Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis
Abstract
1. Introduction
2. Ezrin as a Linker between the Plasma Membrane and Cytoskeleton
2.1. Modulation of Membrane–Cytoskeleton Interactions
2.2. Maintenance of Cell Shape and Cell Structure
2.3. Regulation of Cell–Cell Adhesion
2.4. Regulation of Cell Movement
3. Ezrin Interacts with Metastasis-Related Proteins
3.1. ACTB
3.2. ADORA2B
3.3. ADRA1B
3.4. ARF6
3.5. ARHGDIA
3.6. ARHGDIB
3.7. CD44
3.8. CDH1
3.9. CLIC5
3.10. CTNNB1
3.11. EGFR
3.12. FAS
3.13. ICAM1, ICAM2, and ICAM3
3.14. IQGAP1
3.15. L1CAM
3.16. MSN
3.17. NF2
3.18. PALLD
3.19. PRCKA
3.20. PTK2
3.21. RDX
3.22. RHOA
3.23. ROCK1
3.24. S100P
3.25. SCYL3
3.26. SDC2
3.27. SELL and SELP
3.28. SLC9A1
3.29. SLC9A3R1
3.30. SLC9A3R2
3.31. SNX27
3.32. SPN
3.33. TSC1
3.34. VCAM1
4. Role of Ezrin in Immunity to Prevent Immune Attack
5. Ezrin as a Target for Treating Metastatic Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bretscher, A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J. Cell Biol. 1983, 97, 425–432. [Google Scholar] [CrossRef]
- Pakkanen, R.; Hedman, K.; Turunen, O.; Wahlström, T.; Vaheri, A. Microvillus-specific Mr 75,000 plasma membrane protein of human choriocarcinoma cells. J. Histochem. Cytochem. 1987, 35, 809–816. [Google Scholar] [CrossRef]
- Pakkanen, R. Immunofluorescent and immunochemical evidence for the expression of cytovillin in the microvilli of a wide range of cultured human cells. J. Cell. Biochem. 1988, 38, 65–75. [Google Scholar] [CrossRef]
- Urushidani, T.; Hanzel, D.K.; Forte, J.G. Characterization of an 80-kDa phosphoprotein involved in parietal cell stimulation. Am. J. Physiol. 1989, 256, G1070–G1081. [Google Scholar] [CrossRef]
- Hunter, T.; Cooper, J.A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell 1981, 24, 741–752. [Google Scholar] [CrossRef]
- Bretscher, A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J. Cell Biol. 1989, 108, 921–930. [Google Scholar] [CrossRef]
- Gould, K.L.; Bretscher, A.; Esch, F.S.; Hunter, T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989, 8, 4133–4142. [Google Scholar] [CrossRef]
- Gould, K.L.; Cooper, J.A.; Bretscher, A.; Hunter, T. The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. J. Cell Biol. 1986, 102, 660–669. [Google Scholar] [CrossRef]
- Pakkanen, R.; Vaheri, A. Cytovillin and other microvillar proteins of human choriocarcinoma cells. J. Cell. Biochem. 1989, 41, 1–12. [Google Scholar] [CrossRef]
- Turunen, O.; Winqvist, R.; Pakkanen, R.; Grzeschik, K.H.; Wahlström, T.; Vaheri, A. Cytovillin, a microvillar Mr 75,000 protein. cDNA sequence, prokaryotic expression, and chromosomal localization. J. Biol. Chem. 1989, 264, 16727–16732. [Google Scholar] [CrossRef]
- Hanzel, D.; Reggio, H.; Bretscher, A.; Forte, J.G.; Mangeat, P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J. 1991, 10, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A.; Reczek, D.; Berryman, M. Ezrin: A protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 1997, 110 Pt 24, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Elliott, B.E.; Louvard, D.; Arpin, M. Src-dependent ezrin phosphorylation in adhesion-mediated signaling. Mol. Biol. Cell 2005, 16, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Gautreau, A.; Louvard, D.; Arpin, M. ERM proteins and NF2 tumor suppressor: The Yin and Yang of cortical actin organization and cell growth signaling. Curr. Opin. Cell Biol. 2002, 14, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Mangeat, P.; Roy, C.; Martin, M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999, 9, 187–192. [Google Scholar] [CrossRef]
- Pujuguet, P.; Del Maestro, L.; Gautreau, A.; Louvard, D.; Arpin, M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol. Biol. Cell 2003, 14, 2181–2191. [Google Scholar] [CrossRef]
- Berryman, M.; Gary, R.; Bretscher, A. Ezrin oligomers are major cytoskeletal components of placental microvilli: A proposal for their involvement in cortical morphogenesis. J. Cell Biol. 1995, 131, 1231–1242. [Google Scholar] [CrossRef]
- Lamb, R.F.; Ozanne, B.W.; Roy, C.; McGarry, L.; Stipp, C.; Mangeat, P.; Jay, D.G. Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr. Biol. 1997, 7, 682–688. [Google Scholar] [CrossRef]
- Mackay, D.J.; Esch, F.; Furthmayr, H.; Hall, A. Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: An essential role for ezrin/radixin/moesin proteins. J. Cell Biol. 1997, 138, 927–938. [Google Scholar] [CrossRef]
- Crepaldi, T.; Gautreau, A.; Comoglio, P.M.; Louvard, D.; Arpin, M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J. Cell Biol. 1997, 138, 423–434. [Google Scholar] [CrossRef]
- Ng, T.; Parsons, M.; Hughes, W.E.; Monypenny, J.; Zicha, D.; Gautreau, A.; Arpin, M.; Gschmeissner, S.; Verveer, P.J.; Bastiaens, P.I.; et al. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001, 20, 2723–2741. [Google Scholar] [CrossRef]
- Turunen, O.; Sainio, M.; Jääskeläinen, J.; Carpén, O.; Vaheri, A. Structure-function relationships in the ezrin family and the effect of tumor-associated point mutations in neurofibromatosis 2 protein. Biochim. Biophys. Acta 1998, 1387, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A.; Edwards, K.; Fehon, R.G. ERM proteins and merlin: Integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002, 3, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Algrain, M.; Turunen, O.; Vaheri, A.; Louvard, D.; Arpin, M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 1993, 120, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.A.; Reczek, D.; Bretscher, A.; Karplus, P.A. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 2000, 101, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, K.; Yu, H.; Jin, D.; Wang, G.; Yu, B. Prognostic Value of Ezrin in Various Cancers: A Systematic Review and Updated Meta-analysis. Sci. Rep. 2015, 5, 17903. [Google Scholar] [CrossRef]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.C.; Massagué, J. Molecular basis of metastasis. N. Engl. J. Med. 2008, 359, 2814–2823. [Google Scholar] [CrossRef]
- Comen, E.; Norton, L.; Massagué, J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 2011, 8, 369–377. [Google Scholar] [CrossRef]
- Pienta, K.J.; Robertson, B.A.; Coffey, D.S.; Taichman, R.S. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clin. Cancer Res. 2013, 19, 5849–5855. [Google Scholar] [CrossRef] [PubMed]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Yin, L.M.; Schnoor, M. Modulation of membrane-cytoskeleton interactions: Ezrin as key player. Trends Cell Biol. 2022, 32, 94–97. [Google Scholar] [CrossRef]
