Investigating the Role of TGF-β Signaling Pathways in Human Corneal Endothelial Cell Primary Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Crucial Products
2.3. Primary Culture of hCECs
2.4. Immunofluorescence (IF)
2.5. Methods of Quantification
2.6. Statistics
3. Results
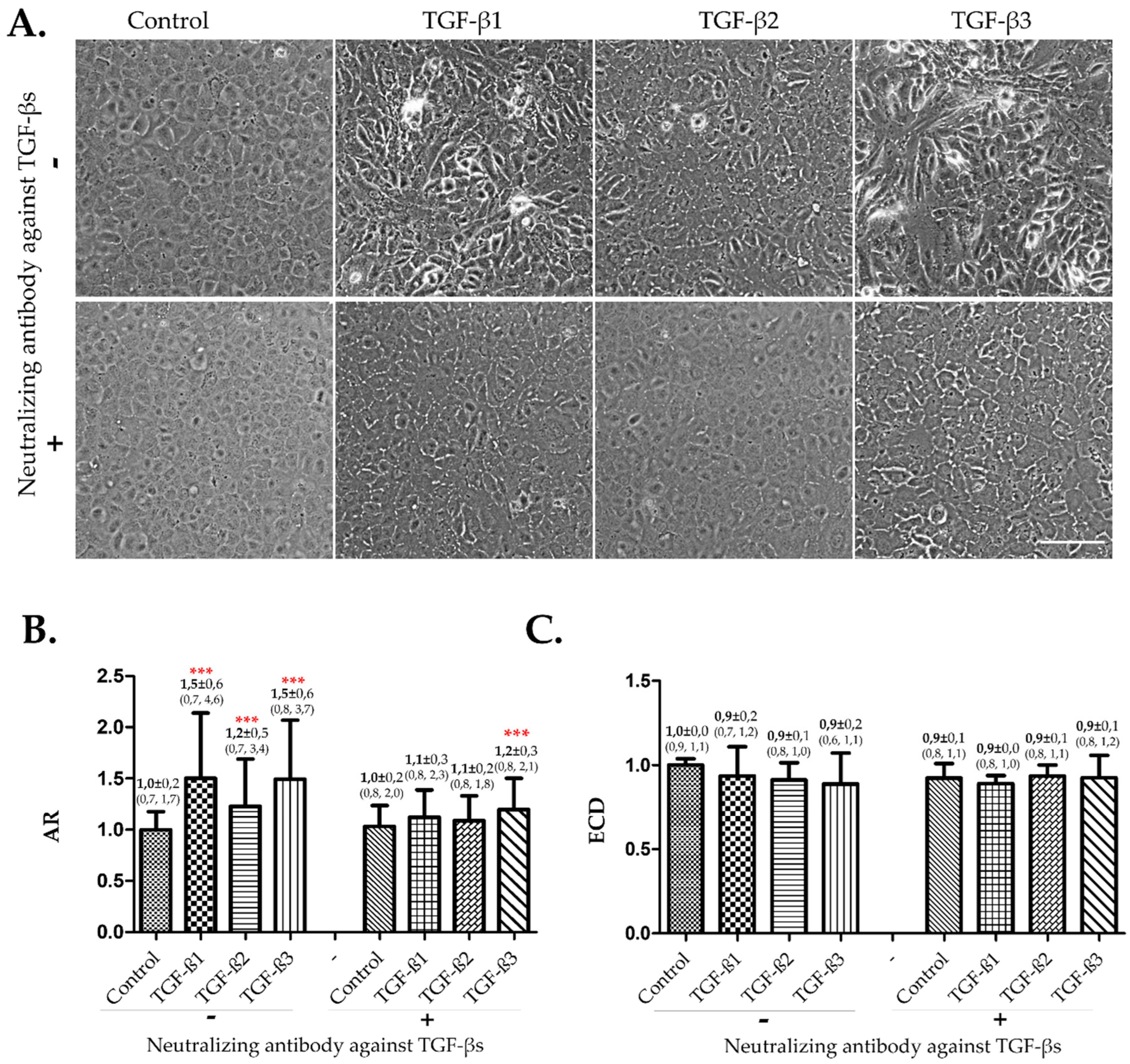
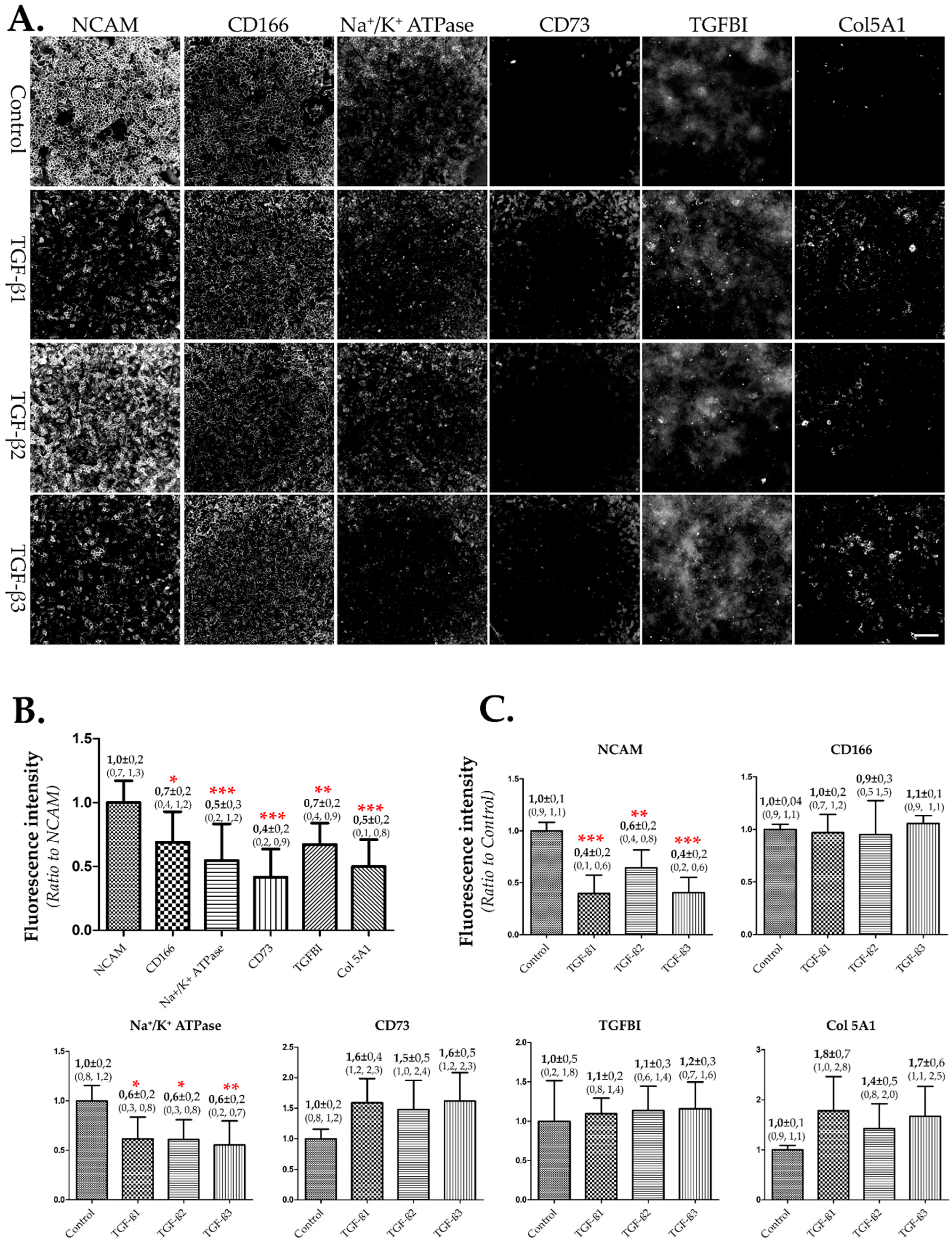
3.1. The Three TGF-β Isforms Induced EndMT on Confluent hCECs in Absence of FBS
3.2. Selection of NCAM as Biomarker to Assess EndMT
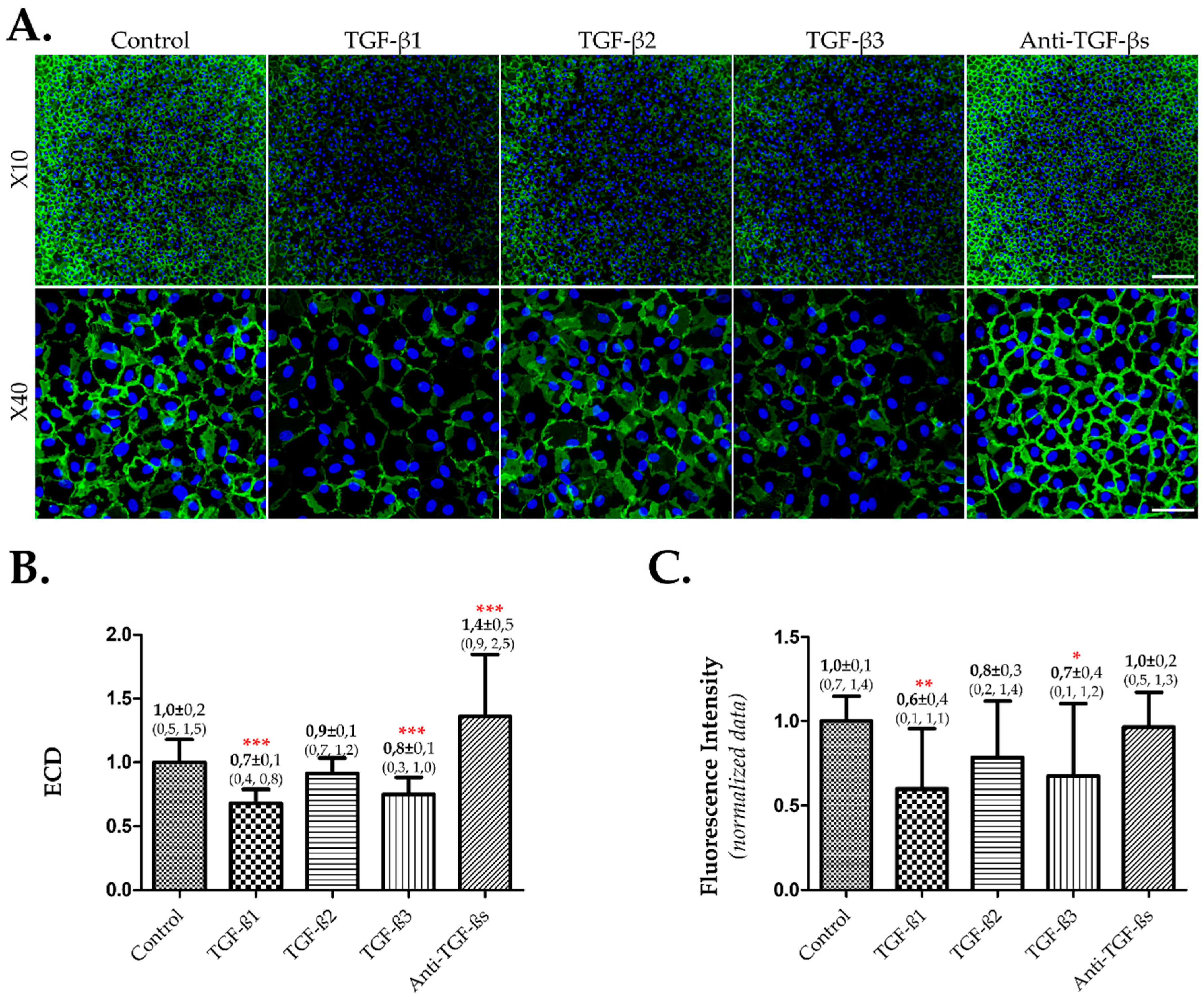
3.3. TGF-β1 and 3 Induced EndMT and Lower ECD on Cultured hCECs in Presence of FBS
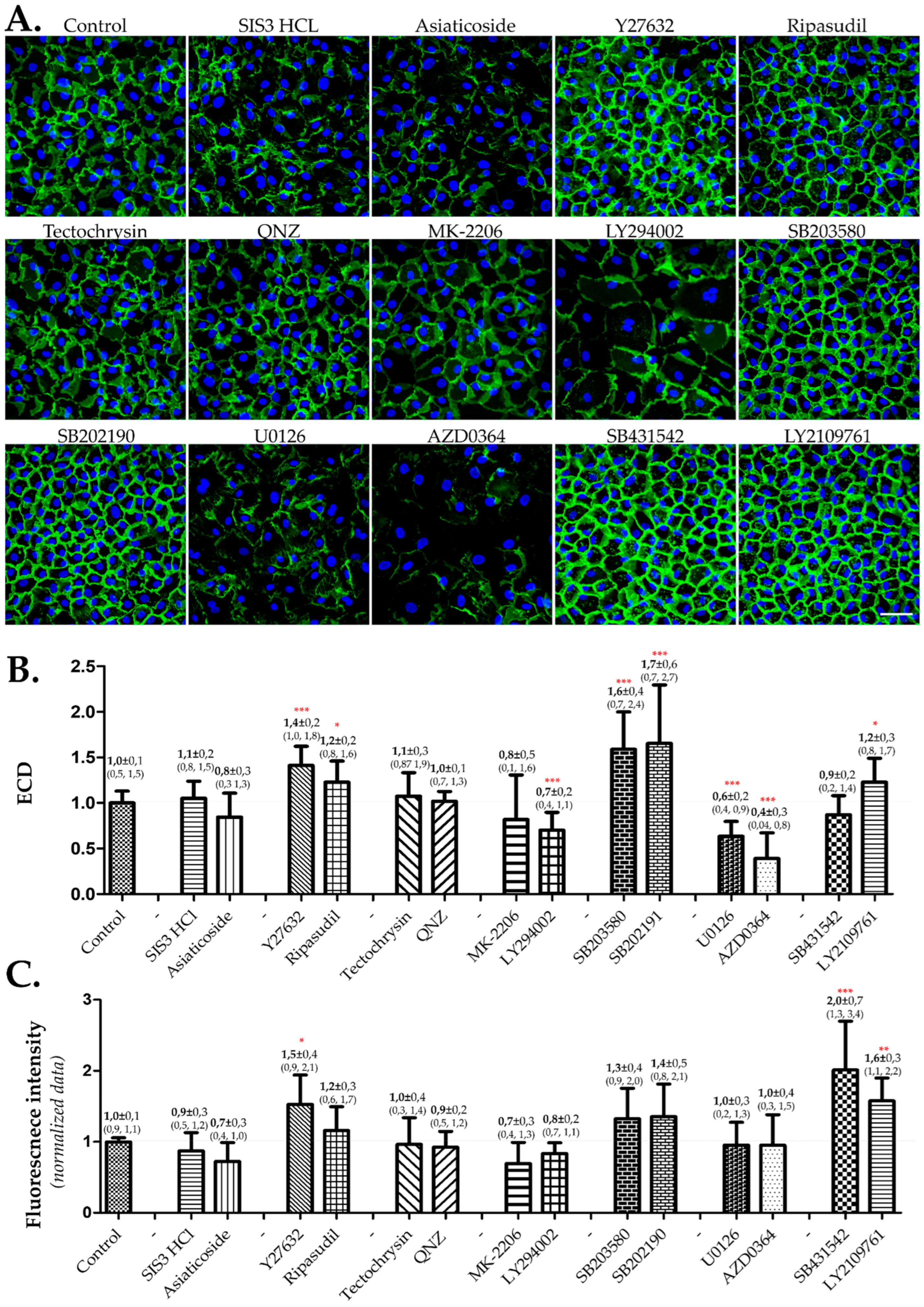
3.4. The Assessment of Inhibitors of TGF-β Signaling Pathways Revealed Positive Effects in Inhibiting P38-MAPK, ROCK, and TGF-β Receptor
4. Discussion
4.1. Effects of TGF-β 1, 2, and 3 on In Vitro hCECs
4.2. Proposal of a Screening System for Molecules to Evaluate Their Effects on hCECs In Vitro
4.3. Effects of Inhibiting Various TGF-β Signaling Pathways on Cultured hCECs
4.4. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ha Thi, B.M.; Campolmi, N.; He, Z.; Pipparelli, A.; Manissolle, C.; Thuret, J.Y.; Piselli, S.; Forest, F.; Peoc’h, M.; Garraud, O.; et al. Microarray analysis of cell cycle gene expression in adult human corneal endothelial cells. PLoS ONE 2014, 9, e94349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Ogita, Y.; Higuchi, S.; Kani, K. Cell movements in a living mammalian tissue: Long-term observation of individual cells in wounded corneal endothelia of cats. J. Morphol. 1982, 174, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, Z.Q.; Li, J.; Zhou, Q. Identification of novel therapeutic targets for Fuchs’ endothelial corneal dystrophy based on gene bioinformatics analysis. PLoS ONE 2022, 17, e0264018. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Chen, X.; Yao, K. Cataract: Advances in surgery and whether surgery remains the only treatment in future. Adv. Ophthalmol. Pract. Res. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Crouzet, E.; He, Z.; Ben Moussa, O.; Mentek, M.; Isard, P.F.; Peyret, B.; Forest, F.; Gain, P.; Koizumi, N.; Okumura, N.; et al. Tissue engineered endothelial keratoplasty in rabbit: Tips and tricks. Acta Ophthalmol. 2022, 100, 690–699. [Google Scholar] [CrossRef]
- Parekh, M.; Romano, V.; Hassanin, K.; Testa, V.; Wongvisavavit, R.; Ferrari, S.; Haneef, A.; Willoughby, C.; Ponzin, D.; Jhanji, V.; et al. Biomaterials for corneal endothelial cell culture and tissue engineering. J. Tissue Eng. 2021, 12, 2041731421990536. [Google Scholar] [CrossRef]
- Teichmann, J.; Valtink, M.; Nitschke, M.; Gramm, S.; Funk, R.H.; Engelmann, K.; Werner, C. Tissue engineering of the corneal endothelium: A review of carrier materials. J. Funct. Biomater. 2013, 4, 178–208. [Google Scholar] [CrossRef] [Green Version]
- Wongvisavavit, R.; Parekh, M.; Ahmad, S.; Daniels, J.T. Challenges in corneal endothelial cell culture. Regen. Med. 2021, 16, 871–891. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Q.; Sun, H.; Zhang, Y.; Tighe, S.; Xu, L.; Zhu, Y. Advances in culture, expansion and mechanistic studies of corneal endothelial cells: A systematic review. J. Biomed. Sci. 2019, 26, 2. [Google Scholar] [CrossRef] [Green Version]
