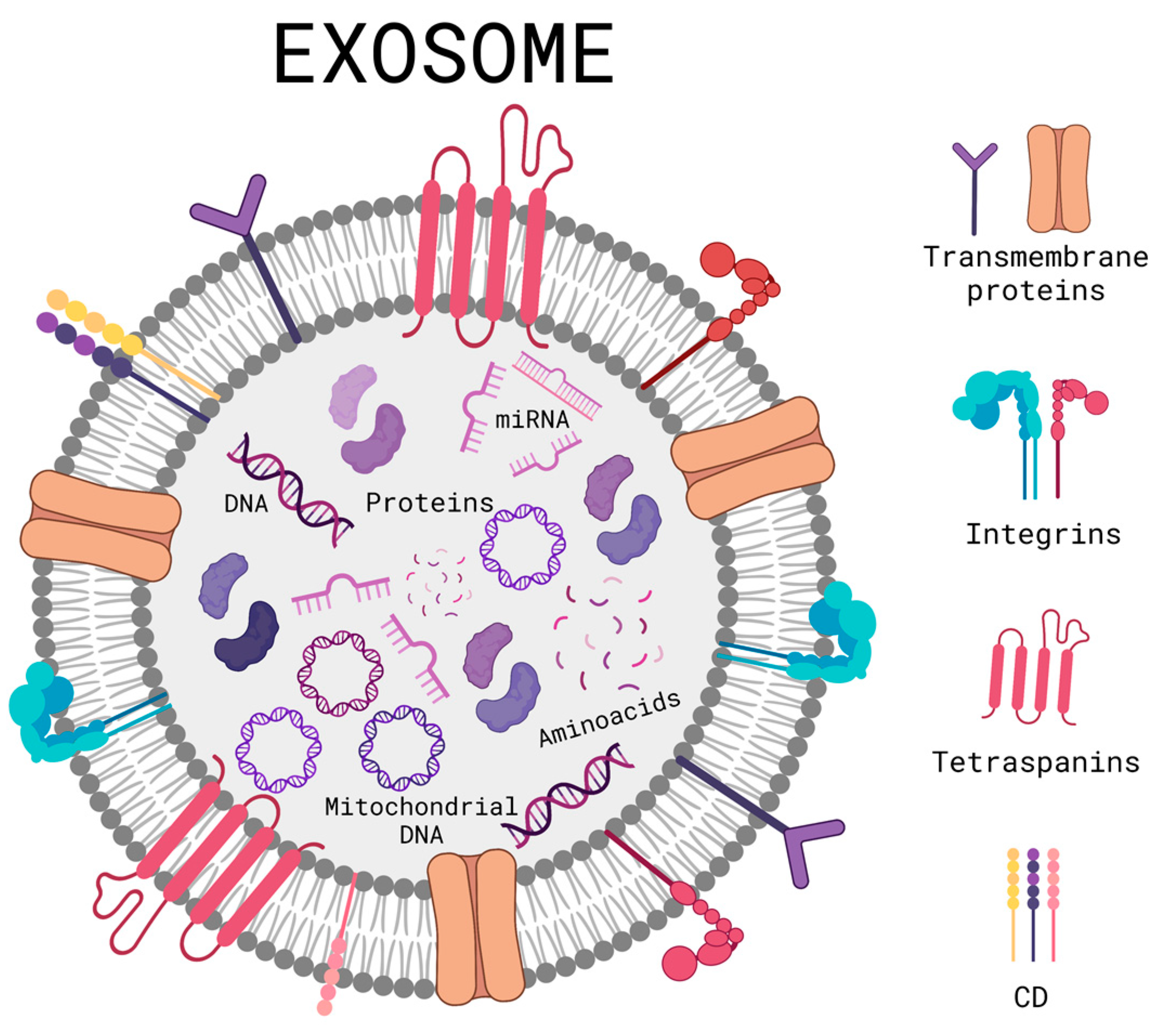
3. Exosome Therapy
3.1. Exosome Purification and Isolation
3.2. Exosomes Derived from Mesenchymal Stem Cells (MSC-EXO) and Their Application in Different Skin Disorders
4. Signaling Pathways Involved in Exosome-Mediated Skin Regeneration
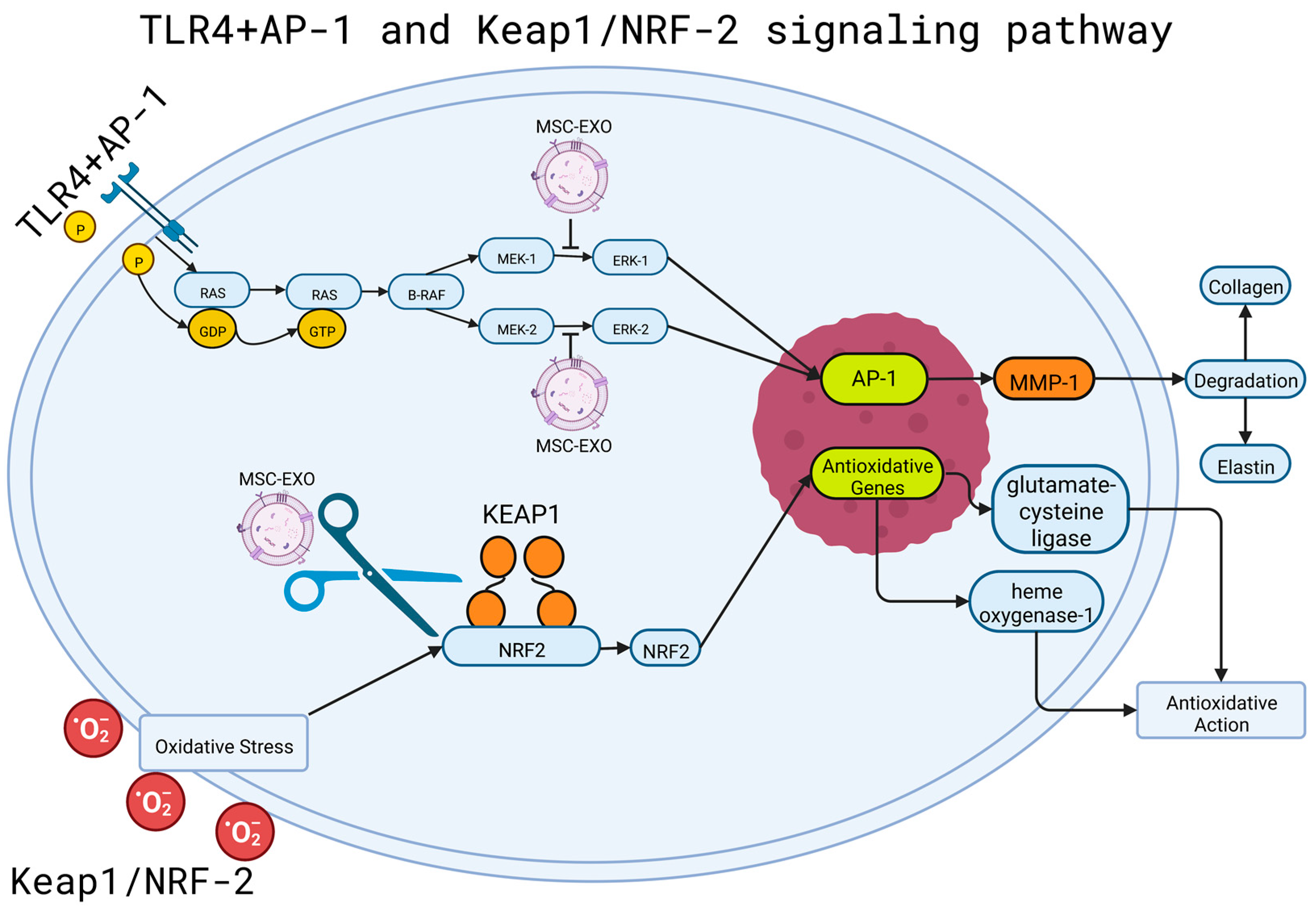
4.1. MSC-EXO and TLR Signaling Pathway
4.2. MSC-EXO and NRF2-KEAP1 Pathway
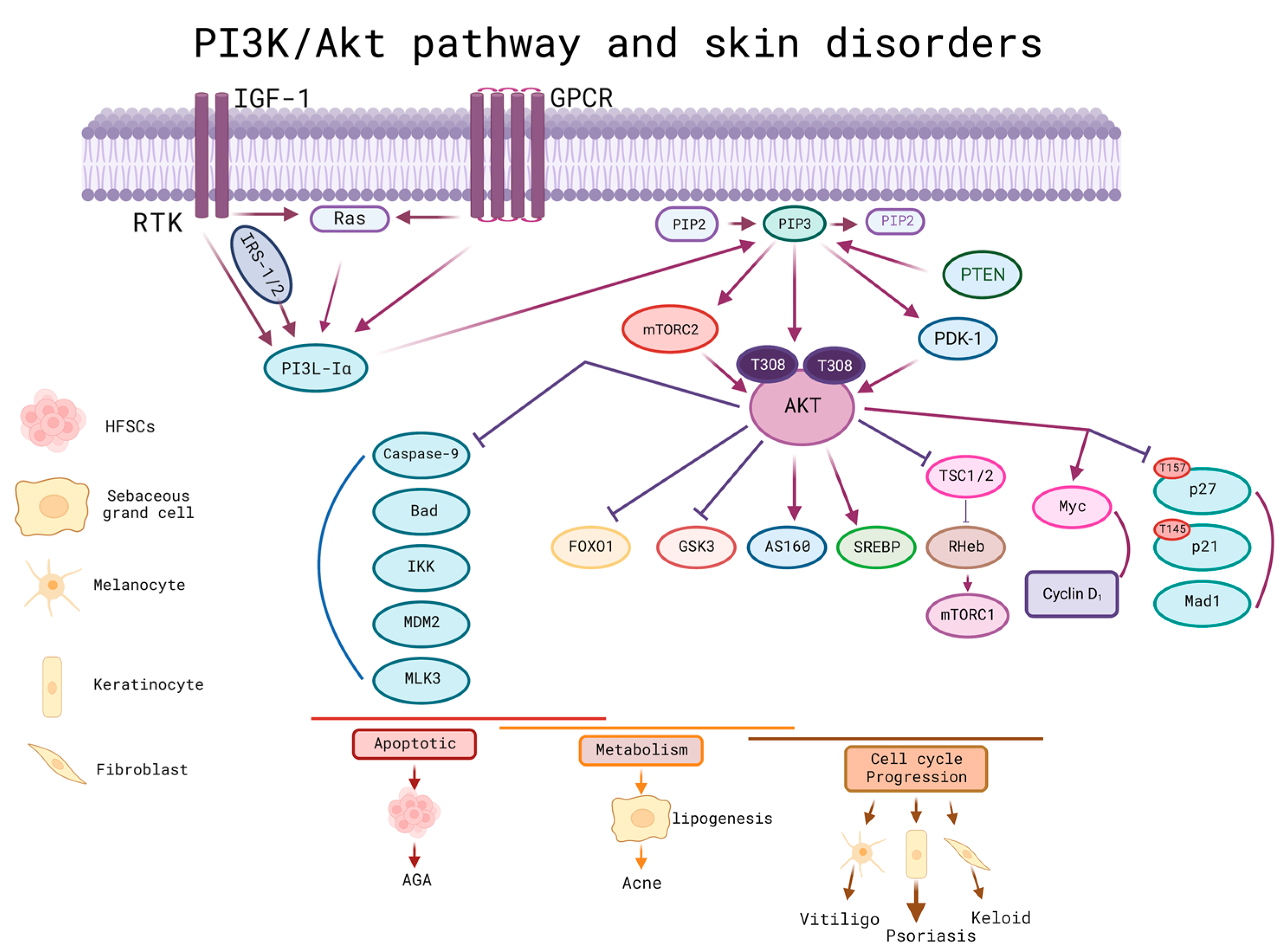
4.3. MSC-EXO and PI3K/Akt Pathway
5. The Role of Exosome in Epigenetics Processes Related to Cell Proliferation, Cell Migration, Differentiation, and Modulation of the Inflammatory Response
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Guo, X. A Review: Therapeutic Potential of Adipose-Derived Stem Cells in Cutaneous Wound Healing and Regeneration. Stem Cell Res. Ther. 2018, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Parveen, S.; Chakravarty, S.; Banerjee, M. Skin Anatomy and Morphology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–10. ISBN 978-981-13-2540-3. [Google Scholar]
- Kerns, M.L.; Chien, A.L.; Kang, S. Skin Aging. In Fitzpatrick’s Dermatology, 9e; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Bennion, S. Structure and Function of the Skin. In Dermatology Secrets E-Book; Elsevier: Amsterdam, The Netherlands, 2020; pp. 4690–4696. [Google Scholar]
- Jia, Q.; Nash, J.F. Pathology of Aging Skin BT—Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 363–385. ISBN 978-3-662-47398-6. [Google Scholar]
- Gökçe, B.; Güngör, S. Nanocarrier-Mediated Follicular Targeting; Elsevier: Amsterdam, The Netherlands, 2020; pp. 305–326. ISBN 9780128222867. [Google Scholar]
- Freeman, S.C.; Sonthalia, S. Histology, Keratohyalin Granules; StarPerarls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin Tissue Regeneration for Burn Injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.M.; Krishnamurthy, K. Histology, Hair and Follicle; StarPerarls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Madden, J.; O’Mahony, C.; Thompson, M.; O’Riordan, A.; Galvin, P. Biosensing in Dermal Interstitial Fluid Using Microneedle Based Electrochemical Devices. Sens. Bio-Sens. Res. 2020, 29, 100348. [Google Scholar] [CrossRef]
- Deniz, A.A.H.; Abdik, E.A.; Abdik, H.; Aydın, S.; Şahin, F.; Taşlı, P.N. Zooming in across the Skin: A Macro-to-Molecular Panorama. In Cell Biology and Translational Medicine; Springer: Berlin/Heidelberg, Germany, 2020; pp. 157–200. [Google Scholar]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.-B.; Mateescu, A.L.; Stan, D. Wound Healing Applications of Creams and “Smart” Hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.; Mendonça, C.; Atayde, L.M.; Maurício, A.C. The Application of Mesenchymal Stem Cells on Wound Repair and Regeneration. Appl. Sci. 2021, 11, 3000. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; LeBleu, V. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The Biogenesis and Secretion of Exosomes and Multivesicular Bodies (MVBs): Intercellular Shuttles and Implications in Human Diseases. Genes Dis. 2022; in press. [Google Scholar] [CrossRef]