- Barik, G.K.; Sahay, O.; Paul, D.; Santra, M.K. Ezrin gone rogue in cancer progression and metastasis: An enticing therapeutic target. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188753. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V.; Morrison, H.; Matzke, A.; Kastilan, T.; Pace, G.; Herrlich, P.; Ponta, H. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol. Biol. Cell 2007, 18, 76–83. [Google Scholar] [CrossRef]
- Huang, L.; Qin, Y.; Zuo, Q.; Bhatnagar, K.; Xiong, J.; Merlino, G.; Yu, Y. Ezrin mediates both HGF/Met autocrine and non-autocrine signaling-induced metastasis in melanoma. Int. J. Cancer 2018, 142, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Saotome, I.; Curto, M.; McClatchey, A.I. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 2004, 6, 855–864. [Google Scholar] [CrossRef]
- Tamura, A.; Kikuchi, S.; Hata, M.; Katsuno, T.; Matsui, T.; Hayashi, H.; Suzuki, Y.; Noda, T.; Tsukita, S.; Tsukita, S. Achlorhydria by ezrin knockdown: Defects in the formation/expansion of apical canaliculi in gastric parietal cells. J. Cell Biol. 2005, 169, 21–28. [Google Scholar] [CrossRef]
- Aoki, K.; Harada, S.; Kawaji, K.; Matsuzawa, K.; Uchida, S.; Ikenouchi, J. STIM-Orai1 signaling regulates fluidity of cytoplasm during membrane blebbing. Nat. Commun. 2021, 12, 480. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Wang, H.; Yuan, X.; Yang, F.; Zhao, L.; Mullen, M.; Du, S.; Zohbi, N.; Muthusamy, S.; et al. Acetylation of ezrin regulates membrane-cytoskeleton interaction underlying CCL18-elicited cell migration. J. Mol. Cell Biol. 2020, 12, 424–437. [Google Scholar] [CrossRef]
- Welf, E.S.; Miles, C.E.; Huh, J.; Sapoznik, E.; Chi, J.; Driscoll, M.K.; Isogai, T.; Noh, J.; Weems, A.D.; Pohlkamp, T.; et al. Actin-Membrane Release Initiates Cell Protrusions. Dev. Cell 2020, 55, 723–736.e728. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.C.; Bertin, A.; Bousquet, H.; Manzi, J.; Senju, Y.; Tsai, M.C.; Picas, L.; Miserey-Lenkei, S.; Lappalainen, P.; Lemichez, E.; et al. Ezrin enrichment on curved membranes requires a specific conformation or interaction with a curvature-sensitive partner. eLife 2018, 7, e37262. [Google Scholar] [CrossRef]
- Berryman, M.; Franck, Z.; Bretscher, A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 1993, 105 Pt 4, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, V.L.; Finnemann, S.C.; Rodriguez-Boulan, E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J. Cell Biol. 1999, 147, 1533–1548. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, J.B.; Saotome, I.; Curto, M.; McClatchey, A.I. Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc. Natl. Acad. Sci. USA 2011, 108, 11924–11929. [Google Scholar] [CrossRef]
- Diz-Muñoz, A.; Krieg, M.; Bergert, M.; Ibarlucea-Benitez, I.; Muller, D.J.; Paluch, E.; Heisenberg, C.P. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010, 8, e1000544. [Google Scholar] [CrossRef]
- Liu, Y.; Belkina, N.V.; Park, C.; Nambiar, R.; Loughhead, S.M.; Patino-Lopez, G.; Ben-Aissa, K.; Hao, J.J.; Kruhlak, M.J.; Qi, H.; et al. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood 2012, 119, 445–453. [Google Scholar] [CrossRef]
- Rouven Brückner, B.; Pietuch, A.; Nehls, S.; Rother, J.; Janshoff, A. Ezrin is a Major Regulator of Membrane Tension in Epithelial Cells. Sci. Rep. 2015, 5, 14700. [Google Scholar] [CrossRef]
- Braunger, J.A.; Brückner, B.R.; Nehls, S.; Pietuch, A.; Gerke, V.; Mey, I.; Janshoff, A.; Steinem, C. Phosphatidylinositol 4,5-bisphosphate alters the number of attachment sites between ezrin and actin filaments: A colloidal probe study. J. Biol. Chem. 2014, 289, 9833–9843. [Google Scholar] [CrossRef]
- Roberts, R.E.; Dewitt, S.; Hallett, M.B. Membrane Tension and the Role of Ezrin During Phagocytosis. Adv. Exp. Med. Biol. 2020, 1246, 83–102. [Google Scholar] [CrossRef]
- Jia, M.; Yan, X.; Jiang, X.; Wu, Y.; Xu, J.; Meng, Y.; Yang, Y.; Shan, X.; Zhang, X.; Mao, S.; et al. Ezrin, a Membrane Cytoskeleton Cross-Linker Protein, as a Marker of Epithelial Damage in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 496–507. [Google Scholar] [CrossRef]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, G.L.; Dewitt, S.; Hallett, M.B. Ezrin and talin relocates from the plasma membrane to cytosol during neutrophil extravasation. Eur. J. Clin. Investig. 2011, 41, 47. [Google Scholar] [CrossRef]
- Elumalai, G.L. Cytosolic Signalling and Behaviour of Oral Neutrophils “Search for Biochemical Memory”. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2012. [Google Scholar]
- Peskin, C.S.; Odell, G.M.; Oster, G.F. Cellular motions and thermal fluctuations: The Brownian ratchet. Biophys. J. 1993, 65, 316–324. [Google Scholar] [CrossRef]
- Takeuchi, K.; Sato, N.; Kasahara, H.; Funayama, N.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J. Cell Biol. 1994, 125, 1371–1384. [Google Scholar] [CrossRef]
- Tsukita, S.; Oishi, K.; Sato, N.; Sagara, J.; Kawai, A.; Tsukita, S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 1994, 126, 391–401. [Google Scholar] [CrossRef]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S.; Tsukita, S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [CrossRef]
- Heiska, L.; Alfthan, K.; Grönholm, M.; Vilja, P.; Vaheri, A.; Carpén, O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J. Biol. Chem. 1998, 273, 21893–21900. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Asano, S. Pathophysiological Roles of Actin-Binding Scaffold Protein, Ezrin. Int. J. Mol. Sci. 2022, 23, 3246. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Vega, A.; Riedl, M.; Collins, R.F.; Ostrowski, P.P.; Woods, E.C.; Bertozzi, C.R.; Tammi, M.I.; Lidke, D.S.; Johnson, P.; et al. Transmembrane Pickets Connect Cyto- and Pericellular Skeletons Forming Barriers to Receptor Engagement. Cell 2018, 172, 305–317.e310. [Google Scholar] [CrossRef]
- Rey-Gallardo, A.; Tomlins, H.; Joachim, J.; Rahman, I.; Kitscha, P.; Frudd, K.; Parsons, M.; Ivetic, A. Sequential binding of ezrin and moesin to L-selectin regulates monocyte protrusive behaviour during transendothelial migration. J. Cell Sci. 2018, 131, jcs215541. [Google Scholar] [CrossRef]
- Khan, K.; Long, B.; Deshpande, G.M.; Fox, P.L. Bidirectional Tumor-Promoting Activities of Macrophage Ezrin. Int. J. Mol. Sci. 2020, 21, 7716. [Google Scholar] [CrossRef]