- Spinozzi, D.; Miron, A.; Bruinsma, M.; Lie, J.T.; Dapena, I.; Oellerich, S.; Melles, G.R.J. Improving the success rate of human corneal endothelial cell cultures from single donor corneas with stabilization medium. Cell Tissue Bank. 2018, 19, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, O.; Leclerc, V.B.; Bourget, J.M.; Theriault, M.; Proulx, S. Understanding the process of corneal endothelial morphological change in vitro. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1228–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frausto, R.F.; Swamy, V.S.; Peh, G.S.L.; Boere, P.M.; Hanser, E.M.; Chung, D.D.; George, B.L.; Morselli, M.; Kao, L.; Azimov, R.; et al. Phenotypic and functional characterization of corneal endothelial cells during in vitro expansion. Sci. Rep. 2020, 10, 7402. [Google Scholar] [CrossRef]
- Parekh, M.; Peh, G.; Mehta, J.S.; Ramos, T.; Ponzin, D.; Ahmad, S.; Ferrari, S. Passaging capability of human corneal endothelial cells derived from old donors with and without accelerating cell attachment. Exp. Eye Res. 2019, 189, 107814. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.; Chng, Z.; Ang, H.P.; Cheng, T.Y.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Peh, G.S.; Toh, K.P.; Ang, H.P.; Seah, X.Y.; George, B.L.; Mehta, J.S. Optimization of human corneal endothelial cell culture: Density dependency of successful cultures in vitro. BMC Res. Notes 2013, 6, 176. [Google Scholar] [CrossRef] [Green Version]
- Oida, T.; Weiner, H.L. Depletion of TGF-beta from fetal bovine serum. J. Immunol. Methods 2010, 362, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Wendt, M.K.; Allington, T.M.; Schiemann, W.P. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009, 5, 1145–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavadil, J.; Bottinger, E.P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 2005, 24, 5764–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.; Winter, S.L.; Alexandrow, M.G. Cell cycle arrest by transforming growth factor beta1 near G1/S is mediated by acute abrogation of prereplication complex activation involving an Rb-MCM interaction. Mol. Cell. Biol. 2010, 30, 845–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.B.; Hocevar, B.A.; Howe, P.H. Inhibition of G1 phase cyclin dependent kinases by transforming growth factor beta 1. J. Cell. Biochem. 1994, 56, 418–425. [Google Scholar] [CrossRef]
- Chen, K.H.; Hsu, W.M.; Lee, S.M. Differential effects of transforming growth factor-beta2 on corneal endothelial cell proliferation-A role of serum factors. Exp. Eye Res. 2002, 75, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C.; Harris, D.L.; Mello, D.M. Mechanisms of mitotic inhibition in corneal endothelium: Contact inhibition and TGF-beta2. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2152–2159. [Google Scholar]
