Apathy in Parkinson’s Disease: Clinical Patterns and Neurobiological Basis
Abstract
:1. Introduction
2. Prevalence and Clinical Correlates
3. Cognitive, Anatomo-Clinical and Computational Models of Apathy
4. Diagnosis and Assessment
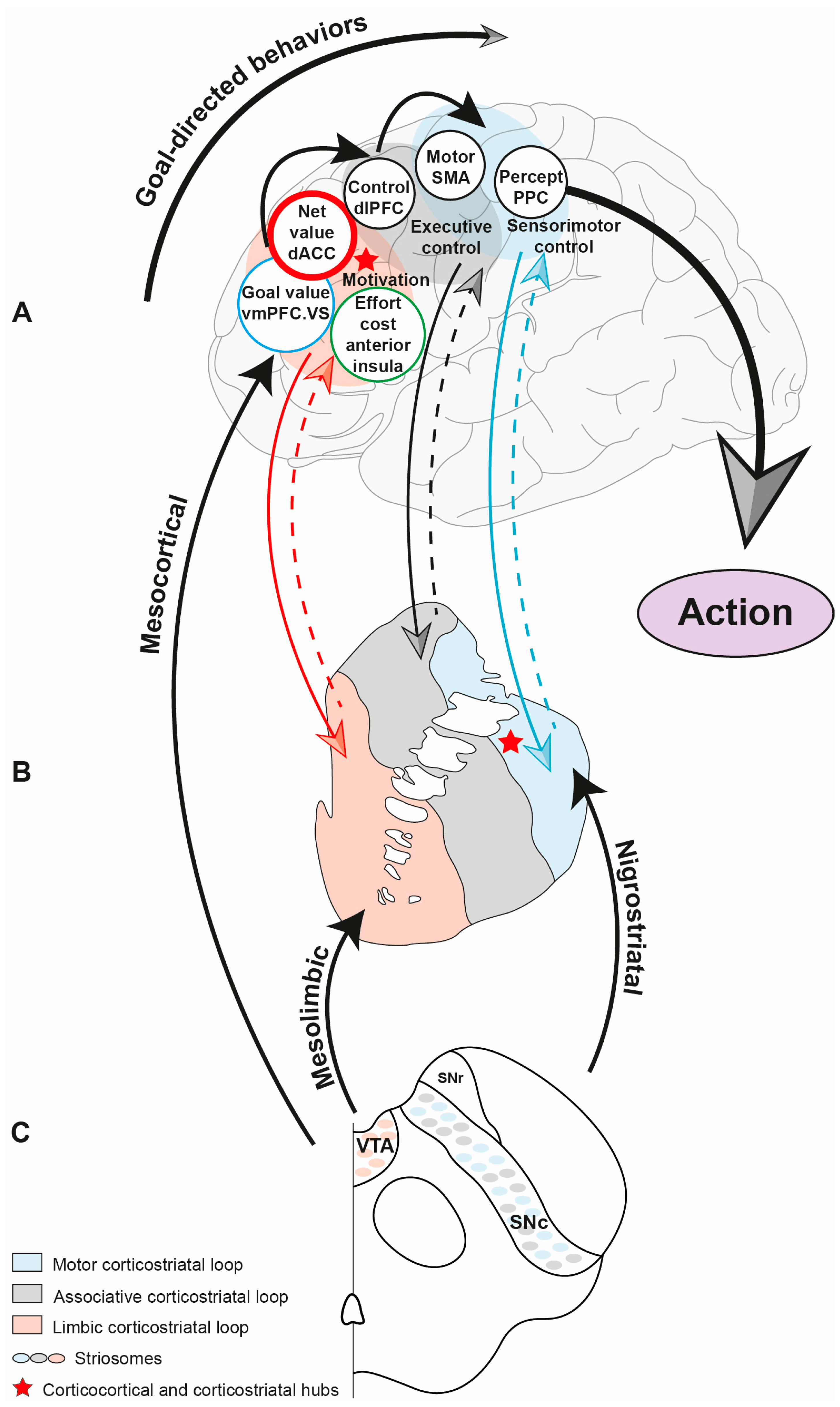
5. Corticobasal Circuits and Motivated Behaviors
6. Neurobiology of Apathy
6.1. Dopamine, Reward and Effort
6.2. The Dopaminergic Behavior Continuum Hypothesis
6.3. The Role of the Subthalamic Nucleus
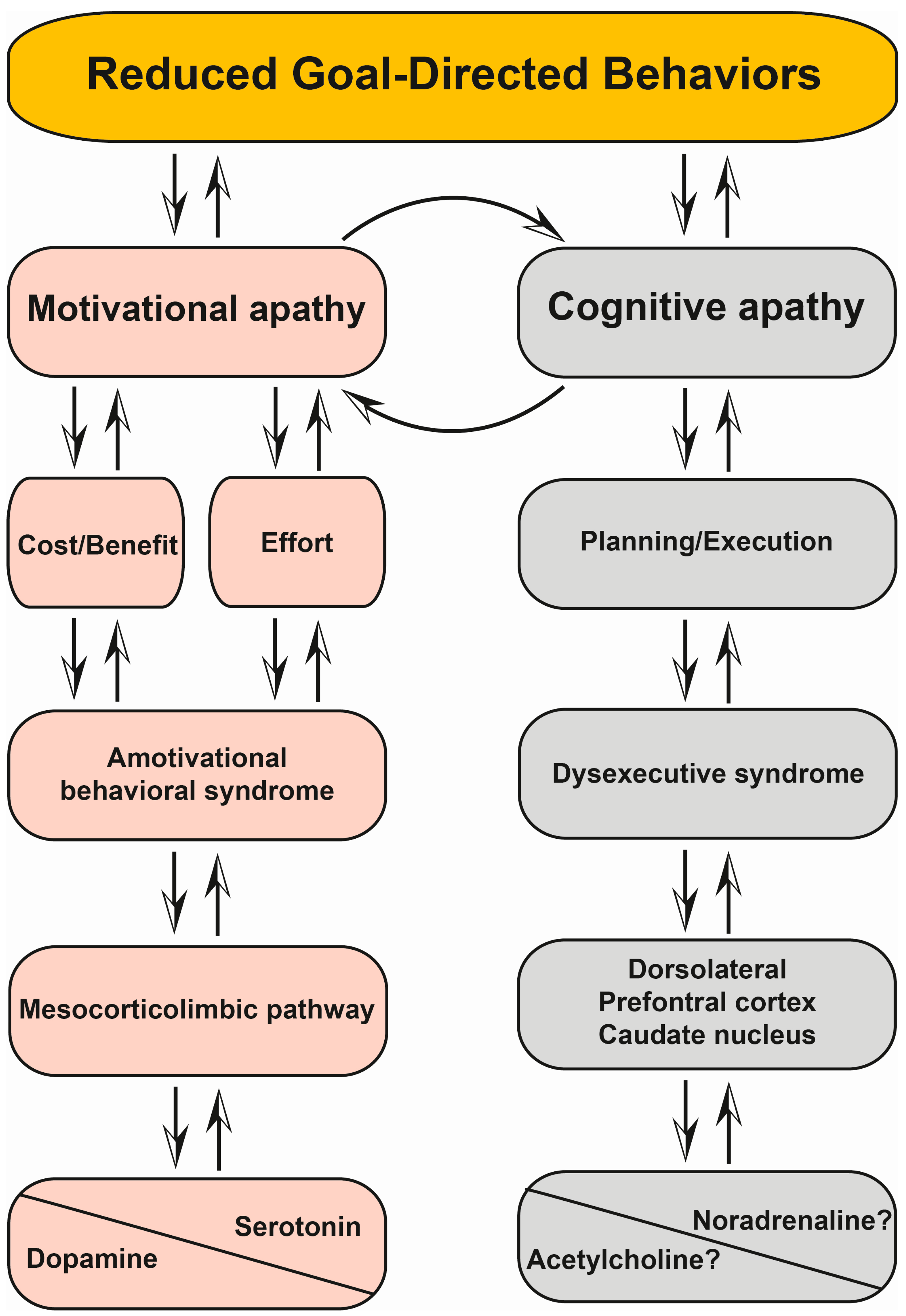
6.4. Motivational and Cognitive Apathy
7. Treatment of Apathy
8. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Anterior Cingulate Cortex |
| AES | Apathy Evaluation Scale |
| AI | Apathy Inventory |
| AS | Apathy Scale |
| ASBPD | Ardouin Scale of Behaviors in Parkinson’s Disease |
| BG | Basal Ganglia |
| DA | Dopaminergic |
| DAT | Dopamine Transporters |
| DBS | Deep Brain Stimulation |
| DLPFC | Dorsolateral Prefrontal Cortex |
| D1Rs, D2Rs, D3Rs | Dopamine D1, D2, D3 Receptors |
| DRT | Dopamine Replacement Therapy |
| EBDM | Effort-Based Decision Making |
| GABA | Gamma-Aminobutyric Acid |
| GDBs | Goal-Directed Behaviors |
| GPe | Globus Pallidus Externus |
| GPi | Globus Pallidus Internus |
| ICD | Impulse Control Disorders |
| ISCTM | International Society for Central Nervous System Clinical Trials Methodology |
| LARS | Lille Apathy Rating Scale |
| MDS | Movement Disorder Society |
| MCL | Mesocorticolimbic |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NAc | Nucleus Accumbens |
| NPI | Neuropsychiatric Inventory |
| OFC | Orbitofrontal Cortex |
| 6-OHDA | 6-hydroxydopamine |
| PD | Parkinson’s Disease |
| PET | Positron Emission Tomography |
| PFFs | Preformed α-synuclein Fibril(s) |
| PSP | Progressive Supranuclear Palsy |
| RCT | Randomized Controlled Trial |
| r-TMS | Repetitive Transcranial Magnetic Stimulation |
| SERT | Serotonin Transporters |
| SNc | Substantia Nigra Pars Compacta |
| SSRI | Selective Serotonin Reuptake Inhibitors |
| STN | Subthalamic Nucleus |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VS | Ventral Striatum |
| vmPFC | Ventromedial Prefrontal Cortex |
| VTA | Ventral Tegmental Area |
| VTAc | Volume of Tissue Activated |
References
- Weintraub, D.; Burn, D.J. Parkinson’s disease: The quintessential neuropsychiatric disorder. Mov. Disord. 2011, 26, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Mamikonyan, E. The Neuropsychiatry of Parkinson Disease: A Perfect Storm. Am. J.Geriatr. Psychiatry 2019, 27, 998–1018. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Pagonabarraga, J.; Kulisevsky, J.; Strafella, A.P.; Krack, P. Apathy in Parkinson’s disease: Clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015, 14, 518–531. [Google Scholar] [CrossRef] [PubMed]
- den Brok, M.G.H.E.; van Dalen, J.W.; van Gool, W.A.; Moll van Charante, E.P.; de Bie, R.M.A.; Richard, E. Apathy in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2015, 30, 759–769. [Google Scholar] [CrossRef]
- Lazcano-Ocampo, C.; Wan, Y.M.; van Wamelen, D.J.; Batzu, L.; Boura, I.; Titova, N.; Leta, V.; Qamar, M.; Martinez-Martin, P.; Ray Chaudhuri, K. Identifying and responding to fatigue and apathy in Parkinson’s disease: A review of current practice. Expert Rev. Neurother. 2020, 20, 477–495. [Google Scholar] [CrossRef]
- Robert, P.; Lanctôt, K.L.; Agüera-Ortiz, L.; Aalten, P.; Bremond, F.; Defrancesco, M.; Hanon, C.; David, R.; Dubois, B.; Dujardin, K.; et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur. Psychiatry 2018, 54, 71–76. [Google Scholar] [CrossRef]
- Miller, D.S.; Robert, P.; Ereshefsky, L.; Adler, L.; Bateman, D.; Cummings, J.; DeKosky, S.T.; Fischer, C.E.; Husain, M.; Ismail, Z.; et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 2021, 17, 1892–1904. [Google Scholar] [CrossRef]
- De Waele, S.; Cras, P.; Crosiers, D. Apathy in Parkinson’s Disease: Defining the Park Apathy Subtype. Brain Sci. 2022, 12, 923. [Google Scholar] [CrossRef]
- Castrioto, A.; Thobois, S.; Carnicella, S.; Maillet, A.; Krack, P. Emotional manifestations of PD: Neurobiological basis. Mov. Disord. 2016, 31, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Castrioto, A.; Lhommée, E.; Moro, E.; Krack, P. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014, 13, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Le Heron, C.; Holroyd, C.B.; Salamone, J.; Husain, M. Brain mechanisms underlying apathy. J. Neurol. Neurosurg. Psychiatry 2019, 90, 302–312. [Google Scholar] [CrossRef]
- Simpson, E.H.; Balsam, P.D. The Behavioral Neuroscience of Motivation: An Overview of Concepts, Measures, and Translational Applications. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2016; Volume 27, pp. 1–12. [Google Scholar]
- Thobois, S.; Ardouin, C.; Lhommée, E.; Klinger, H.; Lagrange, C.; Xie, J.; Fraix, V.; Coelho Braga, M.C.; Hassani, R.; Kistner, A.; et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: Predictors and underlying mesolimbic denervation. Brain 2010, 133 Pt 4, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; Le Bars, D.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 2016, 139 Pt 9, 2486–2502. [Google Scholar] [CrossRef] [PubMed]
- Drapier, D.; Drapier, S.; Sauleau, P.; Haegelen, C.; Raoul, S.; Biseul, I.; Peron, J.; Lallement, F.; Rivier, I.; Reymann, J.M.; et al. Does subthalamic nucleus stimulation induce apathy in Parkinson’s disease? J. Neurol. 2006, 253, 1083–1091. [Google Scholar] [CrossRef]