- Platet, N.; Prévostel, C.; Derocq, D.; Joubert, D.; Rochefort, H.; Garcia, M. Breast cancer cell invasiveness: Correlation with protein kinase C activity and differential regulation by phorbol ester in estrogen receptor-positive and -negative cells. Int. J. Cancer 1998, 75, 750–756. [Google Scholar] [CrossRef]
- Sun, X.G.; Rotenberg, S.A. Overexpression of protein kinase Calpha in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation, and motility. Cell Growth Differ. 1999, 10, 343–352. [Google Scholar] [PubMed]
- Zhang, X.; Flores, L.R.; Keeling, M.C.; Sliogeryte, K.; Gavara, N. Ezrin Phosphorylation at T567 Modulates Cell Migration, Mechanical Properties, and Cytoskeletal Organization. Int. J. Mol. Sci. 2020, 21, 435. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
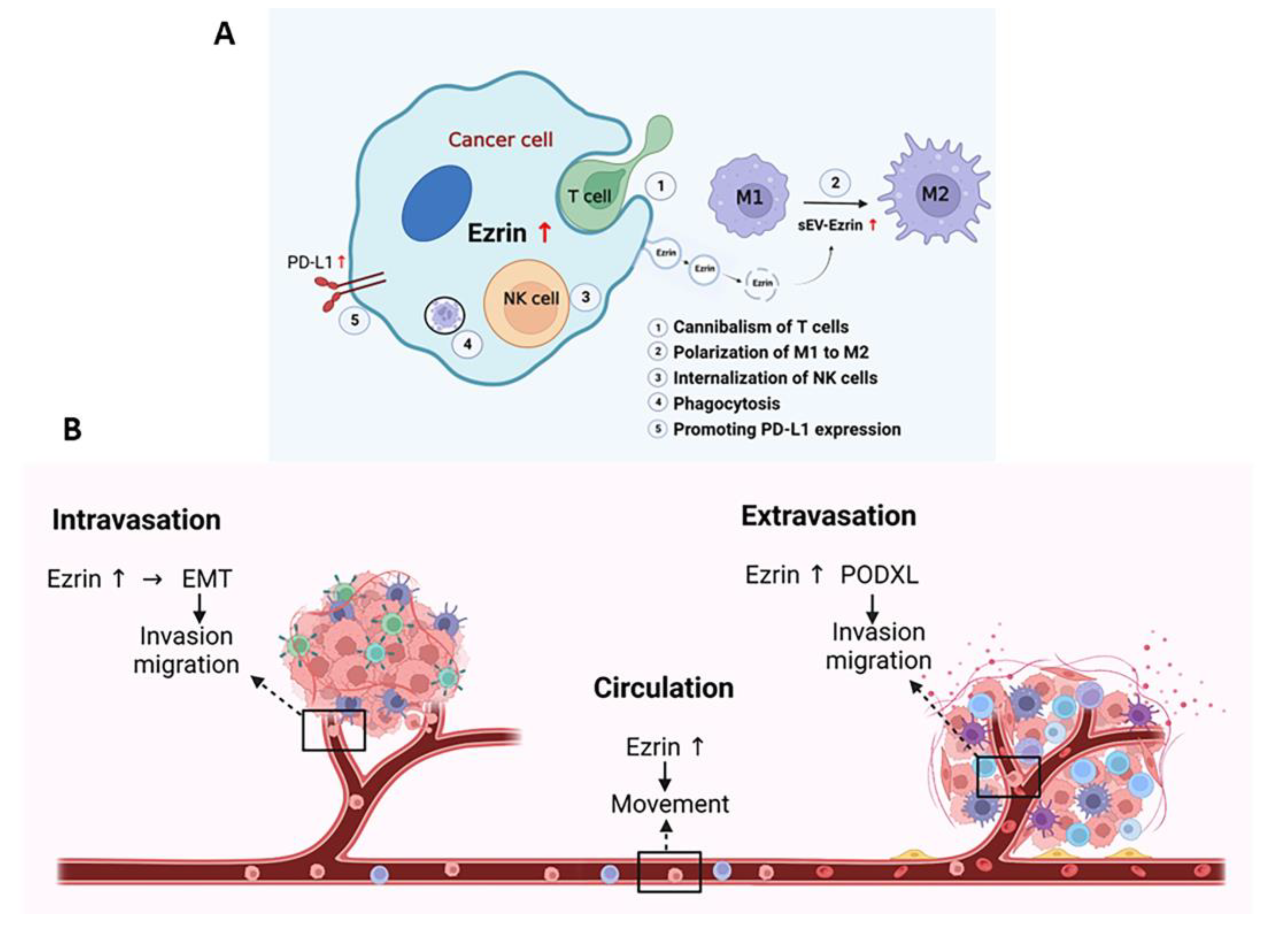
- Fröse, J.; Chen, M.B.; Hebron, K.E.; Reinhardt, F.; Hajal, C.; Zijlstra, A.; Kamm, R.D.; Weinberg, R.A. Epithelial-Mesenchymal Transition Induces Podocalyxin to Promote Extravasation via Ezrin Signaling. Cell Rep. 2018, 24, 962–972. [Google Scholar] [CrossRef]
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. β-Actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 2011, 22, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Tang, S.; Wang, Z.; Cai, L.; Lian, H.; Shen, Y.; Zhou, Y. A pan-cancer analysis of the prognostic and immunological role of β-actin (ACTB) in human cancers. Bioengineered 2021, 12, 6166–6185. [Google Scholar] [CrossRef]
- Shuster, C.B.; Herman, I.M. Indirect association of ezrin with F-actin: Isoform specificity and calcium sensitivity. J. Cell Biol. 1995, 128, 837–848. [Google Scholar] [CrossRef]
- Gao, Z.G.; Inoue, A.; Jacobson, K.A. On the G protein-coupling selectivity of the native A(2B) adenosine receptor. Biochem. Pharmacol. 2018, 151, 201–213. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Role of adenosine A2b receptor overexpression in tumor progression. Life Sci. 2016, 166, 92–99. [Google Scholar] [CrossRef]
- Sitaraman, S.V.; Wang, L.; Wong, M.; Bruewer, M.; Hobert, M.; Yun, C.H.; Merlin, D.; Madara, J.L. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J. Biol. Chem. 2002, 277, 33188–33195. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.F.; Lefkowitz, R.J.; Caron, M.G.; Cotecchia, S. G-protein-coupled receptor genes as protooncogenes: Constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc. Natl. Acad. Sci. USA 1991, 88, 11354–11358. [Google Scholar] [CrossRef]
- Stanasila, L.; Abuin, L.; Diviani, D.; Cotecchia, S. Ezrin directly interacts with the alpha1b-adrenergic receptor and plays a role in receptor recycling. J. Biol. Chem. 2006, 281, 4354–4363. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C.; Chavrier, P. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Macia, E.; Luton, F.; Partisani, M.; Cherfils, J.; Chardin, P.; Franco, M. The GDP-bound form of Arf6 is located at the plasma membrane. J. Cell Sci. 2004, 117, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Miao, Y.; Ding, X.; Deng, H.; Liu, S.; Wang, F.; Zhou, R.; Watson, C.; Fu, C.; Hu, Q.; et al. Proteomic identification and functional characterization of a novel ARF6 GTPase-activating protein, ACAP4. Mol. Cell Proteom. 2006, 5, 1437–1449. [Google Scholar] [CrossRef]
- Ding, X.; Deng, H.; Wang, D.; Zhou, J.; Huang, Y.; Zhao, X.; Yu, X.; Wang, M.; Wang, F.; Ward, T.; et al. Phospho-regulated ACAP4-Ezrin interaction is essential for histamine-stimulated parietal cell secretion. J. Biol. Chem. 2010, 285, 18769–18780. [Google Scholar] [CrossRef]
- Takai, Y.; Sasaki, T.; Tanaka, K.; Nakanishi, H. Rho as a regulator of the cytoskeleton. Trends Biochem. Sci. 1995, 20, 227–231. [Google Scholar] [CrossRef]
- Takahashi, K.; Sasaki, T.; Mammoto, A.; Takaishi, K.; Kameyama, T.; Tsukita, S.; Takai, Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem. 1997, 272, 23371–23375. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, C.; Zhang, J.; Lu, D.; Zhuang, J.; Xing, S.; Feng, J.; Yang, D.; Yan, X. Recognition of CD146 as an ERM-binding protein offers novel mechanisms for melanoma cell migration. Oncogene 2012, 31, 306–321. [Google Scholar] [CrossRef]
- Ota, T.; Maeda, M.; Suto, S.; Tatsuka, M. LyGDI functions in cancer metastasis by anchoring Rho proteins to the cell membrane. Mol. Carcinog. 2004, 39, 206–220. [Google Scholar] [CrossRef]
- Sainio, M.; Zhao, F.; Heiska, L.; Turunen, O.; den Bakker, M.; Zwarthoff, E.; Lutchman, M.; Rouleau, G.A.; Jääskeläinen, J.; Vaheri, A.; et al. Neurofibromatosis 2 tumor suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J. Cell Sci. 1997, 110 Pt 18, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhao, L.; Ma, Z.; An, H.; Marrink, S.J.; Sun, F. Molecular basis of PIP2-dependent conformational switching of phosphorylated CD44 in binding FERM. Biophys. J. 2023. In Press. [Google Scholar] [CrossRef]
- Herrlich, P.; Morrison, H.; Sleeman, J.; Orian-Rousseau, V.; König, H.; Weg-Remers, S.; Ponta, H. CD44 acts both as a growth- and invasiveness-promoting molecule and as a tumor-suppressing cofactor. Ann. N. Y. Acad. Sci. 2000, 910, 106–118; discussion 118–120. [Google Scholar] [CrossRef]
- Zohar, R.; Suzuki, N.; Suzuki, K.; Arora, P.; Glogauer, M.; McCulloch, C.A.; Sodek, J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J. Cell Physiol. 2000, 184, 118–130. [Google Scholar] [CrossRef]
- Martin, T.A.; Harrison, G.; Mansel, R.E.; Jiang, W.G. The role of the CD44/ezrin complex in cancer metastasis. Crit. Rev. Oncol. Hematol. 2003, 46, 165–186. [Google Scholar] [CrossRef]
- Takeichi, M. Cadherins in cancer: Implications for invasion and metastasis. Curr. Opin. Cell Biol. 1993, 5, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, S.; Jiang, W.G. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J. Cell Sci. 1999, 112 Pt 18, 3081–3090. [Google Scholar] [CrossRef]
- Yao, W.; Feng, D.; Bian, W.; Yang, L.; Li, Y.; Yang, Z.; Xiong, Y.; Zheng, J.; Zhai, R.; He, J. EBP50 inhibits EGF-induced breast cancer cell proliferation by blocking EGFR phosphorylation. Amino Acids 2012, 43, 2027–2035. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, M.; Jin, F.; Xiao, Q.; He, M.; Wu, H.; Ren, J.; Zhao, L.; Zhao, H.; Yao, W.; et al. Combined expression of ezrin and E-cadherin is associated with lymph node metastasis and poor prognosis in breast cancer. Oncol. Rep. 2015, 34, 165–174. [Google Scholar] [CrossRef]
- Guedj, N.; Vaquero, J.; Clapéron, A.; Mergey, M.; Chrétien, Y.; Paradis, V.; Fouassier, L. Loss of ezrin in human intrahepatic cholangiocarcinoma is associated with ectopic expression of E-cadherin. Histopathology 2016, 69, 211–221. [Google Scholar] [CrossRef]