- Kumari, M.; Anji, A. Small but Mighty—Exosomes, Novel Intercellular Messengers. Biology 2022, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV Damage to Cellular DNA: From Mechanisms to Biological Effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Gao, W.; Yuan, L.M.; Zhang, Y.; Huang, F.Z.; Gao, F.; Li, J.; Xu, F.; Wang, H.; Wang, Y.S. MiR-1246-Overexpressing Exosomes Suppress UVB-Induced Photoaging via Regulation of TGF-β/Smad and Attenuation of MAPK/AP-1 Pathway. Photochem. Photobiol. Sci. 2022, 22, 135–146. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight Damage to Cellular DNA: Focus on Oxidatively Generated Lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Krutmann, J. Infrared A-Induced Skin Aging. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–7. [Google Scholar]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous Effects of Infrared Radiation: From Clinical Observations to Molecular Response Mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef]
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.-E.; Lee, Y.M.; Park, C.-H.; Chung, J.H. Effects of Infrared Radiation and Heat on Human Skin Aging In Vivo. J. Investig. Dermatology Symp. Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Lee, D.H.; Cho, S.; Chung, J.H. Minimal Heating Dose: A Novel Biological Unit to Measure Infrared Irradiation. Photodermatol. Photoimmunol. Photomed. 2006, 22, 148–152. [Google Scholar] [CrossRef]
- Schieke, S.M.; Stege, H.; Kürten, V.; Grether-Beck, S.; Sies, H.; Krutmann, J. Infrared-A Radiation-Induced Matrix Metalloproteinase 1 Expression Is Mediated Through Extracellular Signal-Regulated Kinase 1/2 Activation in Human Dermal Fibroblasts. J. Investig. Dermatol. 2002, 119, 1323–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-S.; Kim, Y.K.; Cho, K.H.; Chung, J.H. Regulation of Type I Procollagen and MMP-1 Expression after Single or Repeated Exposure to Infrared Radiation in Human Skin. Mech. Ageing Dev. 2006, 127, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, Y.K.; Cho, K.H.; Chung, J.H. Infrared Exposure Induces an Angiogenic Switch in Human Skin That Is Partially Mediated by Heat. Br. J. Dermatol. 2006, 155, 1131–1138. [Google Scholar] [CrossRef]
- Chen, Z.; Seo, J.Y.; Kim, Y.K.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Heat Modulation of Tropoelastin, Fibrillin-1, and Matrix Metalloproteinase-12 in Human Skin In Vivo. J. Investig. Dermatol. 2005, 124, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Ozsvar, J.; Yang, C.; Cain, S.A.; Baldock, C.; Tarakanova, A.; Weiss, A.S. Tropoelastin and Elastin Assembly. Front. Bioeng. Biotechnol. 2021, 9, 643110. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.S.; Zuk, A.V.; Sengle, G. The Fibrillin Microfibril/Elastic Fibre Network: A Critical Extracellular Supramolecular Scaffold to Balance Skin Homoeostasis. Exp. Dermatol. 2021, 30, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, S.; Storey, A. Repair of UV-Induced Thymine Dimers Is Compromised in Cells Expressing the E6 Protein from Human Papillomaviruses Types 5 and 18. Br. J. Cancer 2004, 90, 2203–2209. [Google Scholar] [CrossRef]
- Chiu, H.-W.; Chen, C.-H.; Chen, Y.-J.; Hsu, Y.-H. Far-Infrared Suppresses Skin Photoaging in Ultraviolet B-Exposed Fibroblasts and Hairless Mice. PLoS ONE 2017, 12, e0174042. [Google Scholar] [CrossRef] [Green Version]
- Barolet, D.; Christiaens, F.; Hamblin, M.R. Infrared and Skin: Friend or Foe. J. Photochem. Photobiol. B Biol. 2016, 155, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Quondamatteo, F. Skin and Diabetes Mellitus: What Do We Know? Cell Tissue Res. 2014, 355, 1–21. [Google Scholar] [CrossRef]