- Le Jeune, F.; Drapier, D.; Bourguignon, A.; Péron, J.; Mesbah, H.; Drapier, S.; Sauleau, P.; Haegelen, C.; Travers, D.; Garin, E.; et al. Subthalamic nucleus stimulation in Parkinson disease induces apathy: A PET study. Neurology 2009, 73, 1746–1751. [Google Scholar] [CrossRef]
- Funkiewiez, A.; Ardouin, C.; Krack, P.; Fraix, V.; Van Blercom, N.; Xie, J.; Moro, E.; Benabid, A.-L.; Pollak, P. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson’s disease. Mov. Disord. 2003, 18, 524–530. [Google Scholar] [CrossRef]
- Ulla, M.; Thobois, S.; Lemaire, J.-J.; Schmitt, A.; Derost, P.; Broussolle, E.; Llorca, P.-M.; Durif, F. Manic behaviour induced by deep-brain stimulation in Parkinson’s disease: Evidence of substantia nigra implication? J. Neurol. Neurosurg. Psychiatry 2006, 77, 1363–1366. [Google Scholar] [CrossRef]
- Dujardin, K.; Sockeel, P.; Delliaux, M.; Destée, A.; Defebvre, L. Apathy may herald cognitive decline and dementia in Parkinson’s disease. Mov. Disord. 2009, 24, 2391–2397. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Maltête, D.; Lefaucheur, R.; Kreisler, A.; Eusebio, A.; Defer, G.; Ouk, T.; Azulay, J.-P.; Krystkowiak, P.; et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: A double-blind, placebo-controlled, randomised clinical trial. J. Neurol. Neurosurg. Psychiatry 2014, 85, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Loued-Khenissi, L.; Preuschoff, K. Apathy and noradrenaline: Silent partners to mild cognitive impairment in Parkinson’s disease? Curr. Opin. Neurol. 2015, 28, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Hezemans, F.H.; Wolpe, N.; O’Callaghan, C.; Ye, R.; Rua, C.; Jones, P.S.; Murley, A.G.; Holland, N.; Regenthal, R.; Tsvetanov, K.A.; et al. Noradrenergic deficits contribute to apathy in Parkinson’s disease through the precision of expected outcomes. PLoS Comput. Biol. 2022, 18, e1010079. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; O’Callaghan, C.; Rua, C.; Hezemans, F.H.; Holland, N.; Malpetti, M.; Jones, P.S.; Barker, R.A.; Williams-Gray, C.H.; Robbins, T.W.; et al. Locus Coeruleus Integrity from 7 T MRI Relates to Apathy and Cognition in Parkinsonian Disorders. Mov. Disord. 2022, 37, 1663–1672. [Google Scholar] [CrossRef]
- Chagraoui, A.; Boukhzar, L.; Thibaut, F.; Anouar, Y.; Maltête, D. The pathophysiological mechanisms of motivational deficits in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 138–152. [Google Scholar] [CrossRef]
- Mele, B.; Van, S.; Holroyd-Leduc, J.; Ismail, Z.; Pringsheim, T.; Goodarzi, Z. Diagnosis, treatment and management of apathy in Parkinson’s disease: A scoping review. BMJ Open 2020, 10, e037632. [Google Scholar] [CrossRef]
- Dujardin, K.; Sockeel, P.; Devos, D.; Delliaux, M.; Krystkowiak, P.; Destée, A.; Defebvre, L. Characteristics of apathy in Parkinson’s disease. Mov. Disord. 2007, 22, 778–784. [Google Scholar] [CrossRef]
- Leentjens, A.F.G.; Dujardin, K.; Marsh, L.; Martinez-Martin, P.; Richard, I.H.; Starkstein, S.E.; Weintraub, D.; Sampaio, C.; Poewe, W.; Rascol, O.; et al. Apathy and anhedonia rating scales in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2008, 23, 2004–2014. [Google Scholar] [CrossRef]
- Hagell, P.; Brundin, L. Towards an understanding of fatigue in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 489–492. [Google Scholar] [CrossRef]
- Dujardin, K.; Langlois, C.; Plomhause, L.; Carette, A.-S.; Delliaux, M.; Duhamel, A.; Defebvre, L. Apathy in untreated early-stage Parkinson disease: Relationship with other non-motor symptoms. Mov. Disord. 2014, 29, 1796–1801. [Google Scholar] [CrossRef]
- Foley, J.A.; Cipolotti, L. Apathy in Parkinson’s Disease: A Retrospective Study of Its Prevalence and Relationship With Mood, Anxiety, and Cognitive Function. Front. Psychol. 2021, 12, 749624. [Google Scholar] [CrossRef] [PubMed]
- Loas, G.; Duru, C.; Godefroy, O.; Krystkowiak, P. Hedonic deficits in Parkinson’s disease: Is consummatory anhedonia specific? Front. Neurol. 2014, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Houeto, J.-L.; Magnard, R.; Dalley, J.W.; Belin, D.; Carnicella, S. Trait Impulsivity and Anhedonia: Two Gateways for the Development of Impulse Control Disorders in Parkinson’s Disease? Front. Psychiatry 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Meira, B.; Lhommée, E.; Schmitt, E.; Klinger, H.; Bichon, A.; Pélissier, P.; Anheim, M.; Tranchant, C.; Fraix, V.; Meoni, S.; et al. Early Parkinson’s Disease Phenotypes Tailored by Personality, Behavior, and Motor Symptoms. J. Park. Dis. 2022, 12, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000, 179, 34–42. [Google Scholar] [CrossRef]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic presentations of Parkinson’s disease in primary care: A case-control study. Lancet Neurol. 2015, 14, 57–64. [Google Scholar] [CrossRef]
- Skorvanek, M.; Gdovinova, Z.; Rosenberger, J.; Saeedian, R.G.; Nagyova, I.; Groothoff, J.W.; van Dijk, J.P. The associations between fatigue, apathy, and depression in Parkinson’s disease. Acta Neurol. Scand. 2015, 131, 80–87. [Google Scholar] [CrossRef]
- Ongre, S.O.; Larsen, J.P.; Tysnes, O.B.; Herlofson, K. Fatigue in early Parkinson’s disease: The Norwegian ParkWest study. Eur. J. Neurol. 2017, 24, 105–111. [Google Scholar]
- Siciliano, M.; Trojano, L.; De Micco, R.; Giordano, A.; Russo, A.; Tedeschi, G.; Chiorri, C.; Tessitore, A. Predictors of fatigue severity in early, de novo Parkinson disease patients: A 1-year longitudinal study. Park. Relat. Disord. 2020, 79, 3–8. [Google Scholar] [CrossRef]
- Kang, S.Y.; Ma, H.-I.; Lim, Y.-M.; Hwang, S.H.; Kim, Y.J. Fatigue in drug-naïve Parkinson’s disease. Eur. Neurol. 2013, 70, 59–64. [Google Scholar] [CrossRef]