- Chen, K.H.; Harris, D.L.; Joyce, N.C. TGF-beta2 in aqueous humor suppresses S-phase entry in cultured corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2513–2519. [Google Scholar]
- Zhang, Z.H.; Miao, Y.Y.; Ke, B.L.; Liu, K.; Xu, X. LY2109761, Transforming Growth Factor beta Receptor Type I and Type II Dual Inhibitor, is a Novel Approach to Suppress Endothelial Mesenchymal Transformation in Human Corneal Endothelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 963–972. [Google Scholar] [CrossRef]
- Okumura, N.; Kay, E.P.; Nakahara, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS ONE 2013, 8, e58000. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Okumura, N.; Sato, M.; Komori, Y.; Nakahara, M.; Gain, P.; Koizumi, N.; Thuret, G. Corneal endothelial cell therapy: Feasibility of cell culture from corneas stored in organ culture. Cell Tissue Bank. 2021, 22, 551–562. [Google Scholar] [CrossRef]
- Li, J.; Yang, B.; Zhou, Q.; Wu, Y.; Shang, D.; Guo, Y.; Song, Z.; Zheng, Q.; Xiong, J. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis 2013, 34, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Zhu, B.; Liang, Y.; Bi, L.; Hu, Z.; Chen, B.; Zhang, K.; Zhu, J. Asiaticoside suppresses collagen expression and TGF-beta/Smad signaling through inducing Smad7 and inhibiting TGF-betaRI and TGF-betaRII in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Isobe, T.; Mizuno, K.; Kaneko, Y.; Ohta, M.; Koide, T.; Tanabe, S. Effects of K-115, a rho-kinase inhibitor, on aqueous humor dynamics in rabbits. Curr. Eye Res. 2014, 39, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.E.; Park, E.S.; Yoon, H.S.; Seo, D.W.; Hyun, B.K.; Han, S.B.; Ham, Y.W.; Hwang, B.Y.; Hong, J.T. Anticancer effect of tectochrysin in colon cancer cell via suppression of NF-kappaB activity and enhancement of death receptor expression. Mol. Cancer 2015, 14, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Ren, X.; Zhang, Y.; Patel, R.; Sharma, A.; Wu, H.; Robertson, G.P.; Yan, L.; Rubin, E.; Yang, J.M. eEF-2 kinase dictates cross-talk between autophagy and apoptosis induced by Akt Inhibition, thereby modulating cytotoxicity of novel Akt inhibitor MK-2206. Cancer Res. 2011, 71, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorlick, R.; Maris, J.M.; Houghton, P.J.; Lock, R.; Carol, H.; Kurmasheva, R.T.; Kolb, E.A.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; et al. Testing of the Akt/PKB inhibitor MK-2206 by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2012, 59, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Semba, S.; Itoh, N.; Ito, M.; Harada, M.; Yamakawa, M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3’-kinase, in human colon cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 1957–1963. [Google Scholar]