- Berryman, M.; Bretscher, A. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol. Biol. Cell 2000, 11, 1509–1521. [Google Scholar] [CrossRef]
- Wegner, B.; Al-Momany, A.; Kulak, S.C.; Kozlowski, K.; Obeidat, M.; Jahroudi, N.; Paes, J.; Berryman, M.; Ballermann, B.J. CLIC5A, a component of the ezrin-podocalyxin complex in glomeruli, is a determinant of podocyte integrity. Am. J. Physiol. Renal Physiol. 2010, 298, F1492–F1503. [Google Scholar] [CrossRef]
- Pierchala, B.A.; Muñoz, M.R.; Tsui, C.C. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 2010, 78, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Al-Momany, A.; Li, L.; Alexander, R.T.; Ballermann, B.J. Clustered PI(4,5)P2 accumulation and ezrin phosphorylation in response to CLIC5A. J. Cell Sci. 2014, 127, 5164–5178. [Google Scholar] [CrossRef] [PubMed]
- Flores-Téllez, T.N.; Lopez, T.V.; Vásquez Garzón, V.R.; Villa-Treviño, S. Co-Expression of Ezrin-CLIC5-Podocalyxin Is Associated with Migration and Invasiveness in Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0131605. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Ben-Ze’ev, A. beta-Catenin signaling in biological control and cancer. J. Cell Biochem. 2007, 102, 820–828. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Shmuel, A.; Lemmon, V.; Brabletz, T.; Ben-Ze’ev, A. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J. Cell Sci. 2010, 123, 2135–2143. [Google Scholar] [CrossRef]
- Khazaie, K.; Schirrmacher, V.; Lichtner, R.B. EGF receptor in neoplasia and metastasis. Cancer Metastasis Rev. 1993, 12, 255–274. [Google Scholar] [CrossRef]
- Chiasson-MacKenzie, C.; Morris, Z.S.; Baca, Q.; Morris, B.; Coker, J.K.; Mirchev, R.; Jensen, A.E.; Carey, T.; Stott, S.L.; Golan, D.E.; et al. NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin. J. Cell Biol. 2015, 211, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Saygideğer-Kont, Y.; Minas, T.Z.; Jones, H.; Hour, S.; Çelik, H.; Temel, I.; Han, J.; Atabey, N.; Erkizan, H.V.; Toretsky, J.A.; et al. Ezrin Enhances EGFR Signaling and Modulates Erlotinib Sensitivity in Non-Small Cell Lung Cancer Cells. Neoplasia 2016, 18, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Parlato, S.; Giammarioli, A.M.; Logozzi, M.; Lozupone, F.; Matarrese, P.; Luciani, F.; Falchi, M.; Malorni, W.; Fais, S. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: A novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 2000, 19, 5123–5134. [Google Scholar] [CrossRef]
- Kuo, W.C.; Yang, K.T.; Hsieh, S.L.; Lai, M.Z. Ezrin is a negative regulator of death receptor-induced apoptosis. Oncogene 2010, 29, 1374–1383. [Google Scholar] [CrossRef]
- Gahmberg, C.G.; Tolvanen, M.; Kotovuori, P. Leukocyte adhesion—Structure and function of human leukocyte beta2-integrins and their cellular ligands. Eur. J. Biochem. 1997, 245, 215–232. [Google Scholar] [CrossRef]
- Helander, T.S.; Carpén, O.; Turunen, O.; Kovanen, P.E.; Vaheri, A.; Timonen, T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature 1996, 382, 265–268. [Google Scholar] [CrossRef]
- Serrador, J.M.; Alonso-Lebrero, J.L.; del Pozo, M.A.; Furthmayr, H.; Schwartz-Albiez, R.; Calvo, J.; Lozano, F.; Sánchez-Madrid, F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J. Cell Biol. 1997, 138, 1409–1423. [Google Scholar] [CrossRef]
- Kong, J.; Yao, C.; Dong, S.; Wu, S.; Xu, Y.; Li, K.; Ji, L.; Shen, Q.; Zhang, Q.; Zhan, R.; et al. ICAM-1 Activates Platelets and Promotes Endothelial Permeability through VE-Cadherin after Insufficient Radiofrequency Ablation. Adv. Sci. 2021, 8, 2002228. [Google Scholar] [CrossRef]
- White, C.D.; Erdemir, H.H.; Sacks, D.B. IQGAP1 and its binding proteins control diverse biological functions. Cell Signal 2012, 24, 826–834. [Google Scholar] [CrossRef]
- Peng, X.; Wang, T.; Gao, H.; Yue, X.; Bian, W.; Mei, J.; Zhang, Y. The interplay between IQGAP1 and small GTPases in cancer metastasis. Biomed. Pharmacother. 2021, 135, 111243. [Google Scholar] [CrossRef] [PubMed]
- Nammalwar, R.C.; Heil, A.; Gerke, V. Ezrin interacts with the scaffold protein IQGAP1 and affects its cortical localization. Biochim. Biophys. Acta 2015, 1853, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guidry, J.J.; Worthylake, D.K. Conserved sequence repeats of IQGAP1 mediate binding to Ezrin. J. Proteome Res. 2014, 13, 1156–1166. [Google Scholar] [CrossRef]
- Weinspach, D.; Seubert, B.; Schaten, S.; Honert, K.; Sebens, S.; Altevogt, P.; Krüger, A. Role of L1 cell adhesion molecule (L1CAM) in the metastatic cascade: Promotion of dissemination, colonization, and metastatic growth. Clin. Exp. Metastasis 2014, 31, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Xie, Y.M.; Ran, L.Q.; Cao, H.H.; Sun, C.; Wu, J.Y.; Wu, Z.Y.; Liao, L.D.; Zhao, W.J.; Fang, W.K.; et al. L1CAM drives oncogenicity in esophageal squamous cell carcinoma by stimulation of ezrin transcription. J. Mol. Med. 2017, 95, 1355–1368. [Google Scholar] [CrossRef]
- Kiefel, H.; Bondong, S.; Pfeifer, M.; Schirmer, U.; Erbe-Hoffmann, N.; Schäfer, H.; Sebens, S.; Altevogt, P. EMT-associated up-regulation of L1CAM provides insights into L1CAM-mediated integrin signalling and NF-κB activation. Carcinogenesis 2012, 33, 1919–1929. [Google Scholar] [CrossRef]
- Maccio, U.; Mihic, A.; Lenggenhager, D.; Kolm, I.; Mittmann, C.; Heikenwälder, M.; Lorentzen, A.; Mihic-Probst, D. Hypoxia and Ezrin Expression in Primary Melanoma Have High Prognostic Relevance. Int. J. Mol. Sci. 2022, 23, 10745. [Google Scholar] [CrossRef]
- Shcherbina, A.; Bretscher, A.; Kenney, D.M.; Remold-O’Donnell, E. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 1999, 443, 31–36. [Google Scholar] [CrossRef]
- Parameswaran, N.; Gupta, N. Re-defining ERM function in lymphocyte activation and migration. Immunol. Rev. 2013, 256, 63–79. [Google Scholar] [CrossRef]
- Xu, H.M.; Gutmann, D.H. Merlin differentially associates with the microtubule and actin cytoskeleton. J. Neurosci. Res. 1998, 51, 403–415. [Google Scholar] [CrossRef]
- Stamenkovic, I.; Yu, Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr. Protein Pept. Sci. 2010, 11, 471–484. [Google Scholar] [CrossRef]
- Ye, K. Phosphorylation of merlin regulates its stability and tumor suppressive activity. Cell Adh. Migr. 2007, 1, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Muranen, T.; Grönholm, M.; Lampin, A.; Lallemand, D.; Zhao, F.; Giovannini, M.; Carpén, O. The tumor suppressor merlin interacts with microtubules and modulates Schwann cell microtubule cytoskeleton. Hum. Mol. Genet. 2007, 16, 1742–1751. [Google Scholar] [CrossRef]