- Ardouin, C.; Chéreau, I.; Llorca, P.-M.; Lhommée, E.; Durif, F.; Pollak, P.; Krack, P.; Groupe Évaluation Comportementale de la Maladie de Parkinson. Assessment of hyper- and hypodopaminergic behaviors in Parkinson’s disease. Rev. Neurol. 2009, 165, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Béreau, M.; Castrioto, A.; Lhommée, E.; Maillet, A.; Gérazime, A.; Bichon, A.; Pélissier, P.; Schmitt, E.; Klinger, H.; Longato, N.; et al. Fatigue in de novo Parkinson’s Disease: Expanding the Neuropsychiatric Triad? J. Park. Dis. 2022, 12, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.F.; Alves, G.; Aarsland, D.; Larsen, J.P. Occurrence and risk factors for apathy in Parkinson disease: A 4-year prospective longitudinal study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.S. Apathy: A neuropsychiatric syndrome. J. Neuropsychiatry Clin. Neurosci. 1991, 3, 243–254. [Google Scholar] [PubMed]
- Brown, R.G.; Pluck, G. Negative symptoms: The “pathology” of motivation and goal-directed behaviour. Trends Neurosci. 2000, 23, 412–417. [Google Scholar] [CrossRef]
- Levy, R.; Dubois, B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex 2006, 16, 916–928. [Google Scholar] [CrossRef]
- Husain, M.; Roiser, J.P. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat. Rev. Neurosci. 2018, 19, 470–484. [Google Scholar] [CrossRef]
- Le Bouc, R.; Rigoux, L.; Schmidt, L.; Degos, B.; Welter, M.-L.; Vidailhet, M.; Daunizeau, J.; Pessiglione, M. Computational Dissection of Dopamine Motor and Motivational Functions in Humans. J. Neurosci. 2016, 36, 6623–6633. [Google Scholar] [CrossRef]
- Pessiglione, M.; Vinckier, F.; Bouret, S.; Daunizeau, J.; Le Bouc, R. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain 2018, 141, 629–650. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Salamone, J.D.; Yohn, S.E.; López-Cruz, L.; San Miguel, N.; Correa, M. Activational and effort-related aspects of motivation: Neural mechanisms and implications for psychopathology. Brain 2016, 139 Pt 5, 1325–1347. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Mayberg, H.S.; Preziosi, T.J.; Andrezejewski, P.; Leiguarda, R.; Robinson, R.G. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 134–139. [Google Scholar] [PubMed]
- Marin, R.S.; Biedrzycki, R.C.; Firinciogullari, S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991, 38, 143–162. [Google Scholar] [CrossRef]
- Robert, P.H.; Clairet, S.; Benoit, M.; Koutaich, J.; Bertogliati, C.; Tible, O.; Caci, H.; Borg, M.; Brocker, P.; Bedoucha, P. The apathy inventory: Assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. Int. J. Geriatr. Psychiatry 2002, 17, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef]
- Sockeel, P.; Dujardin, K.; Devos, D.; Denève, C.; Destée, A.; Defebvre, L. The Lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 579–584. [Google Scholar] [CrossRef]
- Rieu, I.; Martinez-Martin, P.; Pereira, B.; De Chazeron, I.; Verhagen Metman, L.; Jahanshahi, M.; Ardouin, C.; Chéreau, I.; Brefel-Courbon, C.; Ory-Magne, F.; et al. International validation of a behavioral scale in Parkinson’s disease without dementia. Mov. Disord. 2015, 30, 705–713. [Google Scholar] [CrossRef]
- Yelnik, J. Functional anatomy of the basal ganglia. Mov. Disord. 2002, 17 (Suppl. S3), S15–S21. [Google Scholar]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Behrens, T.E.J. The neural network underlying incentive-based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron 2014, 83, 1019–1039. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Choi, E.Y.; Heilbronner, S.R.; Haber, S.N. Nonhuman primate meso-circuitry data: A translational tool to understand brain networks across species. Brain Struct. Funct. 2021, 226, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 2014, 282, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lehéricy, S.; Ducros, M.; Van de Moortele, P.-F.; Francois, C.; Thivard, L.; Poupon, C.; Swindale, N.; Ugurbil, K.; Kim, D.-S. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann. Neurol. 2004, 55, 522–529. [Google Scholar] [CrossRef]
- Wiesendanger, E.; Clarke, S.; Kraftsik, R.; Tardif, E. Topography of cortico-striatal connections in man: Anatomical evidence for parallel organization. Eur. J. Neurosci. 2004, 20, 1915–1922. [Google Scholar] [CrossRef]
- Draganski, B.; Kherif, F.; Klöppel, S.; Cook, P.A.; Alexander, D.C.; Parker, G.J.M.; Deichmann, R.; Ashburner, J.; Frackowiak, R.S.J. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J. Neurosci. 2008, 28, 7143–7152. [Google Scholar] [CrossRef]
- Plantinga, B.R.; Temel, Y.; Duchin, Y.; Uludağ, K.; Patriat, R.; Roebroeck, A.; Kuijf, M.; Jahanshahi, A.; Ter Haar Romenij, B.; Vitek, J.; et al. Individualized parcellation of the subthalamic nucleus in patients with Parkinson’s disease with 7T MRI. Neuroimage 2018, 168, 403–411. [Google Scholar] [CrossRef]
- Rodriguez-Rojas, R.; Pineda-Pardo, J.A.; Mañez-Miro, J.; Sanchez-Turel, A.; Martinez-Fernandez, R.; Del Alamo, M.; DeLong, M.; Obeso, J.A. Functional Topography of the Human Subthalamic Nucleus: Relevance for Subthalamotomy in Parkinson’s Disease. Mov. Disord. 2022, 37, 279–290. [Google Scholar] [CrossRef]