- Hongo, A.; Okumura, N.; Nakahara, M.; Kay, E.P.; Koizumi, N. The Effect of a p38 Mitogen-Activated Protein Kinase Inhibitor on Cellular Senescence of Cultivated Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3325–3334. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, Y.; Jiang, X.; Zhang, X.; Xia, L.; Lin, K.; Xu, Y. The Effect of Quercetin on the Osteogenesic Differentiation and Angiogenic Factor Expression of Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0129605. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.C.; Huang, W.C.; Pang, J.H.S.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1beta-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef] [Green Version]
- Jasek-Gajda, E.; Jurkowska, H.; Jasinska, M.; Lis, G.J. Targeting the MAPK/ERK and PI3K/AKT Signaling Pathways Affects NRF2, Trx and GSH Antioxidant Systems in Leukemia Cells. Antioxidants 2020, 9, 633. [Google Scholar] [CrossRef]
- He, Z.; Campolmi, N.; Ha Thi, B.M.; Dumollard, J.M.; Peoc’h, M.; Garraud, O.; Piselli, S.; Gain, P.; Thuret, G. Optimization of immunolocalization of cell cycle proteins in human corneal endothelial cells. Mol. Vis. 2011, 17, 3494–3511. [Google Scholar]
- Joko, T.; Shiraishi, A.; Akune, Y.; Tokumaru, S.; Kobayashi, T.; Miyata, K.; Ohashi, Y. Involvement of P38MAPK in human corneal endothelial cell migration induced by TGF-beta(2). Exp. Eye Res. 2013, 108, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, Z.; Reinach, P.; Zhang, J.; Dai, W.; Lu, L.; Xu, M. TGF-beta2 inhibits AKT activation and FGF-2-induced corneal endothelial cell proliferation. Exp. Cell Res. 2006, 312, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, W.I.; Smith, R.E.; Kay, E.D. Role of p27(Kip1) in cAMP- and TGF-beta2-mediated antiproliferation in rabbit corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3142–3149. [Google Scholar]
- Harris, D.L.; Joyce, N.C. Transforming growth factor-beta suppresses proliferation of rabbit corneal endothelial cells in vitro. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 1999, 19, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Moulin, A.; Mathieu, M.; Lawrence, C.; Bigelow, R.; Levine, M.; Hamel, C.; Marquette, J.P.; Le Parc, J.; Loux, C.; Ferrari, P.; et al. Structures of a pan-specific antagonist antibody complexed to different isoforms of TGFbeta reveal structural plasticity of antibody-antigen interactions. Protein Sci. A Publ. Protein Soc. 2014, 23, 1698–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.C.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Reiss, M.; Hsu, F.J.; Berzofsky, J.A.; Lawrence, D.P. Phase I study of GC1008 (fresolimumab): A human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 2014, 9, e90353. [Google Scholar] [CrossRef]
- Giannoni, E.; Parri, M.; Chiarugi, P. EMT and oxidative stress: A bidirectional interplay affecting tumor malignancy. Antioxid. Redox Signal. 2012, 16, 1248–1263. [Google Scholar] [CrossRef]
- Beaulieu Leclerc, V.; Roy, O.; Santerre, K.; Proulx, S. TGF-beta1 promotes cell barrier function upon maturation of corneal endothelial cells. Sci. Rep. 2018, 8, 4438. [Google Scholar] [CrossRef] [Green Version]