- Luo, Z.L.; Cheng, S.Q.; Shi, J.; Zhang, H.L.; Zhang, C.Z.; Chen, H.Y.; Qiu, B.J.; Tang, L.; Hu, C.L.; Wang, H.Y.; et al. A splicing variant of Merlin promotes metastasis in hepatocellular carcinoma. Nat. Commun. 2015, 6, 8457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Feng, Y.; Tao, K.; Su, Z.; Yu, X.; Zheng, J.; Zhang, L.; Yang, D. The expression and phosphorylation of ezrin and merlin in human pancreatic cancer. Int. J. Oncol. 2014, 44, 2059–2067. [Google Scholar] [CrossRef]
- Morales, F.C.; Molina, J.R.; Hayashi, Y.; Georgescu, M.M. Overexpression of ezrin inactivates NF2 tumor suppressor in glioblastoma. Neuro Oncol. 2010, 12, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Otey, C.A.; Rachlin, A.; Moza, M.; Arneman, D.; Carpen, O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int. Rev. Cytol. 2005, 246, 31–58. [Google Scholar] [CrossRef]
- Mykkänen, O.M.; Grönholm, M.; Rönty, M.; Lalowski, M.; Salmikangas, P.; Suila, H.; Carpén, O. Characterization of human palladin, a microfilament-associated protein. Mol. Biol. Cell 2001, 12, 3060–3073. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J.; Kim, W.; Dann, P.; Takyar, F.; Gefter, J.V.; Friedman, P.A.; Wysolmerski, J.J. Inhibition of ezrin causes PKCα-mediated internalization of erbb2/HER2 tyrosine kinase in breast cancer cells. J. Biol. Chem. 2019, 294, 887–901. [Google Scholar] [CrossRef]
- Schwock, J.; Dhani, N.; Hedley, D.W. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin. Ther. Targets 2010, 14, 77–94. [Google Scholar] [CrossRef]
- Golubovskaya, V.M. Targeting FAK in human cancer: From finding to first clinical trials. Front. Biosci. 2014, 19, 687–706. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Luo, M.; Mo, X.; Lu, J.; Yeo, S.K.; Guan, J.L. FAK activates AKT-mTOR signaling to promote the growth and progression of MMTV-Wnt1-driven basal-like mammary tumors. Breast Cancer Res. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Poullet, P.; Gautreau, A.; Kadaré, G.; Girault, J.A.; Louvard, D.; Arpin, M. Ezrin interacts with focal adhesion kinase and induces its activation independently of cell-matrix adhesion. J. Biol. Chem. 2001, 276, 37686–37691. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W. EZR promotes pancreatic cancer proliferation and metastasis by activating FAK/AKT signaling pathway. Cancer Cell Int. 2021, 21, 521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.H.; Wang, A.X.; Chen, Y. Radixin enhances colon cancer cell invasion by increasing MMP-7 production via Rac1-ERK pathway. Sci. World J. 2014, 2014, 340271. [Google Scholar] [CrossRef]
- Bukong, T.N.; Kodys, K.; Szabo, G. Human ezrin-moesin-radixin proteins modulate hepatitis C virus infection. Hepatology 2013, 58, 1569–1579. [Google Scholar] [CrossRef]
- Qin, J.J.; Wang, J.M.; Du, J.; Zeng, C.; Han, W.; Li, Z.D.; Xie, J.; Li, G.L. Radixin knockdown by RNA interference suppresses human glioblastoma cell growth in vitro and in vivo. Asian Pac. J. Cancer Prev. 2014, 15, 9805–9812. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Yan, J.K.; Li, J.J.; Ou, Y.M.; Yang, Q. Knockdown of Radixin Suppresses Gastric Cancer Metastasis In Vitro by Up-Regulation of E-Cadherin via NF-κB/Snail Pathway. Cell Physiol. Biochem. 2016, 39, 2509–2521. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, C.; Lu, H.; Yu, H.; Hong, H.; Guo, C.; Wu, Z. miR-200b regulates breast cancer cell proliferation and invasion by targeting radixin. Exp. Ther. Med. 2020, 19, 2741–2750. [Google Scholar] [CrossRef]
- Schmieder, S.; Nagai, M.; Orlando, R.A.; Takeda, T.; Farquhar, M.G. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J. Am. Soc. Nephrol. 2004, 15, 2289–2298. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.P.; Zhang, X.H.; Geng, C.Z.; Li, Z.H. Relationship of RhoA signaling activity with ezrin expression and its significance in the prognosis for breast cancer patients. Chin. Med. J. 2013, 126, 242–247. [Google Scholar]
- Ma, L.; Liu, Y.P.; Zhang, X.H.; Xing, L.X.; Wang, J.L.; Geng, C.Z. Effect of RhoA signaling transduction on expression of Ezrin in breast cancer cell lines. Ai Zheng 2009, 28, 108–111. [Google Scholar] [PubMed]
- Chiappetta, C.; Leopizzi, M.; Censi, F.; Puggioni, C.; Petrozza, V.; Rocca, C.D.; Di Cristofano, C. Correlation of the Rac1/RhoA pathway with ezrin expression in osteosarcoma. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Xiong, D.; Huang, H.; Wen, Z.Y. Ezrin Promotes the Proliferation, Migration, and Invasion of Ovarian Cancer Cells. Biomed. Environ. Sci. 2021, 34, 139–151. [Google Scholar] [CrossRef]
- Rath, N.; Olson, M.F. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012, 13, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Makino, Y.; Iwahara, T.; Nishihara, H.; Sawa, H.; Nagashima, K.; Hanafusa, H.; Tanaka, S. Crk associates with ERM proteins and promotes cell motility toward hyaluronic acid. J. Biol. Chem. 2004, 279, 46843–46850. [Google Scholar] [CrossRef]
- Ding, N.; Li, P.; Li, H.; Lei, Y.; Zhang, Z. The ROCK-ezrin signaling pathway mediates LPS-induced cytokine production in pulmonary alveolar epithelial cells. Cell Commun. Signal 2022, 20, 65. [Google Scholar] [CrossRef]
- Hébert, M.; Potin, S.; Sebbagh, M.; Bertoglio, J.; Bréard, J.; Hamelin, J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J. Immunol. 2008, 181, 5963–5973. [Google Scholar] [CrossRef]
- Koltzscher, M.; Neumann, C.; König, S.; Gerke, V. Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P. Mol. Biol Cell 2003, 14, 2372–2384. [Google Scholar] [CrossRef]
- Austermann, J.; Nazmi, A.R.; Müller-Tidow, C.; Gerke, V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J. Biol. Chem. 2008, 283, 29331–29340. [Google Scholar] [CrossRef]
- Sullivan, A.; Uff, C.R.; Isacke, C.M.; Thorne, R.F. PACE-1, a novel protein that interacts with the C-terminal domain of ezrin. Exp. Cell Res. 2003, 284, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Granés, F.; Berndt, C.; Roy, C.; Mangeat, P.; Reina, M.; Vilaró, S. Identification of a novel Ezrin-binding site in syndecan-2 cytoplasmic domain. FEBS Lett. 2003, 547, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Mytilinaiou, M.; Nikitovic, D.; Berdiaki, A.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.; Tzanakakis, G.N. IGF-I regulates HT1080 fibrosarcoma cell migration through a syndecan-2/Erk/ezrin signaling axis. Exp. Cell Res. 2017, 361, 9–18. [Google Scholar] [CrossRef]