- Magnard, R.; Vachez, Y.; Carcenac, C.; Krack, P.; David, O.; Savasta, M.; Boulet, S.; Carnicella, S. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl. Psychiatry 2016, 6, e753. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Yang, J.-H.; Rotolo, R.; Presby, R. Dopamine, Effort-Based Choice, and Behavioral Economics: Basic and Translational Research. Front. Behav. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Fischbach-Weiss, S.; Reese, R.M.; Janak, P.H. Inhibiting Mesolimbic Dopamine Neurons Reduces the Initiation and Maintenance of Instrumental Responding. Neuroscience 2018, 372, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Favier, M.; Carcenac, C.; Savasta, M.; Carnicella, S. Dopamine D3 Receptors: A Potential Target to Treat Motivational Deficits in Parkinson’s Disease. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2023; Volume 60, pp. 109–132. [Google Scholar]
- Favier, M.; Duran, T.; Carcenac, C.; Drui, G.; Savasta, M.; Carnicella, S. Pramipexole reverses Parkinson’s disease-related motivational deficits in rats. Mov. Disord. 2014, 29, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Carnicella, S.; Drui, G.; Boulet, S.; Carcenac, C.; Favier, M.; Duran, T.; Savasta, M. Implication of dopamine D3 receptor activation in the reversion of Parkinson’s disease-related motivational deficits. Transl. Psychiatry 2014, 4, e401. [Google Scholar] [CrossRef] [PubMed]
- Drui, G.; Carnicella, S.; Carcenac, C.; Favier, M.; Bertrand, A.; Boulet, S.; Savasta, M. Loss of dopaminergic nigrostriatal neurons accounts for the motivational and affective deficits in Parkinson’s disease. Mol. Psychiatry 2014, 19, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; d’Arc, B.F.; Lafargue, G.; Galanaud, D.; Czernecki, V.; Grabli, D.; Schüpbach, M.; Hartmann, A.; Lévy, R.; Dubois, B.; et al. Disconnecting force from money: Effects of basal ganglia damage on incentive motivation. Brain 2008, 131 Pt 5, 1303–1310. [Google Scholar] [CrossRef]
- Grabli, D.; McCairn, K.; Hirsch, E.C.; Agid, Y.; Féger, J.; François, C.; Tremblay, L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain 2004, 127 Pt 9, 2039–2054. [Google Scholar] [CrossRef]
- Baup, N.; Grabli, D.; Karachi, C.; Mounayar, S.; François, C.; Yelnik, J.; Féger, J.; Tremblay, L. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J. Neurosci. 2008, 28, 8785–8788. [Google Scholar] [CrossRef]
- Worbe, Y.; Baup, N.; Grabli, D.; Chaigneau, M.; Mounayar, S.; McCairn, K.; Féger, J.; Tremblay, L. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb. Cortex 2009, 19, 1844–1856. [Google Scholar] [CrossRef]
- Tremblay, L.; Worbe, Y.; Thobois, S.; Sgambato-Faure, V.; Féger, J. Selective dysfunction of basal ganglia subterritories: From movement to behavioral disorders. Mov. Disord. 2015, 30, 1155–1170. [Google Scholar] [CrossRef]
- Brown, C.A.; Campbell, M.C.; Karimi, M.; Tabbal, S.D.; Loftin, S.K.; Tian, L.L.; Moerlein, S.M.; Perlmutter, J.S. Dopamine pathway loss in nucleus accumbens and ventral tegmental area predicts apathetic behavior in MPTP-lesioned monkeys. Exp. Neurol. 2012, 236, 190–197. [Google Scholar] [CrossRef]
- Tian, L.; Xia, Y.; Flores, H.P.; Campbell, M.C.; Moerlein, S.M.; Perlmutter, J.S. Neuroimaging Analysis of the Dopamine Basis for Apathetic Behaviors in an MPTP-Lesioned Primate Model. PLoS ONE 2015, 10, e0132064. [Google Scholar] [CrossRef] [PubMed]
- Laplane, D.; Baulac, M.; Widlöcher, D.; Dubois, B. Pure psychic akinesia with bilateral lesions of basal ganglia. J. Neurol. Neurosurg. Psychiatry 1984, 47, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Poncet, M. Loss of vitality, of interest and of the affect (athymhormia syndrome) in lacunar lesions of the corpus striatum. Rev. Neurol. 1988, 144, 571–577. [Google Scholar] [PubMed]
- Starkstein, S.E.; Berthier, M.L.; Leiguarda, R. Psychic akinesia following bilateral pallidal lesions. Int. J. Psychiatry Med. 1989, 19, 155–164. [Google Scholar] [CrossRef]
- Reijnders, J.S.A.M.; Scholtissen, B.; Weber, W.E.J.; Aalten, P.; Verhey, F.R.J.; Leentjens, A.F.G. Neuroanatomical correlates of apathy in Parkinson’s disease: A magnetic resonance imaging study using voxel-based morphometry. Mov. Disord. 2010, 25, 2318–2325. [Google Scholar] [CrossRef]
- Kos, C.; van Tol, M.-J.; Marsman, J.-B.C.; Knegtering, H.; Aleman, A. Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci. Biobehav. Rev. 2016, 69, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, E.; Santin, M.D.; Valabrègue, R.; Mangone, G.; Gaurav, R.; Pyatigorskaya, N.; Hutchison, M.; Yahia-Cherif, L.; Villain, N.; Habert, M.-O.; et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 2021, 144, 3114–3125. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A Neural Substrate of Prediction and Reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Schultz, W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013, 23, 229–238. [Google Scholar] [CrossRef]
- Frank, M.J.; Seeberger, L.C.; O’reilly, R.C. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science 2004, 306, 1940–1943. [Google Scholar] [CrossRef]
- Cox, S.M.L.; Frank, M.J.; Larcher, K.; Fellows, L.K.; Clark, C.A.; Leyton, M.; Dagher, A. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage 2015, 109, 95–101. [Google Scholar] [CrossRef]
- Kirschner, M.; Rabinowitz, A.; Singer, N.; Dagher, A. From apathy to addiction: Insights from neurology and psychiatry. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 101, 109926. [Google Scholar] [CrossRef]
- Sokoloff, P.; Le Foll, B. The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 2017, 45, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, D.; Martres, M.P.; Diaz, J.; Griffon, N.; Lammers, C.H.; Sokoloff, P.; Schwartz, J.C. A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 1719–1723. [Google Scholar] [CrossRef]