- Yaseen, H.; Khamaisi, M. Skin Well-Being in Diabetes: Role of Macrophages. Cell. Immunol. 2020, 356, 104154. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef]
- De Macedo, G.M.C.; Nunes, S.; Barreto, T. Skin Disorders in Diabetes Mellitus: An Epidemiology and Physiopathology Review. Diabetol. Metab. Syndr. 2016, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Sierawska, O.; Małkowska, P.; Taskin, C.; Hrynkiewicz, R.; Mertowska, P.; Grywalska, E.; Korzeniowski, T.; Torres, K.; Surowiecka, A.; Niedźwiedzka-Rystwej, P.; et al. Innate Immune System Response to Burn Damage—Focus on Cytokine Alteration. Int. J. Mol. Sci. 2022, 23, 716. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn Injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage Polarization and Diabetic Wound Healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.-K.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Huda, M.N.; Nafiujjaman, M.; Deaguero, I.G.; Okonkwo, J.; Hill, M.L.; Kim, T.; Nurunnabi, M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2106–2149. [Google Scholar] [CrossRef]
- McBride, J.D.; Rodriguez-Menocal, L.; Badiavas, E.V. Extracellular Vesicles as Biomarkers and Therapeutics in Dermatology: A Focus on Exosomes. J. Investig. Dermatol. 2017, 137, 1622–1629. [Google Scholar] [CrossRef]
- Girón, J.; Maurmann, N.; Pranke, P. The Role of Stem Cell-Derived Exosomes in the Repair of Cutaneous and Bone Tissue. J. Cell Biochem. 2022, 123, 183–201. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A Review on Exosomes Application in Clinical Trials: Perspective, Questions, and Challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The Novel Mechanisms and Applications of Exosomes in Dermatology and Cutaneous Medical Aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Street, J.M.; Koritzinsky, E.H.; Glispie, D.M.; Yuen, P.S.T. Urine Exosome Isolation and Characterization. Methods Mol. Biol. 2017, 1641, 413–423. [Google Scholar] [CrossRef]
- Li, K.; Wong, D.K.; Hong, K.Y.; Raffai, R.L. Cushioned–Density Gradient Ultracentrifugation (C-DGUC): A Refined and High Performance Method for the Isolation, Characterization, and Use of Exosomes. Extracell. RNA Methods Protoc. 2018, 1740, 69–83. [Google Scholar]
- Duong, P.; Chung, A.; Bouchareychas, L.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC) Improves the Isolation Efficiency of Extracellular Vesicles. PLoS ONE 2019, 14, e0215324. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. BioMed Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal Stem Cells-Derived Exosomes for Drug Delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- de la Torre Gomez, C.; Goreham, R.V.; Bech Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liu, Y.; Wang, T.; Jiang, Q.; Xu, F.; Liu, Z. Living Cell for Drug Delivery. Eng. Regen. 2022, 3, 131–148. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes Derived from Human Adipose Tissue-Derived Mesenchymal Stem Cells Alleviate Atopic Dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes Released from Educated Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing via Promoting Angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-Preconditioned Mesenchymal Stromal Cells Modify Macrophage Polarization for Resolution of Chronic Inflammation via Exosome-Shuttled Let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-Stimulated MSC-Derived Exosomes Improve Diabetic Wound Healing through Regulating Macrophage M1 and M2 Polarization by Targeting the PTEN/AKT Pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Eckert, R.L.; Adhikary, G.; Young, C.A.; Jans, R.; Crish, J.F.; Xu, W.; Rorke, E.A. AP1 Transcription Factors in Epidermal Differentiation and Skin Cancer. J. Skin Cancer 2013, 2013, 537028. [Google Scholar] [CrossRef] [Green Version]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small Molecule Inhibitors Targeting Activator Protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef]
- Agron, M.; Brekhman, V.; Morgenstern, D.; Lotan, T. Regulation of AP-1 by MAPK Signaling in Metal-Stressed Sea Anemone. Cell Physiol. Biochem. 2017, 42, 952–964. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ngo, H.T.T.; Hwang, E.; Wei, X.; Liu, Y.; Liu, J.; Yi, T.H. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents Uvb-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-Derived Exosomes Attenuate Cell Death through Suppressing AIF Nucleus Translocation and Enhance Cutaneous Wound Healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of Metastatic Melanoma Patients with Autologous Dendritic Cell (DC) Derived-Exosomes: Results of the First Phase 1 Clinical Trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, E.Y.; Chiou, T.W.; Harn, H.J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising Effects of Exosomes Isolated from Menstrual Blood-Derived Mesenchymal Stem Cell on Wound-Healing Process in Diabetic Mouse Model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef]
- Tao, S.-C.; Guo, S.-C.; Li, M.; Guo, Y.-P.; Zhang, C.-Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl Med. 2017, 6, 736–747. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia Adipose Stem Cell-Derived Exosomes Promote High-Quality Healing of Diabetic Wound Involves Activation of PI3K/Akt Pathways. J. Nanobiotechnology 2021, 19, 202. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes Derived from Pioglitazone-Pretreated MSCs Accelerate Diabetic Wound Healing through Enhancing Angiogenesis. J. Nanobiotechnology 2021, 19, 150. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomedicine 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B.; Bolontrade, M.F. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-Induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSc-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells Stimulate Regenerative Wound Healing via Transforming Growth Factor-β Receptor Inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.A.; Kim, S.Y.; Park, J.H.; Jo, D.G.; Cho, Y.W. Functional Recovery in Photo-Damaged Human Dermal Fibroblasts by Human Adipose-Derived Stem Cell Extracellular Vesicles. J. Extracell. Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jian, Z.; Baskys, A.; Yang, J.; Li, J.; Guo, H.; Hei, Y.; Xian, P.; He, Z.; Li, Z.; et al. MSC-Derived Exosomes Protect against Oxidative Stress-Induced Skin Injury via Adaptive Regulation of the NRF2 Defense System. Biomaterials 2020, 257, 120264. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.-U.; Cheng, K. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano 2022, 13, 139–148. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Han, X.; Sun, Y.; Sun, Z.; Li, L.; Jin, Q.; Fu, P.; Xu, W.; Qian, H. HucMSC Exosome-Delivered 14-3-3ζ Alleviates Ultraviolet Radiation-Induced Photodamage via SIRT1 Pathway Modulation. Aging 2021, 13, 11542–11563. [Google Scholar] [CrossRef]