- Ivetic, A.; Florey, O.; Deka, J.; Haskard, D.O.; Ager, A.; Ridley, A.J. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J. Biol. Chem. 2004, 279, 33263–33272. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Chen, E.J.; Clarke, F.; Lyck, R.; Affentranger, S.; Burkhardt, J.K.; Niggli, V. Ezrin/Radixin/Moesin proteins and flotillins cooperate to promote uropod formation in T cells. Front. Immunol. 2013, 4, 84. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.; Chou, C.Y.; Hsu, K.F.; Huang, Y.F.; Shen, M.R. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J. Cell Physiol. 2008, 214, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, G.; Stock, C.M.; Lemaire, J.; Lund, S.F.; Jensen, M.F.; Damsgaard, B.; Petersen, K.S.; Wiwel, M.; Rønnov-Jessen, L.; Schwab, A.; et al. The Na+/H+ exchanger NHE1, but not the Na+, HCO3(-) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 2012, 317, 172–183. [Google Scholar] [CrossRef]
- Stock, C.; Ludwig, F.T.; Schwab, A. Is the multifunctional Na(+)/H(+) exchanger isoform 1 a potential therapeutic target in cancer? Curr. Med. Chem. 2012, 19, 647–660. [Google Scholar] [CrossRef]
- Frontzek, F.; Nitzlaff, S.; Horstmann, M.; Schwab, A.; Stock, C. Functional interdependence of NHE1 and merlin in human melanoma cells. Biochem. Cell Biol. 2014, 92, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Barber, D.L. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 2002, 159, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Huang, D.C.; Orlowski, J.; Furthmayr, H.; Barber, D.L. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol. Cell 2000, 6, 1425–1436. [Google Scholar] [CrossRef]
- Wu, K.L.; Khan, S.; Lakhe-Reddy, S.; Jarad, G.; Mukherjee, A.; Obejero-Paz, C.A.; Konieczkowski, M.; Sedor, J.R.; Schelling, J.R. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J. Biol. Chem. 2004, 279, 26280–26286. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.E.; Fliegel, L. Physiological role and regulation of the Na+/H+ exchanger. Can. J. Physiol. Pharmacol. 2006, 84, 1081–1095. [Google Scholar] [CrossRef]
- Bera, K.; Kiepas, A.; Godet, I.; Li, Y.; Mehta, P.; Ifemembi, B.; Paul, C.D.; Sen, A.; Serra, S.A.; Stoletov, K.; et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature 2022, 611, 365–373. [Google Scholar] [CrossRef]
- Vaquero, J.; Nguyen Ho-Bouldoires, T.H.; Clapéron, A.; Fouassier, L. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: From signaling regulation to clinical relevance. Oncogene 2017, 36, 3067–3079. [Google Scholar] [CrossRef]
- Vondriska, T.M.; Pass, J.M.; Ping, P. Scaffold proteins and assembly of multiprotein signaling complexes. J. Mol. Cell Cardiol. 2004, 37, 391–397. [Google Scholar] [CrossRef]
- Dard, N.; Peter, M. Scaffold proteins in MAP kinase signaling: More than simple passive activating platforms. Bioessays 2006, 28, 146–156. [Google Scholar] [CrossRef]
- Clapéron, A.; Therrien, M. KSR and CNK: Two scaffolds regulating RAS-mediated RAF activation. Oncogene 2007, 26, 3143–3158. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lin, W.L.; Hou, Y.T.; Pu, Y.S.; Shun, C.T.; Chen, C.L.; Wu, Y.Y.; Chen, J.Y.; Chen, T.H.; Jou, T.S. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am. J. Pathol. 2010, 176, 3050–3061. [Google Scholar] [CrossRef]
- Yun, C.H.; Lamprecht, G.; Forster, D.V.; Sidor, A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J. Biol. Chem. 1998, 273, 25856–25863. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, G.; Weinman, E.J.; Yun, C.H. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J. Biol. Chem. 1998, 273, 29972–29978. [Google Scholar] [CrossRef]
- Zizak, M.; Lamprecht, G.; Steplock, D.; Tariq, N.; Shenolikar, S.; Donowitz, M.; Yun, C.H.; Weinman, E.J. cAMP-induced phosphorylation and inhibition of Na(+)/H(+) exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor. J. Biol. Chem. 1999, 274, 24753–24758. [Google Scholar] [CrossRef] [PubMed]
- Ingraffea, J.; Reczek, D.; Bretscher, A. Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur. J. Cell Biol. 2002, 81, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Boratkó, A.; Csortos, C. NHERF2 is crucial in ERM phosphorylation in pulmonary endothelial cells. Cell Commun. Signal 2013, 11, 99. [Google Scholar] [CrossRef]
- Orlando, R.A.; Takeda, T.; Zak, B.; Schmieder, S.; Benoit, V.M.; McQuistan, T.; Furthmayr, H.; Farquhar, M.G. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J. Am. Soc. Nephrol. 2001, 12, 1589–1598. [Google Scholar] [CrossRef]
- Hatano, R.; Takeda, A.; Abe, Y.; Kawaguchi, K.; Kazama, I.; Matsubara, M.; Asano, S. Loss of ezrin expression reduced the susceptibility to the glomerular injury in mice. Sci. Rep. 2018, 8, 4512. [Google Scholar] [CrossRef]
- Fukusumi, Y.; Yasuda, H.; Zhang, Y.; Kawachi, H. Nephrin-Ephrin-B1-Na(+)/H(+) Exchanger Regulatory Factor 2-Ezrin-Actin Axis Is Critical in Podocyte Injury. Am. J. Pathol. 2021, 191, 1209–1226. [Google Scholar] [CrossRef]
- Ghai, R.; Tello-Lafoz, M.; Norwood, S.J.; Yang, Z.; Clairfeuille, T.; Teasdale, R.D.; Mérida, I.; Collins, B.M. Phosphoinositide binding by the SNX27 FERM domain regulates its localization at the immune synapse of activated T-cells. J. Cell Sci. 2015, 128, 553–565. [Google Scholar] [CrossRef]
- Tello-Lafoz, M.; Ghai, R.; Collins, B.; Mérida, I. A role for novel lipid interactions in the dynamic recruitment of SNX27 to the T-cell immune synapse. Bioarchitecture 2014, 4, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Mobli, M.; Norwood, S.J.; Bugarcic, A.; Teasdale, R.D.; King, G.F.; Collins, B.M. Phox homology band 4.1/ezrin/radixin/moesin-like proteins function as molecular scaffolds that interact with cargo receptors and Ras GTPases. Proc. Natl. Acad. Sci. USA 2011, 108, 7763–7768. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Kendall, A.K.; Jackson, L.P. Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease. Front. Cell Dev. Biol. 2021, 9, 642378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, K.; Zhang, Y.; Lu, R.; Wu, S.; Tang, J.; Xia, Y.; Sun, J. Deletion of sorting nexin 27 suppresses proliferation in highly aggressive breast cancer MDA-MB-231 cells in vitro and in vivo. BMC Cancer 2019, 19, 555. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Allenspach, E.J.; Takahashi, S.M.; Mody, P.D.; Park, C.; Burkhardt, J.K.; Sperling, A.I. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J. Exp. Med. 2004, 199, 1277–1283. [Google Scholar] [CrossRef]