- Bézard, E.; Ferry, S.; Mach, U.; Stark, H.; Leriche, L.; Boraud, T.; Gross, C.; Sokoloff, P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat. Med. 2003, 9, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Boileau, I.; Guttman, M.; Rusjan, P.; Adams, J.R.; Houle, S.; Tong, J.; Hornykiewicz, O.; Furukawa, Y.; Wilson, A.A.; Kapur, S.; et al. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson’s disease. Brain 2009, 132 Pt 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Czernecki, V.; Schüpbach, M.; Yaici, S.; Lévy, R.; Bardinet, E.; Yelnik, J.; Dubois, B.; Agid, Y. Apathy following subthalamic stimulation in Parkinson disease: A dopamine responsive symptom. Mov. Disord. 2008, 23, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Thobois, S.; Lhommée, E.; Klinger, H.; Ardouin, C.; Schmitt, E.; Bichon, A.; Kistner, A.; Castrioto, A.; Xie, J.; Fraix, V.; et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain 2013, 136 Pt 5, 1568–1577. [Google Scholar] [CrossRef]
- Adam, R.; Leff, A.; Sinha, N.; Turner, C.; Bays, P.; Draganski, B.; Husain, M. Dopamine reverses reward insensitivity in apathy following globus pallidus lesions. Cortex 2013, 49, 1292–1303. [Google Scholar] [CrossRef]
- Manohar, S.G.; Husain, M. Reduced pupillary reward sensitivity in Parkinson’s disease. NPJ Park. Dis. 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, K.; Ben Yehuda, M.; Drew, D.; Manohar, S.; Husain, M. Reward sensitivity and action in Parkinson’s disease patients with and without apathy. Brain Commun. 2021, 3, fcab022. [Google Scholar] [CrossRef]
- Chong, T.T.-J.; Bonnelle, V.; Manohar, S.; Veromann, K.-R.; Muhammed, K.; Tofaris, G.K.; Hu, M.; Husain, M. Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex 2015, 69, 40–46. [Google Scholar] [CrossRef]
- Skvortsova, V.; Degos, B.; Welter, M.-L.; Vidailhet, M.; Pessiglione, M. A Selective Role for Dopamine in Learning to Maximize Reward But Not to Minimize Effort: Evidence from Patients with Parkinson’s Disease. J. Neurosci. 2017, 37, 6087–6097. [Google Scholar] [CrossRef]
- Walton, M.E.; Bouret, S. What Is the Relationship between Dopamine and Effort? Trends Neurosci. 2019, 42, 79–91. [Google Scholar] [CrossRef]
- Sierra, M.; Carnicella, S.; Strafella, A.P.; Bichon, A.; Lhommée, E.; Castrioto, A.; Chabardes, S.; Thobois, S.; Krack, P. Apathy and Impulse Control Disorders: Yin & Yang of Dopamine Dependent Behaviors. J. Park. Dis. 2015, 5, 625–636. [Google Scholar]
- Béreau, M.; Fleury, V.; Bouthour, W.; Castrioto, A.; Lhommée, E.; Krack, P. Hyperdopaminergic behavioral spectrum in Parkinson’s disease: A review. Rev. Neurol. 2018, 174, 653–663. [Google Scholar] [CrossRef]
- Bódi, N.; Kéri, S.; Nagy, H.; Moustafa, A.; Myers, C.E.; Daw, N.; Dibó, G.; Takáts, A.; Bereczki, D.; Gluck, M.A. Reward-learning and the novelty-seeking personality: A between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain 2009, 132 Pt 9, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Lhommée, E.; Boyer, F.; Wack, M.; Pélissier, P.; Klinger, H.; Schmitt, E.; Bichon, A.; Fraix, V.; Chabardès, S.; Mertens, P.; et al. Personality, dopamine, and Parkinson’s disease: Insights from subthalamic stimulation. Mov. Disord. 2017, 32, 1191–1200. [Google Scholar] [CrossRef]
- Steeves, T.D.L.; Miyasaki, J.; Zurowski, M.; Lang, A.E.; Pellecchia, G.; Van Eimeren, T.; Rusjan, P.; Houle, S.; Strafella, A.P. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: A [11C] raclopride PET study. Brain 2009, 132 Pt 5, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.H.; Pavese, N.; Lawrence, A.D.; Tai, Y.F.; Appel, S.; Doder, M.; Brooks, D.J.; Lees, A.J.; Piccini, P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann. Neurol. 2006, 59, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Castrioto, A.; Carnicella, S.; Fraix, V.; Chabardes, S.; Moro, E.; Krack, P. Reversing dopaminergic sensitization. Mov. Disord. 2017, 32, 1679–1683. [Google Scholar] [CrossRef]
- Castrioto, A.; Kistner, A.; Klinger, H.; Lhommée, E.; Schmitt, E.; Fraix, V.; Chabardès, S.; Mertens, P.; Quesada, J.-L.; Broussolle, E.; et al. Psychostimulant effect of levodopa: Reversing sensitisation is possible. J. Neurol. Neurosurg. Psychiatry 2013, 84, 18–22. [Google Scholar] [CrossRef]
- Abbes, M.; Lhommée, E.; Thobois, S.; Klinger, H.; Schmitt, E.; Bichon, A.; Castrioto, A.; Xie, J.; Fraix, V.; Kistner, A.; et al. Subthalamic stimulation and neuropsychiatric symptoms in Parkinson’s disease: Results from a long-term follow-up cohort study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Volkmann, J.; Fasano, A.; Kühn, A.; Krack, P.; Deuschl, G. Changing Gears—DBS For Dopaminergic Desensitization in Parkinson’s Disease? Ann. Neurol. 2021, 90, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.P.; Tsivos, D.; Smith, L.; Knight, B.E.; Bogacz, R.; Whone, A.; Coulthard, E.J. Effects of dopamine on reinforcement learning and consolidation in Parkinson’s disease. Elife 2017, 6, e26801. [Google Scholar] [CrossRef]
- Castrioto, A.; Thobois, S.; Anheim, M.; Quesada, J.L.; Lhommée, E.; Klinger, H.; Bichon, A.; Schmitt, E.; Durif, F.; Azulay, J.P.; et al. A randomized controlled double-blind study of rotigotine on neuropsychiatric symptoms in de novo PD. NPJ Park. Dis. 2020, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.M.; Eisinger, R.S.; Burns, M.R.; Lopes, J.; Okun, M.S.; Gunduz, A.; Bowers, D. Co-occurrence of apathy and impulse control disorders in Parkinson disease. Neurology 2020, 95, e2769–e2780. [Google Scholar] [CrossRef]
- Petitet, P.; Scholl, J.; Attaallah, B.; Drew, D.; Manohar, S.; Husain, M. The relationship between apathy and impulsivity in large population samples. Sci. Rep. 2021, 11, 4830. [Google Scholar] [CrossRef] [PubMed]