- Deng, M.; Yu, Z.; Li, D.; Wang, X.; Zhou, G.; Liu, W.; Cao, Y.; Xia, W.; Li, W.; Jie Zhang, W. Human Umbilical Cord Mesenchymal Stem Cell-Derived and Dermal Fibroblast-Derived Extracellular Vesicles Protect Dermal Fibroblasts from Ultraviolet Radiation-Induced Photoaging: In Vitro. Photochem. Photobiol. Sci. 2020, 19, 406–414. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Mojzych, M.; Kontek, R. Focus on Uv-Induced Dna Damage and Repair—Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21, 7264. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, X.; Li, Y.; Wang, X.; Tan, S.; Chen, F. LPS Enhances Platelets Aggregation via TLR4, Which Is Related to Mitochondria Damage Caused by Intracellular ROS, but Not Extracellular ROS. Cell. Immunol. 2018, 328, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kaczanowska, S.; Davila, E. IL-1 Receptor-Associated Kinase Signaling and Its Role in Inflammation, Cancer Progression, and Therapy Resistance. Front. Immunol. 2014, 5, 553. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Wirth, A.K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 Pathway Promotes Cell Proliferation and Diminishes Ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The Pi3k/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Chen, C.-C.; Jeon, S.-M.; Bhaskar, P.T.; Nogueira, V.; Sundararajan, D.; Tonic, I.; Park, Y.; Hay, N. FoxOs Inhibit MTORC1 and Activate Akt by Inducing the Expression of Sestrin3 and Rictor. NIH Public Access 2010, 18, 592–604. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, H.; Tian, R.; Zhang, Y.; Drutskaya, M.S.; Wang, C.; Ge, J.; Fan, Z.; Kong, D.; Wang, X.; et al. Macrophages Induce AKT/β-Catenin-Dependent Lgr5+ Stem Cell Activation and Hair Follicle Regeneration through TNF. Nat. Commun. 2017, 8, 14091. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-Free Therapy Based on Adipose Tissue Stem Cell-Derived Exosomes Promotes Wound Healing via the PI3K/Akt Signaling Pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Jenuwein, T. The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.Z.; Jiang, J.; Duan, C.G. The Crosstalk Between Epigenetic Mechanisms and Alternative RNA Processing Regulation. Front. Genet. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Chromatin Remodeling and Epigenetic Regulation in Plant DNA Damage Repair. Int. J. Mol. Sci. 2019, 20, 4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.; Schneider, R. Histone Post-Translational Modifications—Cause and Consequence of Genome Function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The Roles of MicroRNAs in Epigenetic Regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef]
- Lee, Y.S.; Liang, Y.C.; Wu, P.; Kulber, D.A.; Tanabe, K.; Chuong, C.M.; Widelitz, R.; Tuan, T.L. STAT3 Signalling Pathway Is Implicated in Keloid Pathogenesis by Preliminary Transcriptome and Open Chromatin Analyses. Exp. Dermatol. 2019, 28, 480–484. [Google Scholar] [CrossRef]
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860. [Google Scholar] [CrossRef]