- Santerre, K.; Cortez Ghio, S.; Proulx, S. TGF-beta-Mediated Modulation of Cell-Cell Interactions in Postconfluent Maturing Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chou, H.C.; Ho, Y.J.; Chang, S.J.; Liao, E.C.; Wei, Y.S.; Lin, M.W.; Wang, Y.S.; Chien, Y.A.; Yu, X.R.; et al. Characterization of TGF-beta by Induced Oxidative Stress in Human Trabecular Meshwork Cells. Antioxidants 2021, 10, 107. [Google Scholar] [CrossRef]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. mTOR-Mediated Antioxidant Activation in Solid Tumor Radioresistance. J. Oncol. 2019, 2019, 5956867. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Wang, H.; Wu, X.; He, L.; Zhou, Q.; Wang, F.; Chen, S.; Huang, L.; Chen, J.; Wang, H.; et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 2019, 10, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartakova, A.; Kuzmenko, O.; Alvarez-Delfin, K.; Kunzevitzky, N.J.; Goldberg, J.L. A Cell Culture Approach to Optimized Human Corneal Endothelial Cell Function. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1617–1629. [Google Scholar] [CrossRef] [Green Version]
- Bartakova, A.; Alvarez-Delfin, K.; Weisman, A.D.; Salero, E.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kunzevitzky, N.J.; Goldberg, J.L. Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2749–2762. [Google Scholar] [CrossRef] [Green Version]
- Forest, F.; Thuret, G.; Gain, P.; Dumollard, J.M.; Peoc’h, M.; Perrache, C.; He, Z. Optimization of immunostaining on flat-mounted human corneas. Mol. Vis. 2015, 21, 1345–1356. [Google Scholar] [PubMed]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’h, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef] [Green Version]
- Okumura, N.; Hirano, H.; Numata, R.; Nakahara, M.; Ueno, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Cell surface markers of functional phenotypic corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7610–7618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chng, Z.; Peh, G.S.; Herath, W.B.; Cheng, T.Y.; Ang, H.P.; Toh, K.P.; Robson, P.; Mehta, J.S.; Colman, A. High throughput gene expression analysis identifies reliable expression markers of human corneal endothelial cells. PLoS ONE 2013, 8, e67546. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, D.; Liu, Z.; Li, C.; Liu, Y.; Zhou, J.; Wan, P.; Mou, Y.G.; Wang, Z. Enhanced survival in vitro of human corneal endothelial cells using mouse embryonic stem cell conditioned medium. Mol. Vis. 2010, 16, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Rinkoski, T.A.; Bahler, C.K.; Pacheco, J.M.; Khanna, M.L.; Holmes, D.M.; Roy Chowdhury, U.; Baratz, K.H.; Patel, S.V.; Maguire, L.J.; Wieben, E.D.; et al. Characterization of a dual media system for culturing primary normal and Fuchs endothelial corneal dystrophy (FECD) endothelial cells. PLoS ONE 2021, 16, e0258006. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, R.; Li, H.; Wang, B.; Chen, Y.; Chen, S.; Ou, K.; Wang, X. Secreted TGF-beta-induced protein promotes aggressive progression in bladder cancer cells. Cancer Manag. Res. 2019, 11, 6995–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, G.S.; Kline, H.L.; Lee, C.H.; Wilkes, D.S.; Zhang, C. Regulation of Collagen V Expression and Epithelial-Mesenchymal Transition by miR-185 and miR-186 during Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2016, 186, 2310–2316. [Google Scholar] [CrossRef] [Green Version]