- Serrador, J.M.; Nieto, M.; Alonso-Lebrero, J.L.; del Pozo, M.A.; Calvo, J.; Furthmayr, H.; Schwartz-Albiez, R.; Lozano, F.; González-Amaro, R.; Sánchez-Mateos, P.; et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 1998, 91, 4632–4644. [Google Scholar] [CrossRef]
- Cannon, J.L.; Mody, P.D.; Blaine, K.M.; Chen, E.J.; Nelson, A.D.; Sayles, L.J.; Moore, T.V.; Clay, B.S.; Dulin, N.O.; Shilling, R.A.; et al. CD43 interaction with ezrin-radixin-moesin (ERM) proteins regulates T-cell trafficking and CD43 phosphorylation. Mol. Biol. Cell 2011, 22, 954–963. [Google Scholar] [CrossRef]
- Yonemura, S.; Tsukita, S.; Tsukita, S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J. Cell Biol. 1999, 145, 1497–1509. [Google Scholar] [CrossRef]
- Lamb, R.F.; Roy, C.; Diefenbach, T.J.; Vinters, H.V.; Johnson, M.W.; Jay, D.G.; Hall, A. The TSC1 tumour suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat. Cell Biol. 2000, 2, 281–287. [Google Scholar] [CrossRef]
- Ognibene, M.; Vanni, C.; Segalerba, D.; Mancini, P.; Merello, E.; Torrisi, M.R.; Bosco, M.C.; Varesio, L.; Eva, A. The tumor suppressor hamartin enhances Dbl protein transforming activity through interaction with ezrin. J. Biol. Chem. 2011, 286, 29973–29983. [Google Scholar] [CrossRef]
- Barreiro, O.; Yanez-Mo, M.; Serrador, J.M.; Montoya, M.C.; Vicente-Manzanares, M.; Tejedor, R.; Furthmayr, H.; Sanchez-Madrid, F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002, 157, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.H.; Massagué, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr. Opin. Cell Biol. 1999, 11, 109–116. [Google Scholar] [CrossRef]
- Fais, S. A role for ezrin in a neglected metastatic tumor function. Trends Mol. Med. 2004, 10, 249–250. [Google Scholar] [CrossRef]
- Marin-Padilla, M. Erythrophagocytosis by epithelial cells of a breast carcinoma. Cancer 1977, 39, 1085–1089. [Google Scholar] [CrossRef]
- DeSimone, P.A.; East, R.; Powell, R.D., Jr. Phagocytic tumor cell activity in oat cell carcinoma of the lung. Hum. Pathol. 1980, 11, 535–539. [Google Scholar] [PubMed]
- Defacque, H.; Egeberg, M.; Habermann, A.; Diakonova, M.; Roy, C.; Mangeat, P.; Voelter, W.; Marriott, G.; Pfannstiel, J.; Faulstich, H.; et al. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000, 19, 199–212. [Google Scholar] [CrossRef]
- Lugini, L.; Lozupone, F.; Matarrese, P.; Funaro, C.; Luciani, F.; Malorni, W.; Rivoltini, L.; Castelli, C.; Tinari, A.; Piris, A.; et al. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: A key role of ezrin. Lab. Investig. 2003, 83, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Matarrese, P.; Tinari, A.; Lozupone, F.; Federici, C.; Iessi, E.; Gentile, M.; Luciani, F.; Parmiani, G.; Rivoltini, L.; et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006, 66, 3629–3638. [Google Scholar] [CrossRef]
- Chang, Y.T.; Peng, H.Y.; Hu, C.M.; Huang, S.C.; Tien, S.C.; Jeng, Y.M. Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis. Am. J. Cancer Res. 2020, 10, 12–37. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Alcover, A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front. Biosci. 2006, 11, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, A.; Serrador, J.M. ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion. Int. J. Mol. Sci. 2020, 21, 1502. [Google Scholar] [CrossRef] [PubMed]
- Kakurina, G.V.; Stakheeva, M.N.; Bakhronov, I.A.; Sereda, E.E.; Cheremisina, O.V.; Choynzonov, E.L.; Kondakova, I.V. Circulating Actin-Binding Proteins in Laryngeal Cancer: Its Relationship with Circulating Tumor Cells and Cells of the Immune System. Acta Nat. 2021, 13, 64–68. [Google Scholar] [CrossRef]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S.; et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef]
- Tu, X.; Qin, B.; Zhang, Y.; Zhang, C.; Kahila, M.; Nowsheen, S.; Yin, P.; Yuan, J.; Pei, H.; Li, H.; et al. PD-L1 (B7-H1) Competes with the RNA Exosome to Regulate the DNA Damage Response and Can Be Targeted to Sensitize to Radiation or Chemotherapy. Mol. Cell 2019, 74, 1215–1226.e1214. [Google Scholar] [CrossRef]
- Kobori, T.; Tanaka, C.; Tameishi, M.; Urashima, Y.; Ito, T.; Obata, T. Role of Ezrin/Radixin/Moesin in the Surface Localization of Programmed Cell Death Ligand-1 in Human Colon Adenocarcinoma LS180 Cells. Pharmaceuticals 2021, 14, 864. [Google Scholar] [CrossRef]
- Tanaka, C.; Kobori, T.; Tameishi, M.; Urashima, Y.; Ito, T.; Obata, T. Ezrin Modulates the Cell Surface Expression of Programmed Cell Death Ligand-1 in Human Cervical Adenocarcinoma Cells. Molecules 2021, 26, 5648. [Google Scholar] [CrossRef]
- Tanaka, C.; Kobori, T.; Okada, R.; Doukuni, R.; Tameishi, M.; Urashima, Y.; Ito, T.; Takagaki, N.; Obata, T. Ezrin Regulates the Cell Surface Localization of PD-L1 in HEC-151 Cells. J. Clin. Med. 2022, 11, 2226. [Google Scholar] [CrossRef]
- Yu, Y. The Function of NK Cells in Tumor Metastasis and NK Cell-Based Immunotherapy. Cancers 2023, 15, 2323. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Z.; Xia, P.; Liu, T.; Wang, J.; Li, S.; Sun, L.; Lu, J.; Wen, Q.; Zhou, M.; et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009, 19, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Matsui, M.; Ichioka, S.; Nakamura, Y.; Hirata, T. The ERM protein moesin regulates natural killer cell homeostasis in vivo. Cell Immunol. 2022, 371, 104456. [Google Scholar] [CrossRef]
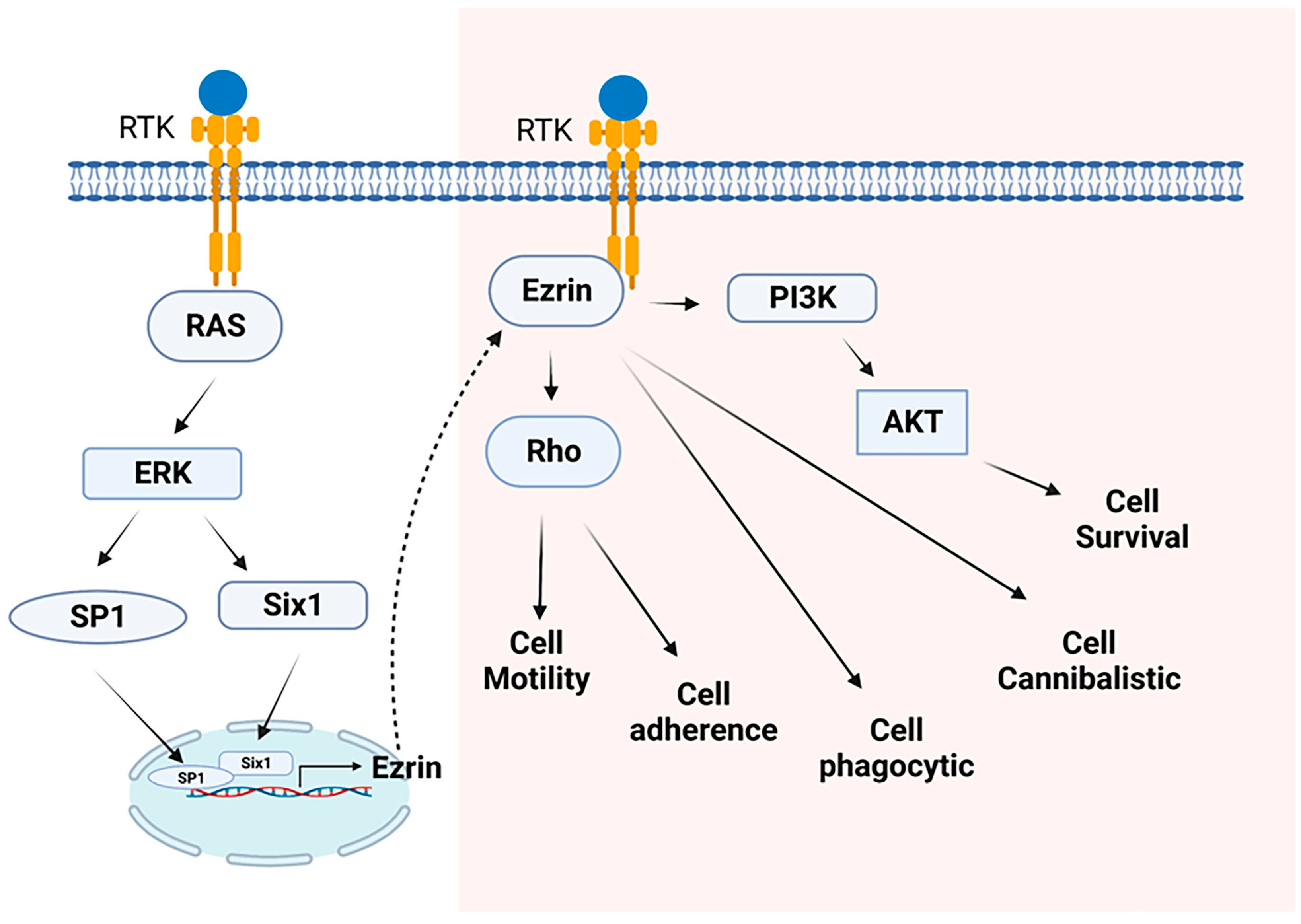
- Yu, Y.; Khan, J.; Khanna, C.; Helman, L.; Meltzer, P.S.; Merlino, G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat. Med. 2004, 10, 175–181. [Google Scholar] [CrossRef]
- Kong, J.; Di, C.; Piao, J.; Sun, J.; Han, L.; Chen, L.; Yan, G.; Lin, Z. Ezrin contributes to cervical cancer progression through induction of epithelial-mesenchymal transition. Oncotarget 2016, 7, 19631–19642. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Chen, B.; Chen, S.; Jiang, Z.; Zhou, T.; Hou, Z.; Wang, Y. Ezrin/NF-kB activation regulates epithelial-mesenchymal transition induced by EGF and promotes metastasis of colorectal cancer. Biomed. Pharmacother. 2017, 92, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.X.; Feng, S.D.; Shen, R.; Wu, Z.Y.; Chen, F.; Zhu, X. The clinical significance of the Ezrin gene and circulating tumor cells in osteosarcoma. OncoTargets Ther. 2017, 10, 527–533. [Google Scholar] [CrossRef]
- Hoskin, V.; Szeto, A.; Ghaffari, A.; Greer, P.A.; Côté, G.P.; Elliott, B.E. Ezrin regulates focal adhesion and invadopodia dynamics by altering calpain activity to promote breast cancer cell invasion. Mol. Biol. Cell 2015, 26, 3464–3479. [Google Scholar] [CrossRef]