- Remy, P.; Doder, M.; Lees, A.; Turjanski, N.; Brooks, D. Depression in Parkinson’s disease: Loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005, 128 Pt 6, 1314–1322. [Google Scholar] [CrossRef]
- Prange, S.; Metereau, E.; Maillet, A.; Klinger, H.; Schmitt, E.; Lhommée, E.; Bichon, A.; Lancelot, S.; Meoni, S.; Broussolle, E.; et al. Limbic Serotonergic Plasticity Contributes to the Compensation of Apathy in Early Parkinson’s Disease. Mov. Disord. 2022, 37, 1211–1221. [Google Scholar] [CrossRef]
- Maillet, A.; Météreau, E.; Tremblay, L.; Favre, E.; Klinger, H.; Lhommée, E.; Le Bars, D.; Castrioto, A.; Prange, S.; Sgambato, V.; et al. Serotonergic and Dopaminergic Lesions Underlying Parkinsonian Neuropsychiatric Signs. Mov. Disord. 2021, 36, 2888–2900. [Google Scholar] [CrossRef]
- Prange, S.; Metereau, E.; Maillet, A.; Lhommée, E.; Klinger, H.; Pelissier, P.; Ibarrola, D.; Heckemann, R.A.; Castrioto, A.; Tremblay, L.; et al. Early limbic microstructural alterations in apathy and depression in de novo Parkinson’s disease. Mov. Disord. 2019, 34, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Florence, G.; Heinsen, H.; Plantinga, B.R.; Temel, Y.; Uludag, K.; Alho, E.; Teixeira, M.J.; Amaro, E.; Fonoff, E.T. Subthalamic Nucleus Deep Brain Stimulation: Basic Concepts and Novel Perspectives. eNeuro 2017, 4, ENEURO.0140-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J. Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural. Netw. 2006, 19, 1120–1136. [Google Scholar] [CrossRef]
- Haynes, W.I.A.; Haber, S.N. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for Basal Ganglia models and deep brain stimulation. J. Neurosci. 2013, 33, 4804–4814. [Google Scholar] [CrossRef]
- Lardeux, S.; Pernaud, R.; Paleressompoulle, D.; Baunez, C. Beyond the reward pathway: Coding reward magnitude and error in the rat subthalamic nucleus. J. Neurophysiol. 2009, 102, 2526–2537. [Google Scholar] [CrossRef]
- Lardeux, S.; Paleressompoulle, D.; Pernaud, R.; Cador, M.; Baunez, C. Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. J. Neurophysiol. 2013, 110, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Baunez, C.; Ravel, S. Neurons in the Monkey’s Subthalamic Nucleus Differentially Encode Motivation and Effort. J. Neurosci. 2022, 42, 2539–2551. [Google Scholar] [CrossRef]
- Vachez, Y.; Carcenac, C.; Magnard, R.; Kerkerian-Le Goff, L.; Salin, P.; Savasta, M.; Carnicella, S.; Boulet, S. Subthalamic Nucleus Stimulation Impairs Motivation: Implication for Apathy in Parkinson’s Disease. Mov. Disord. 2020, 35, 616–628. [Google Scholar] [CrossRef]
- Carcenac, C.; Favier, M.; Vachez, Y.; Lacombe, E.; Carnicella, S.; Savasta, M.; Boulet, S. Subthalamic deep brain stimulation differently alters striatal dopaminergic receptor levels in rats. Mov. Disord. 2015, 30, 1739–1749. [Google Scholar] [CrossRef]
- Vachez, Y.M.; Creed, M.C. Deep Brain Stimulation of the Subthalamic Nucleus Modulates Reward-Related Behavior: A Systematic Review. Front. Hum. Neurosci. 2020, 14, 578564. [Google Scholar] [CrossRef]
- Fife, K.H.; Gutierrez-Reed, N.A.; Zell, V.; Bailly, J.; Lewis, C.M.; Aron, A.R.; Hnasko, T.S. Causal role for the subthalamic nucleus in interrupting behavior. Elife 2017, 6, e27689. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019, 184, 293–316. [Google Scholar] [CrossRef]
- Ricciardi, L.; Morgante, L.; Epifanio, A.; Zibetti, M.; Lanotte, M.; Lopiano, L.; Morgante, F. Stimulation of the subthalamic area modulating movement and behavior. Park. Relat. Disord. 2014, 20, 1298–1300. [Google Scholar] [CrossRef]
- Dafsari, H.S.; Petry-Schmelzer, J.N.; Ray-Chaudhuri, K.; Ashkan, K.; Weis, L.; Dembek, T.A.; Samuel, M.; Rizos, A.; Silverdale, M.; Barbe, M.T.; et al. Non-motor outcomes of subthalamic stimulation in Parkinson’s disease depend on location of active contacts. Brain Stimul. 2018, 11, 904–912. [Google Scholar]
- Irmen, F.; Horn, A.; Mosley, P.; Perry, A.; Petry-Schmelzer, J.N.; Dafsari, H.S.; Barbe, M.; Visser-Vandewalle, V.; Schneider, G.-H.; Li, N.; et al. Left Prefrontal Connectivity Links Subthalamic Stimulation with Depressive Symptoms. Ann. Neurol. 2020, 87, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Boon, L.I.; Potters, W.V.; Zoon, T.J.C.; van den Heuvel, O.A.; Prent, N.; de Bie, R.M.A.; Bot, M.; Schuurman, P.R.; van den Munckhof, P.; Geurtsen, G.J.; et al. Structural and functional correlates of subthalamic deep brain stimulation-induced apathy in Parkinson’s disease. Brain Stimul. 2021, 14, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Prange, S.; Lin, Z.; Nourredine, M.; Danaila, T.; Laurencin, C.; Lagha-Boukbiza, O.; Anheim, M.; Klinger, H.; Longato, N.; Phillipps, C.; et al. Limbic Stimulation Drives Mania in STN-DBS in Parkinson Disease: A Prospective Study. Ann. Neurol. 2022, 92, 411–417. [Google Scholar] [CrossRef]
- Bouthour, W.; Béreau, M.; Kibleur, A.; Zacharia, A.; Tomkova Chaoui, E.; Fleury, V.; Benis, D.; Momjian, S.; Bally, J.; Lüscher, C.; et al. Dyskinesia-inducing lead contacts optimize outcome of subthalamic stimulation in Parkinson’s disease. Mov. Disord. 2019, 34, 1728–1734. [Google Scholar] [CrossRef]
- Cools, R.; Froböse, M.; Aarts, E.; Hofmans, L. Dopamine and the motivation of cognitive control. Handb. Clin. Neurol. 2019, 163, 123–143. [Google Scholar]