- Na, J.; Lee, K.; Na, W.; Shin, J.Y.; Lee, M.J.; Yune, T.Y.; Lee, H.K.; Jung, H.S.; Kim, W.S.; Ju, B.G. Histone H3K27 Demethylase JMJD3 in Cooperation with NF-ΚB Regulates Keratinocyte Wound Healing. J. Investig. Dermatol. 2016, 136, 847–858. [Google Scholar] [CrossRef] [Green Version]
- den Dekker, A.; Davis, F.M.; Kunkel, S.L.; Gallagher, K.A. Targeting Epigenetic Mechanisms in Diabetic Wound Healing. Transl. Res. 2019, 204, 39–50. [Google Scholar] [CrossRef]
- Gondaliya, P.; Sayyed, A.A.; Bhat, P.; Mali, M.; Arya, N.; Khairnar, A.; Kalia, K. Mesenchymal Stem Cell-Derived Exosomes Loaded with MiR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol. Pharm. 2022, 19, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Miao, J.; Liu, W.; Cai, K.; Huang, X.; Peng, L. Mesenchymal Stem Cell-Derived Exosomes Carry Microrna-125a to Protect against Diabetic Nephropathy by Targeting Histone Deacetylase 1 and Downregulating Endothelin-1. Diabetes Metab. Syndr. Obes. 2021, 14, 1405–1418. [Google Scholar] [CrossRef] [PubMed]

| Exosome Source | Model | Mechanism of Action | Type of Application | Reference |
|---|---|---|---|---|
| Human umbilical cord | In vitro (THP-1) + LPS + rats + STZ | MSCs switched macrophages to an M2 state. Macrophage polarization was regulated through TLR4/NF-KB/STAT3/AKT regulatory signaling pathways. | Diabetic wound healing | [65] |
| Menstrual blood sample. | C57BL/6 + STZ | The M2 stage was induced through the downregulation of TLR4 and NF-KB. STAT3 and STAT6 were activated, triggering the expression of M2 genes such as Arg-1 and Arg-2. | Promoting wound-healing processes in diabetic wounds. | [76] |
| hBMSCs | SD rats + STZ | The activation of the AKT/PI3K pathways was inhibited due to the promotion of the expression of the PTEN gene. This lead to the inhibition of the inflammatory phase, leading to a quicker tissue regeneration phase. M1 polarization of macrophages was inhibited, leading to M2. | Diabetic wounds | [66] |
| Synovial membrane | HMEC-1 cells cultured in MCDB131 + 10% FBs + 10 ng/mL epidermal growth factor + 2 mM L-glutamine + 1 mg/mL hydrocortisone. Adult male Sprague Dawley rats. | An increase in the granulation tissue and angiogenesis. Using SMSCs and overexpressing miR-126-3p increased the strength of exosomes (SMSC-126-Exos); it was found that they could trigger the generation of newly formed vessels and also increase their maturation. | Wound healing in diabetic skin | [77] |
| ADSCs | BALB/c mice + high fat diet + STZ + skin injury | Fibroblast proliferation and migration were promoted through the activation of the PI3K/AKT pathway, accelerating the healing of diabetic wounds. TGF-β levels were also enhanced, stimulating fibroblast proliferation. | Promotion of diabetic wound healing | [78] |
| PGZ-exos + HUVECs + BMSCs | Mice + STZ + cutaneous wounds | The expression of p-AKT and p-PI3K was promoted, activating the PI3K/AKT/eNOS pathway and enhancing angiogenesis. PGZ-exos promoted collagen deposition, and re-epithelization accelerated wound healing. | Promotion of diabetic wound healing | [79] |
| hUCMSCs | SD rats + STZ + skin wounds | β-catenin activation in endothelial cells was induced, resulting in the promotion of wound healing. PF-127 hydrogel served as a vessel for the continuous release of exosomes. | Promotion of diabetic wound healing | [80] |