- Bartova, M.; Hlavaty, J.; Tan, Y.; Singer, C.; Pohlodek, K.; Luha, J.; Walter, I. Expression of ezrin and moesin in primary breast carcinoma and matched lymph node metastases. Clin. Exp. Metastasis 2017, 34, 333–344. [Google Scholar] [CrossRef]
- Chen, M.; Pan, Y.; Liu, H.; Ning, F.; Lu, Q.; Duan, Y.; Gan, X.; Lu, S.; Hou, H.; Zhang, M.; et al. Ezrin accelerates breast cancer liver metastasis through promoting furin-like convertase-mediated cleavage of Notch1. Cell Oncol. 2022, 46, 571–587. [Google Scholar] [CrossRef]
- Zhan, X.H.; Jiao, J.W.; Zhang, H.F.; Xu, X.E.; He, J.Z.; Li, R.L.; Zou, H.Y.; Wu, Z.Y.; Wang, S.H.; Wu, J.Y.; et al. LOXL2 Upregulates Phosphorylation of Ezrin to Promote Cytoskeletal Reorganization and Tumor Cell Invasion. Cancer Res. 2019, 79, 4951–4964. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Lu, Y.; Lai, C.; Qu, L.; Zhuo, Y. Ezrin expression in circulating tumor cells is a predictor of prostate cancer metastasis. Bioengineered 2022, 13, 4076–4084. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, X.; Yu, S.; Bie, X.; Wang, J.; Ren, L. Inhibition of Ezrin suppresses cell migration and invasion in human nasopharyngeal carcinoma. Oncol. Lett. 2019, 18, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Davicioni, E.; Triche, T.J.; Merlino, G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006, 66, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Gautreau, A.; Louvard, D.; Arpin, M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J. Cell Biol. 2000, 150, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Khanna, C.; Wan, X.; Bose, S.; Cassaday, R.; Olomu, O.; Mendoza, A.; Yeung, C.; Gorlick, R.; Hewitt, S.M.; Helman, L.J. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat. Med. 2004, 10, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Chuan, Y.C.; Pang, S.T.; Cedazo-Minguez, A.; Norstedt, G.; Pousette, A.; Flores-Morales, A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J. Biol. Chem. 2006, 281, 29938–29948. [Google Scholar] [CrossRef]
- Song, Y.; Ma, X.; Zhang, M.; Wang, M.; Wang, G.; Ye, Y.; Xia, W. Ezrin Mediates Invasion and Metastasis in Tumorigenesis: A Review. Front. Cell Dev. Biol. 2020, 8, 588801. [Google Scholar] [CrossRef]
- Bulut, G.; Hong, S.H.; Chen, K.; Beauchamp, E.M.; Rahim, S.; Kosturko, G.W.; Glasgow, E.; Dakshanamurthy, S.; Lee, H.S.; Daar, I.; et al. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene 2012, 31, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Çelik, H.; Bulut, G.; Han, J.; Graham, G.T.; Minas, T.Z.; Conn, E.J.; Hong, S.H.; Pauly, G.T.; Hayran, M.; Li, X.; et al. Ezrin Inhibition Up-regulates Stress Response Gene Expression. J. Biol. Chem. 2016, 291, 13257–13270. [Google Scholar] [CrossRef]
- Çelik, H.; Hong, S.H.; Colón-López, D.D.; Han, J.; Kont, Y.S.; Minas, T.Z.; Swift, M.; Paige, M.; Glasgow, E.; Toretsky, J.A.; et al. Identification of Novel Ezrin Inhibitors Targeting Metastatic Osteosarcoma by Screening Open Access Malaria Box. Mol. Cancer Ther. 2015, 14, 2497–2507. [Google Scholar] [CrossRef]
- Ghaffari, A.; Hoskin, V.; Turashvili, G.; Varma, S.; Mewburn, J.; Mullins, G.; Greer, P.A.; Kiefer, F.; Day, A.G.; Madarnas, Y.; et al. Intravital imaging reveals systemic ezrin inhibition impedes cancer cell migration and lymph node metastasis in breast cancer. Breast Cancer Res. 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bhushan, B.; Mars, W.M.; Bowen, W.; Tao, J.; Orr, A.; Stoops, J.; Yu, Y.; Luo, J.; Duncan, A.W.; et al. Phosphorylated Ezrin (Thr567) Regulates Hippo Pathway and Yes-Associated Protein (Yap) in Liver. Am. J. Pathol. 2020, 190, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Moodley, S.; Lian, E.Y.; Crupi, M.J.F.; Hyndman, B.D.; Mulligan, L.M. RET isoform-specific interaction with scaffold protein Ezrin promotes cell migration and chemotaxis in lung adenocarcinoma. Lung Cancer 2020, 142, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, V.; Ghaffari, A.; Elliott, B.E. Ezrin, more than a metastatic detERMinant? Oncotarget 2019, 10, 6755–6757. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, V.; Ghaffari, A.; Laight, B.J.; SenGupta, S.; Madarnas, Y.; Nicol, C.J.B.; Elliott, B.E.; Varma, S.; Greer, P.A. Targeting the Ezrin Adaptor Protein Sensitizes Metastatic Breast Cancer Cells to Chemotherapy and Reduces Neoadjuvant Therapy-induced Metastasis. Cancer Res. Commun. 2022, 2, 456–470. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, P.; Xiong, J.; Liu, Z.; Berger, S.L.; Merlino, G. Epigenetic drugs can stimulate metastasis through enhanced expression of the pro-metastatic Ezrin gene. PLoS ONE 2010, 5, e12710. [Google Scholar] [CrossRef]


| Name | Format | Mechanism | Disease | Status | Reference(s) |
|---|---|---|---|---|---|
| NSC305787 | small molecular inhibitor | inhibits Ezrin phosphorylation | osteosarcoma | pre-clinical | [230] |
| NSC668394 | small molecular inhibitor | inhibits Ezrin phosphorylation | osteosarcoma | pre-clinical | [230,231] |
| MMV667492 | small molecular inhibitor | inhibits Ezrin phosphorylation | osteosarcoma | pre-clinical | [232] |
| NSC668394 | small molecular inhibitor | inhibits Ezrin phosphorylation | breast cancer | pre-clinical | [233] |
| NSC668394 | small molecular inhibitor | inhibits Ezrin phosphorylation | melanoma | pre-clinical | [36] |
| NSC668394 | small molecular inhibitor | inhibits Ezrin phosphorylation | hepatocellular carcinoma | pre-clinical | [234] |
| NSC305787 | small molecular inhibitor | inhibits Ezrin phosphorylation | lung adenocarcinoma | in vitro | [235] |
| NSC668394+ Lapatinib | small molecular inhibitor | combination | breast cancer | pre-clinical | [131,236] |
| NSC668394+ DOX or DTX | small molecular inhibitor | combination | breast cancer | pre-clinical | [237] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buenaventura, R.G.M.; Merlino, G.; Yu, Y. Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis. Cells 2023, 12, 1620. https://doi.org/10.3390/cells12121620
Buenaventura RGM, Merlino G, Yu Y. Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis. Cells. 2023; 12(12):1620. https://doi.org/10.3390/cells12121620
Chicago/Turabian StyleBuenaventura, Rand Gabriel M., Glenn Merlino, and Yanlin Yu. 2023. "Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis" Cells 12, no. 12: 1620. https://doi.org/10.3390/cells12121620
APA StyleBuenaventura, R. G. M., Merlino, G., & Yu, Y. (2023). Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis. Cells, 12(12), 1620. https://doi.org/10.3390/cells12121620