- Vittal, R.; Fan, L.; Greenspan, D.S.; Mickler, E.A.; Gopalakrishnan, B.; Gu, H.; Benson, H.L.; Zhang, C.; Burlingham, W.; Cummings, O.W.; et al. IL-17 induces type V collagen overexpression and EMT via TGF-beta-dependent pathways in obliterative bronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L401–L414. [Google Scholar] [CrossRef] [Green Version]
- Khoshakhlagh, M.; Soleimani, A.; Binabaj, M.M.; Avan, A.; Ferns, G.A.; Khazaei, M.; Hassanian, S.M. Therapeutic potential of pharmacological TGF-beta signaling pathway inhibitors in the pathogenesis of breast cancer. Biochem. Pharmacol. 2019, 164, 17–22. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Freitas, J.P.; Mazher Hussain, S.; Glazer, E.S. TGF-beta Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Cancer 2019, 50, 207–213. [Google Scholar] [CrossRef]
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal Modulators of TGF-beta in Cancer. J. Clin. Med. 2017, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Neuzillet, C.; de Gramont, A.; Tijeras-Raballand, A.; de Mestier, L.; Cros, J.; Faivre, S.; Raymond, E. Perspectives of TGF-beta inhibition in pancreatic and hepatocellular carcinomas. Oncotarget 2014, 5, 78–94. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.I.; Choi, M.E. TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease. Kidney Res. Clin. Pract. 2012, 31, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Okumura, N.; Fujii, K.; Kagami, T.; Makiko, N.; Kitahara, M.; Kinoshita, S.; Koizumi, N. Activation of the Rho/Rho Kinase Signaling Pathway Is Involved in Cell Death of Corneal Endothelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6843–6851. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Okumura, N.; Kinoshita, S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp. Eye Res. 2012, 95, 60–67. [Google Scholar] [CrossRef]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Leonel, C.; Ferreira, L.C.; Borin, T.F.; Moschetta, M.G.; Freitas, G.S.; Haddad, M.R.; de Camargos Pinto Robles, J.A.; Aparecida Pires de Campos Zuccari, D. Inhibition of Epithelial-mesenchymal Transition in Response to Treatment with Metformin and Y27632 in Breast Cancer Cell Lines. Anti-Cancer Agents Med. Chem. 2017, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Lotz-Jenne, C.; Luthi, U.; Ackerknecht, S.; Lehembre, F.; Fink, T.; Stritt, M.; Wirth, M.; Pavan, S.; Bill, R.; Regenass, U.; et al. A high-content EMT screen identifies multiple receptor tyrosine kinase inhibitors with activity on TGFbeta receptor. Oncotarget 2016, 7, 25983–26002. [Google Scholar] [CrossRef] [Green Version]
- Kopecny, L.R.; Lee, B.W.H.; Coroneo, M.T. A systematic review on the effects of ROCK inhibitors on proliferation and/or differentiation in human somatic stem cells: A hypothesis that ROCK inhibitors support corneal endothelial healing via acting on the limbal stem cell niche. Ocul. Surf. 2023, 27, 16–29. [Google Scholar] [CrossRef]