| Bone marrow from human jaw and iliac crest | C57BL/6J female adult mice + skin excision | Exosomes enhanced anti-inflammatory responses by secreting miR-223, which led to the M2 polarization of macrophages, accelerating wound healing. The expression of M2 factors RELM-α and Arginase 1 increased. | Dermal wound healing. | [81] |
| hucMSCs | Balb/C mice + excisional wound + peroxide | Stem cells inhibit cell-induced death by suppressing the translation of AIF and the hyperactivation of PARP-1, inhibiting the action of keratinocytes and fibroblasts, which accelerate the process of wound regeneration. CK14’s expression was upregulated, leading to improved integrity in the recently formed epidermis. | Cutaneous wounds | [73] |
| Human umbilical cord: hSFCs and hUCMSCs | SD rats + full thickness burn wounds | hUCMSC exosomes reduced the presence of white blood cells and the suppression of the TLR4 pathway, preventing the release of inflammatory factors such as TNF-α and IL-1β. | Reducing inflammation resulting from severe burns. | [82] |
| FDMSC sand ADFs | BALB/c mice + PBS + FDMSC-exosomes | Activating Notch Signalling using MSC-derived exosomes. | Cutaneous wound healing | [83] |
| Bone marrow mesenchymal stem cells of C57BL/6J mice and human umbilical vein endothelial cells | C57BL/6J + pentobarbitone sodium + dorsal wounds | The expression of p-AKT and p-eNOS was enhanced, stimulating angiogenesis through the activation of the AKT-mediated VEGF pathway. | Cutaneous wounds | [64] |
| hUCMSCs | SD rats + skin deep second-degree burn wounds | The number of epidermal and dermal cells significantly increased, and the rate of re-epithelialization was determined by the enhanced expression of CK19: an epithelial biomarker. PCNA-positive cells were found in the wound area, indicating the proliferation of cells. Exosomes deliver Wnt4, which activates Wnt/b-catenin and inhibits stress-induced skin cell apoptosis by the activation of the AKT pathway. | Accelerating re-epithelialization in burn wounds | [84] |
| Human umbilical cord blood stem cells | SD rats + excisional wound | TGF-β receptors are inhibited via miR-21-5p, and miR-125b-5p, resulting in a lower myofibroblast differentiation, preventing excessive scar formation. Collagen formation is inhibited in the late stages of the healing process. | Scar tissue | [85] |
| Primary human adipose stem cells | In vitro (HDFs) + UVB radiation | MMP production is downregulated, proliferating elastin, TGF-β1, TIMP-1, and collagen types I, II, III, IV, and V, assisting in the recovery of damaged fibroblasts. | Promoting the healing of dermal tissue damaged by UV radiation | [86] |
| hUCMSCs | Mice + UV radiation | The NRF2 (antioxidant defense system) could be regulated, resulting in an enhanced rate of repair of oxidative stress-induced skin injury. Collagen fiber density and epidermal thickness were increased. MSCs restored the calcium concentration in damaged keratinocytes. The expression of NRF2 signaling was decreased, resulting in the mediation of oxidation and inflammation factors. | Treatment of oxidative stress injuries resulting from UV radiation | [87] |