- Badre, D.; Nee, D.E. Frontal Cortex and the Hierarchical Control of Behavior. Trends Cogn. Sci. 2018, 22, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, A.; van den Bosch, R.; Määttä, J.I.; Hofmans, L.; Papadopetraki, D.; Cools, R.; Frank, M.J. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science 2020, 367, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Ballanger, B.; Beaudoin-Gobert, M.; Neumane, S.; Epinat, J.; Metereau, E.; Duperrier, S.; Broussolle, E.; Thobois, S.; Bonnefoi, F.; Tourvielle, C.; et al. Imaging Dopamine and Serotonin Systems on MPTP Monkeys: A Longitudinal PET Investigation of Compensatory Mechanisms. J. Neurosci. 2016, 36, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Apps, M.A.J. Motivational fatigue: A neurocognitive framework for the impact of effortful exertion on subsequent motivation. Neuropsychologia 2019, 123, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kok, A. Cognitive control, motivation and fatigue: A cognitive neuroscience perspective. Brain Cogn. 2022, 160, 105880. [Google Scholar] [CrossRef]
- Pavese, N.; Metta, V.; Bose, S.K.; Chaudhuri, K.R.; Brooks, D.J. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 2010, 133, 3434–3443. [Google Scholar] [CrossRef]
- Gargouri, F.; Gallea, C.; Mongin, M.; Pyatigorskaya, N.; Valabregue, R.; Ewenczyk, C.; Sarazin, M.; Yahia-Cherif, L.; Vidailhet, M.; Lehéricy, S. Multimodal magnetic resonance imaging investigation of basal forebrain damage and cognitive deficits in Parkinson’s disease. Mov. Disord. 2019, 34, 516–525. [Google Scholar] [CrossRef]
- van der Zee, S.; Müller, M.L.T.M.; Kanel, P.; van Laar, T.; Bohnen, N.I. Cholinergic Denervation Patterns Across Cognitive Domains in Parkinson’s Disease. Mov. Disord. 2021, 36, 642–650. [Google Scholar]
- Albin, R.L.; van der Zee, S.; van Laar, T.; Sarter, M.; Lustig, C.; Muller, M.L.T.M.; Bohnen, N.I. Cholinergic systems, attentional-motor integration, and cognitive control in Parkinson’s disease. Prog. Brain Res. 2022, 269, 345–371. [Google Scholar]
- Bohnen, N.I.; Kaufer, D.I.; Hendrickson, R.; Constantine, G.M.; Mathis, C.A.; Moore, R.Y. Cortical cholinergic denervation is associated with depressive symptoms in Parkinson’s disease and parkinsonian dementia. J. Neurol. Neurosurg. Psychiatry 2007, 78, 641–643. [Google Scholar] [CrossRef]
- Ray, N.J.; Bradburn, S.; Murgatroyd, C.; Toseeb, U.; Mir, P.; Kountouriotis, G.K.; Teipel, S.J.; Grothe, M.J. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain 2018, 141, 165–176. [Google Scholar] [CrossRef]
- Gang, M.; Baba, T.; Hosokai, Y.; Nishio, Y.; Kikuchi, A.; Hirayama, K.; Hasegawa, T.; Aoki, M.; Takeda, A.; Mori, E.; et al. Clinical and Cerebral Metabolic Changes in Parkinson’s Disease With Basal Forebrain Atrophy. Mov. Disord. 2020, 35, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.-M.; Romenets, S.R.; Anang, J.B.M.; Latreille, V.; Gagnon, J.-F.; Postuma, R.B. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol. 2015, 72, 863–873. [Google Scholar] [PubMed]
- De Pablo-Fernández, E.; Lees, A.J.; Holton, J.L.; Warner, T.T. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019, 76, 470–479. [Google Scholar] [CrossRef]
- Visanji, N.P.; Brotchie, J.M.; Kalia, L.V.; Koprich, J.B.; Tandon, A.; Watts, J.C.; Lang, A.E. α-Synuclein-Based Animal Models of Parkinson’s Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016, 39, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Kirik, D.; Stoyka, L.E.; Standaert, D.G.; Harms, A.S. How can rAAV-α-synuclein and the fibril α-synuclein models advance our understanding of Parkinson’s disease? J. Neurochem. 2016, 139 (Suppl. S1), 131–155. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front. Pharm. 2020, 11, 356. [Google Scholar] [CrossRef]
- Braak, H.; de Vos, R.A.I.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Chung, H.K.; Ho, H.-A.; Pérez-Acuña, D.; Lee, S.-J. Modeling α-Synuclein Propagation with Preformed Fibril Injections. J. Mov. Disord. 2019, 12, 139–151. [Google Scholar] [CrossRef]
- Thakur, P.; Breger, L.S.; Lundblad, M.; Wan, O.W.; Mattsson, B.; Luk, K.C.; Lee, V.M.Y.; Trojanowski, J.Q.; Björklund, A. Modeling Parkinson’s disease pathology by combination of fibril seeds and α-synuclein overexpression in the rat brain. Proc. Natl. Acad. Sci. USA 2017, 114, E8284–E8293. [Google Scholar] [CrossRef]
- Hayley, S.; Vahid-Ansari, F.; Sun, H.; Albert, P.R. Mood disturbances in Parkinson’s disease: From prodromal origins to application of animal models. Neurobiol. Dis. 2023, 181, 106115. [Google Scholar] [CrossRef]
- Leentjens, A.F.G.; Koester, J.; Fruh, B.; Shephard, D.T.S.; Barone, P.; Houben, J.J.G. The effect of pramipexole on mood and motivational symptoms in Parkinson’s disease: A meta-analysis of placebo-controlled studies. Clin. Ther. 2009, 31, 89–98. [Google Scholar] [CrossRef]
- Ray Chaudhuri, K.; Martinez-Martin, P.; Antonini, A.; Brown, R.G.; Friedman, J.H.; Onofrj, M.; Surmann, E.; Ghys, L.; Trenkwalder, C. Rotigotine and specific non-motor symptoms of Parkinson’s disease: Post hoc analysis of RECOVER. Park. Relat. Disord. 2013, 19, 660–665. [Google Scholar] [CrossRef]
- Moreau, C.; Delval, A.; Defebvre, L.; Dujardin, K.; Duhamel, A.; Petyt, G.; Vuillaume, I.; Corvol, J.-C.; Brefel-Courbon, C.; Ory-Magne, F.; et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: A multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012, 11, 589–596. [Google Scholar]
- Zahodne, L.B.; Bernal-Pacheco, O.; Bowers, D.; Ward, H.; Oyama, G.; Limotai, N.; Velez-Lago, F.; Rodriguez, R.L.; Malaty, I.; McFarland, N.R.; et al. Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson’s disease? J. Neuropsychiatry Clin. Neurosci. 2012, 24, 326–330. [Google Scholar] [CrossRef]