- Naik, M.; Kapur, M.; Gupta, V.; Sethi, H.; Srivastava, K. Ripasudil Endgame: Role of Rho-Kinase Inhibitor as a Last-Ditch-Stand Towards Maximally Tolerated Medical Therapy to a Patient of Advanced Glaucoma. Clin. Ophthalmol. 2021, 15, 2683–2692. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Ripasudil: First global approval. Drugs 2014, 74, 2211–2215. [Google Scholar] [CrossRef]
- Thanuja, M.Y.; Ranganath, S.H.; Srinivas, S.P. Role of Oxidative Stress in the Disruption of the Endothelial Apical Junctional Complex During Corneal Cold Storage. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2022, 38, 664–681. [Google Scholar] [CrossRef]
- Shivanna, M.; Rajashekhar, G.; Srinivas, S.P. Barrier dysfunction of the corneal endothelium in response to TNF-alpha: Role of p38 MAP kinase. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1575–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bureta, C.; Setoguchi, T.; Saitoh, Y.; Tominaga, H.; Maeda, S.; Nagano, S.; Komiya, S.; Yamamoto, T.; Taniguchi, N. TGF-beta Promotes the Proliferation of Microglia In Vitro. Brain Sci. 2019, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Kim, E.; Bonanno, J.A. Fluid transport by the cornea endothelium is dependent on buffering lactic acid efflux. Am. J. Physiol. Cell Physiol. 2016, 311, C116–C126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Kim, E.; Ogando, D.G.; Bonanno, J.A. Corneal Endothelial Pump Coupling to Lactic Acid Efflux in the Rabbit and Mouse. Investig. Ophthalmol. Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Bonanno, J.A. Lactate-H(+) transport is a significant component of the in vivo corneal endothelial pump. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2020–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Jung, E.; Heur, M. Injury induces endothelial to mesenchymal transition in the mouse corneal endothelium in vivo via FGF2. Mol. Vis. 2019, 25, 22–34. [Google Scholar]
- Lee, J.; Jung, E.; Gestoso, K.; Heur, M. ZEB1 Mediates Fibrosis in Corneal Endothelial Mesenchymal Transition Through SP1 and SP3. Investig. Ophthalmol. Vis. Sci. 2020, 61, 41. [Google Scholar] [CrossRef]
- Lee, J.G.; Kay, E.P. NF-kappaB is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1530–1538. [Google Scholar] [CrossRef]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lu, J.; Chen, Y.; Xiong, N.; Li, L.; Zhang, J.; Yang, H.; Wu, C.; Zeng, H.; Liu, Y. MCP-1-induced ERK/GSK-3beta/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell. Mol. Immunol. 2017, 14, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Ho, H.C.; Su, Y.C.; Lee, M.S.; Hung, S.K.; Lin, C.H. MCP1-Induced Epithelial-Mesenchymal Transition in Head and Neck Cancer by AKT Activation. Anticancer Res. 2015, 35, 3299–3306. [Google Scholar]