| Bone marrow derived mesenchymal stem cells | Dermal fibroblasts/mice + UVB radiation | UV-induced MMP1 and MMP9 expressions were greatly diminished. Type I procollagen synthesis was enhanced. MSCs produced growth factors such as VEGF, EGF, IGFBP, and bFGF, which contributed to angiogenesis and injury repair. | Diminishing the effects of aging and UV radiation such as poor collagen production | [88] |
| Human umbilical cord mesenchymal stem cells | Mice + acute skin photodamage | The expression of p-p65 was significantly decreased, and the levels of PCNA expression were increased. The γH2AX and 8-OHDG markers for DNA damage expression were lowered. The proliferation of HaCaT cells was enhanced, and apoptosis was inhibited. SIRT1 expression was also enhanced by the delivery of the 14-3-3ζ protein, leading to the promotion of cell survival via autophagy. | Prevention and repair of oxidative and UV-related damage | [89] |
| Human umbilical cord mesenchymal cells | Fibroblasts + UVB radiation | SA-β-gal-positive cells were reduced, indicating a lower rate of cell aging. The expression levels of MMP1 were decreased, while the expression of Col-1 increased, which suggested the recovery of regular cellular functions. The expression of the GPX-1 gene was upregulated, possibly leading to an increase in antioxidant activity. | Development of anti-aging treatment on dermal fibroblasts affected by UVB-induced photoaging | [90] |
| Adipose-derived stem cells | Human skin fibroblasts/mice + UVB radiation | The expression of MMP-1 was significantly reduced by the inhibition of the MAPK/AP-1 pathway. Exosomes rich with miR-1246 increased the gene expression of TGF-β1, which led to the promotion of collagen and elastin synthesis, as well as the transcription of type I procollagen. | Reducing the effects of photoaging derived from UVB radiation damage. | [25] |
| Colostrum of cows and commercialized milk | HDF + UV, HaCaTs + UV | Col M-exo transport | Damaged skin by UV light | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tienda-Vázquez, M.A.; Hanel, J.M.; Márquez-Arteaga, E.M.; Salgado-Álvarez, A.P.; Scheckhuber, C.Q.; Alanis-Gómez, J.R.; Espinoza-Silva, J.I.; Ramos-Kuri, M.; Hernández-Rosas, F.; Melchor-Martínez, E.M.; et al. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells 2023, 12, 1625. https://doi.org/10.3390/cells12121625
Tienda-Vázquez MA, Hanel JM, Márquez-Arteaga EM, Salgado-Álvarez AP, Scheckhuber CQ, Alanis-Gómez JR, Espinoza-Silva JI, Ramos-Kuri M, Hernández-Rosas F, Melchor-Martínez EM, et al. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells. 2023; 12(12):1625. https://doi.org/10.3390/cells12121625
Chicago/Turabian StyleTienda-Vázquez, Mario Adrián, Juan Manuel Hanel, Elsa Margarita Márquez-Arteaga, Ana Paola Salgado-Álvarez, Christian Quintus Scheckhuber, José Rafael Alanis-Gómez, Janette Ivone Espinoza-Silva, Manuel Ramos-Kuri, Fabiola Hernández-Rosas, Elda M. Melchor-Martínez, and et al. 2023. "Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders" Cells 12, no. 12: 1625. https://doi.org/10.3390/cells12121625
APA StyleTienda-Vázquez, M. A., Hanel, J. M., Márquez-Arteaga, E. M., Salgado-Álvarez, A. P., Scheckhuber, C. Q., Alanis-Gómez, J. R., Espinoza-Silva, J. I., Ramos-Kuri, M., Hernández-Rosas, F., Melchor-Martínez, E. M., & Parra-Saldívar, R. (2023). Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells, 12(12), 1625. https://doi.org/10.3390/cells12121625