- Cugusi, L.; Solla, P.; Serpe, R.; Carzedda, T.; Piras, L.; Oggianu, M.; Gabba, S.; Di Blasio, A.; Bergamin, M.; Cannas, A.; et al. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 2015, 37, 245–254. [Google Scholar] [CrossRef]
- Hashimoto, H.; Takabatake, S.; Miyaguchi, H.; Nakanishi, H.; Naitou, Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: A quasi-randomized pilot trial. Complement. Ther. Med. 2015, 23, 210–219. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Wilhelm, J.; Chen, Y.; Blehm, R.; Nutt, J.; Chen, Z.; Serdar, A.; Horak, F.B. Effects of Group, Individual, and Home Exercise in Persons With Parkinson Disease: A Randomized Clinical Trial. J. Neurol. Phys. Ther. 2015, 39, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Rios Romenets, S.; Anang, J.; Fereshtehnejad, S.-M.; Pelletier, A.; Postuma, R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: A randomized control study. Complement. Ther. Med. 2015, 23, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Hosomi, K.; Shimokawa, T.; Kishima, H.; Oshino, S.; Morris, S.; Kageyama, Y.; Yokoe, M.; Yoshimine, T.; Saitoh, Y. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson’s disease. Brain Stimul. 2013, 6, 884–891. [Google Scholar] [PubMed]
- Wei, W.; Yi, X.; Ruan, J.; Duan, X.; Luo, H. The efficacy of repetitive transcranial magnetic stimulation on emotional processing in apathetic patients with Parkinson’s disease: A Placebo-controlled ERP study. J. Affect. Disord. 2021, 282, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Carriere, N.; Besson, P.; Dujardin, K.; Duhamel, A.; Defebvre, L.; Delmaire, C.; Devos, D. Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: A magnetic resonance imaging shape analysis. Mov. Disord. 2014, 29, 897–903. [Google Scholar] [CrossRef]
- Martínez-Horta, S.; Riba, J.; de Bobadilla, R.F.; Pagonabarraga, J.; Pascual-Sedano, B.; Antonijoan, R.M.; Romero, S.; Mañanas, M.À.; García-Sanchez, C.; Kulisevsky, J. Apathy in Parkinson’s disease: Neurophysiological evidence of impaired incentive processing. J. Neurosci. 2014, 34, 5918–5926. [Google Scholar] [CrossRef]
- Hatz, F.; Meyer, A.; Zimmermann, R.; Gschwandtner, U.; Fuhr, P. Apathy in Patients with Parkinson’s Disease Correlates with Alteration of Left Fronto-Polar Electroencephalographic Connectivity. Front. Aging Neurosci. 2017, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.S.; Bowers, D.; Okun, M.S.; Van Patten, R.; Perlstein, W.M. Apathy, Novelty Processing, and the P3 Potential in Parkinson’s Disease. Front. Neurol. 2016, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Thomas, A.J.; Peraza, L.R.; Firbank, M.; Cromarty, R.; Hamilton, C.A.; Donaghy, P.C.; O’Brien, J.T.; Taylor, J.-P. EEG alpha reactivity and cholinergic system integrity in Lewy body dementia and Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 46. [Google Scholar] [CrossRef]
- Yassine, S.; Gschwandtner, U.; Auffret, M.; Achard, S.; Verin, M.; Fuhr, P.; Hassan, M. Functional Brain Dysconnectivity in Parkinson’s Disease: A 5-Year Longitudinal Study. Mov. Disord. 2022, 37, 1444–1453. [Google Scholar] [CrossRef]
- Kraemmer, J.; Smith, K.; Weintraub, D.; Guillemot, V.; Nalls, M.A.; Cormier-Dequaire, F.; Moszer, I.; Brice, A.; Singleton, A.B.; Corvol, J.-C. Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1106–1111. [Google Scholar] [CrossRef]
- Fagan, E.S.; Pihlstrøm, L. Genetic risk factors for cognitive decline in Parkinson’s disease: A review of the literature. Eur. J. Neurol. 2017, 24, 561-e20. [Google Scholar] [CrossRef]
- Cormier-Dequaire, F.; Bekadar, S.; Anheim, M.; Lebbah, S.; Pelissolo, A.; Krack, P.; Lacomblez, L.; Lhommée, E.; Castrioto, A.; Azulay, J.-P.; et al. Suggestive association between OPRM1 and impulse control disorders in Parkinson’s disease. Mov. Disord. 2018, 33, 1878–1886. [Google Scholar] [CrossRef]
- Pachi, I.; Koros, C.; Simitsi, A.M.; Papadimitriou, D.; Bougea, A.; Prentakis, A.; Papagiannakis, N.; Bozi, M.; Antonelou, R.; Angelopoulou, E.; et al. Apathy: An underestimated feature in GBA and LRRK2 non-manifesting mutation carriers. Park. Relat. Disord. 2021, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Szwedo, A.A.; Dalen, I.; Pedersen, K.F.; Camacho, M.; Bäckström, D.; Forsgren, L.; Tzoulis, C.; Winder-Rhodes, S.; Hudson, G.; Liu, G.; et al. GBA and APOE Impact Cognitive Decline in Parkinson’s Disease: A 10-Year Population-Based Study. Mov. Disord. 2022, 37, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.H.; Alcalay, R.N. Genetics of cognitive dysfunction in Parkinson’s disease. Prog. Brain Res. 2022, 269, 195–226. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béreau, M.; Van Waes, V.; Servant, M.; Magnin, E.; Tatu, L.; Anheim, M. Apathy in Parkinson’s Disease: Clinical Patterns and Neurobiological Basis. Cells 2023, 12, 1599. https://doi.org/10.3390/cells12121599
Béreau M, Van Waes V, Servant M, Magnin E, Tatu L, Anheim M. Apathy in Parkinson’s Disease: Clinical Patterns and Neurobiological Basis. Cells. 2023; 12(12):1599. https://doi.org/10.3390/cells12121599
Chicago/Turabian StyleBéreau, Matthieu, Vincent Van Waes, Mathieu Servant, Eloi Magnin, Laurent Tatu, and Mathieu Anheim. 2023. "Apathy in Parkinson’s Disease: Clinical Patterns and Neurobiological Basis" Cells 12, no. 12: 1599. https://doi.org/10.3390/cells12121599
APA StyleBéreau, M., Van Waes, V., Servant, M., Magnin, E., Tatu, L., & Anheim, M. (2023). Apathy in Parkinson’s Disease: Clinical Patterns and Neurobiological Basis. Cells, 12(12), 1599. https://doi.org/10.3390/cells12121599