- Wang, Z.; Ao, X.; Shen, Z.; Ao, L.; Wu, X.; Pu, C.; Guo, W.; Xing, W.; He, M.; Yuan, H.; et al. TNF-alpha augments CXCL10/CXCR3 axis activity to induce Epithelial-Mesenchymal Transition in colon cancer cell. Int. J. Biol. Sci. 2021, 17, 2683–2702. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.C.; Mercurio, A.M. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol. Biol. Cell 2003, 14, 1790–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleszewska, M.; Moonen, J.R.; Huijkman, N.; van de Sluis, B.; Krenning, G.; Harmsen, M.C. IL-1beta and TGFbeta2 synergistically induce endothelial to mesenchymal transition in an NFkappaB-dependent manner. Immunobiology 2013, 218, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.G.; Ko, M.K.; Kay, E.P. Endothelial mesenchymal transformation mediated by IL-1beta-induced FGF-2 in corneal endothelial cells. Exp. Eye Res. 2012, 95, 35–39. [Google Scholar] [CrossRef] [PubMed]
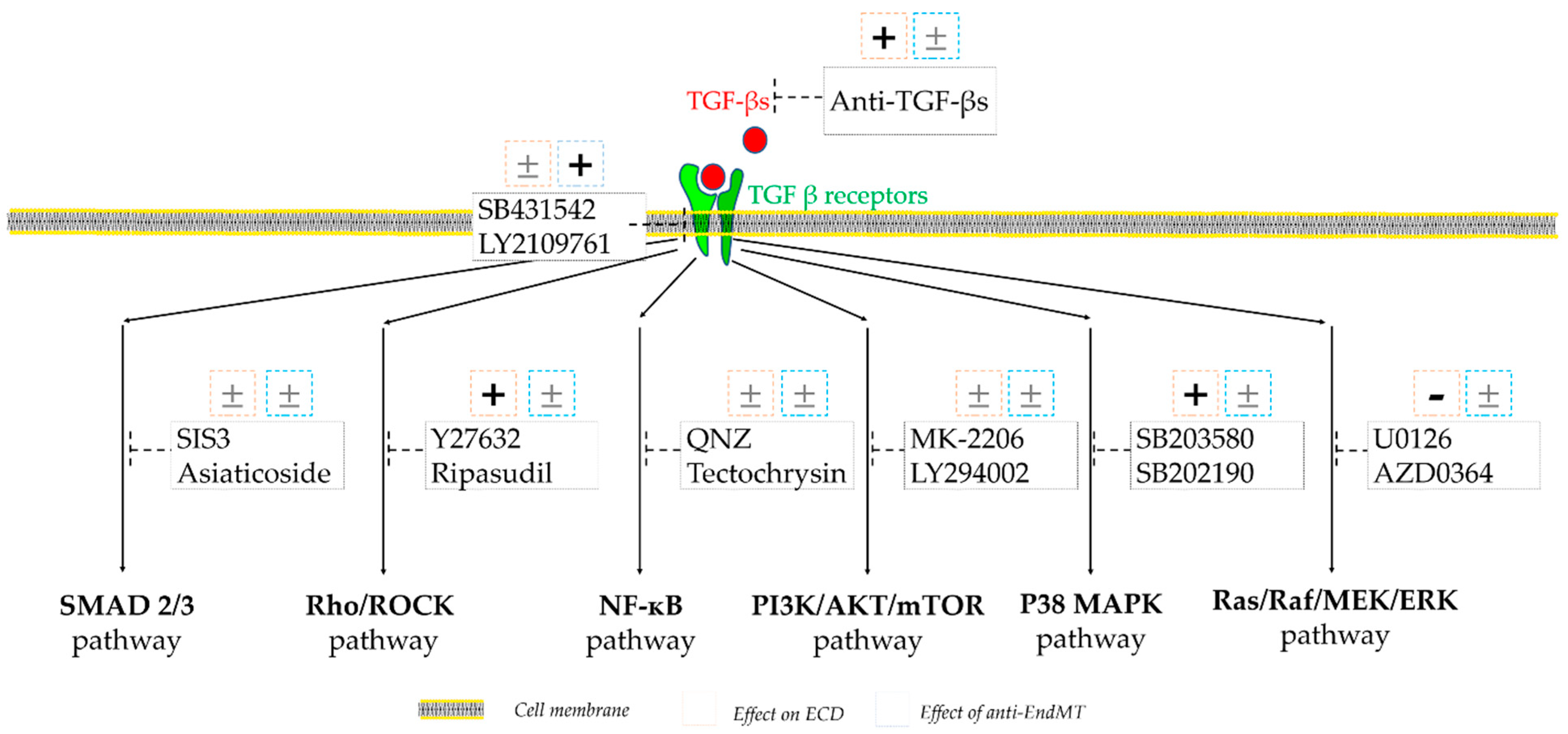
| Inhibitor | Catalog Number | Provider | Final Concentration | Signaling Pathway Inhibition | Reference |
|---|---|---|---|---|---|
| SIS3 HCL | S7959 | SelleckChem | 3 µM | SMAD 2/3 | [30] |
| Asiaticoside | HY N0439 | MedChemExpress | 100 µg/mL | SMAD 2/3 | [31] |
| Y27632 | S1049 | SelleckChem | 10 µM | Rho/ROCK | [32] |
| Ripasudil | HY 15685 | MedChemExpress | 5 µM | Rho/ROCK | [33] |
| Tectochrysin | HY 14592 | MedChemExpress | 10 µg/mL | NF-κB | [34] |
| QNZ | S4902 | SelleckChem | 0.1 µM | NF-κB | [35] |
| MK-2206 | S107811 | SelleckChem | 10 µM | PI3K/AKT/mTOR | [36] |
| LY294002 | L9908 | Sigma-Aldrich | 20 µM | PI3K/AKT/mTOR | [37] |
| SB203580 | S1076 | SelleckChem | 10 µM | P38 MAPK | [38] |
| SB202190 | SYN-1073 | SYNkinase | 10 µM | P38 MAPK | [39] |
| U0126 | 6620056 | CalbioChem | 10 µM | Ras/Raf/MEK/ERK | [40] |
| AZD0364 | S8708 | SelleckChem | 2 µM | Ras/Raf/MEK/ERK | [41] |
| SB431542 | S1067 | SelleckChem | 10 µM | TGFB receptor | [28] |
| LY2109761 | SML 2051 | SelleckChem | 1 µM | TGFB receptor | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouimeur, I.; Sagnial, T.; Coulomb, L.; Maurin, C.; Thomas, J.; Forestier, P.; Ninotta, S.; Perrache, C.; Forest, F.; Gain, P.; et al. Investigating the Role of TGF-β Signaling Pathways in Human Corneal Endothelial Cell Primary Culture. Cells 2023, 12, 1624. https://doi.org/10.3390/cells12121624
Aouimeur I, Sagnial T, Coulomb L, Maurin C, Thomas J, Forestier P, Ninotta S, Perrache C, Forest F, Gain P, et al. Investigating the Role of TGF-β Signaling Pathways in Human Corneal Endothelial Cell Primary Culture. Cells. 2023; 12(12):1624. https://doi.org/10.3390/cells12121624
Chicago/Turabian StyleAouimeur, Inès, Tomy Sagnial, Louise Coulomb, Corantin Maurin, Justin Thomas, Pierre Forestier, Sandrine Ninotta, Chantal Perrache, Fabien Forest, Philippe Gain, and et al. 2023. "Investigating the Role of TGF-β Signaling Pathways in Human Corneal Endothelial Cell Primary Culture" Cells 12, no. 12: 1624. https://doi.org/10.3390/cells12121624
APA StyleAouimeur, I., Sagnial, T., Coulomb, L., Maurin, C., Thomas, J., Forestier, P., Ninotta, S., Perrache, C., Forest, F., Gain, P., Thuret, G., & He, Z. (2023). Investigating the Role of TGF-β Signaling Pathways in Human Corneal Endothelial Cell Primary Culture. Cells, 12(12), 1624. https://doi.org/10.3390/cells12121624










