Organoids as Innovative Models for Bone and Joint Diseases
Abstract
1. Introduction
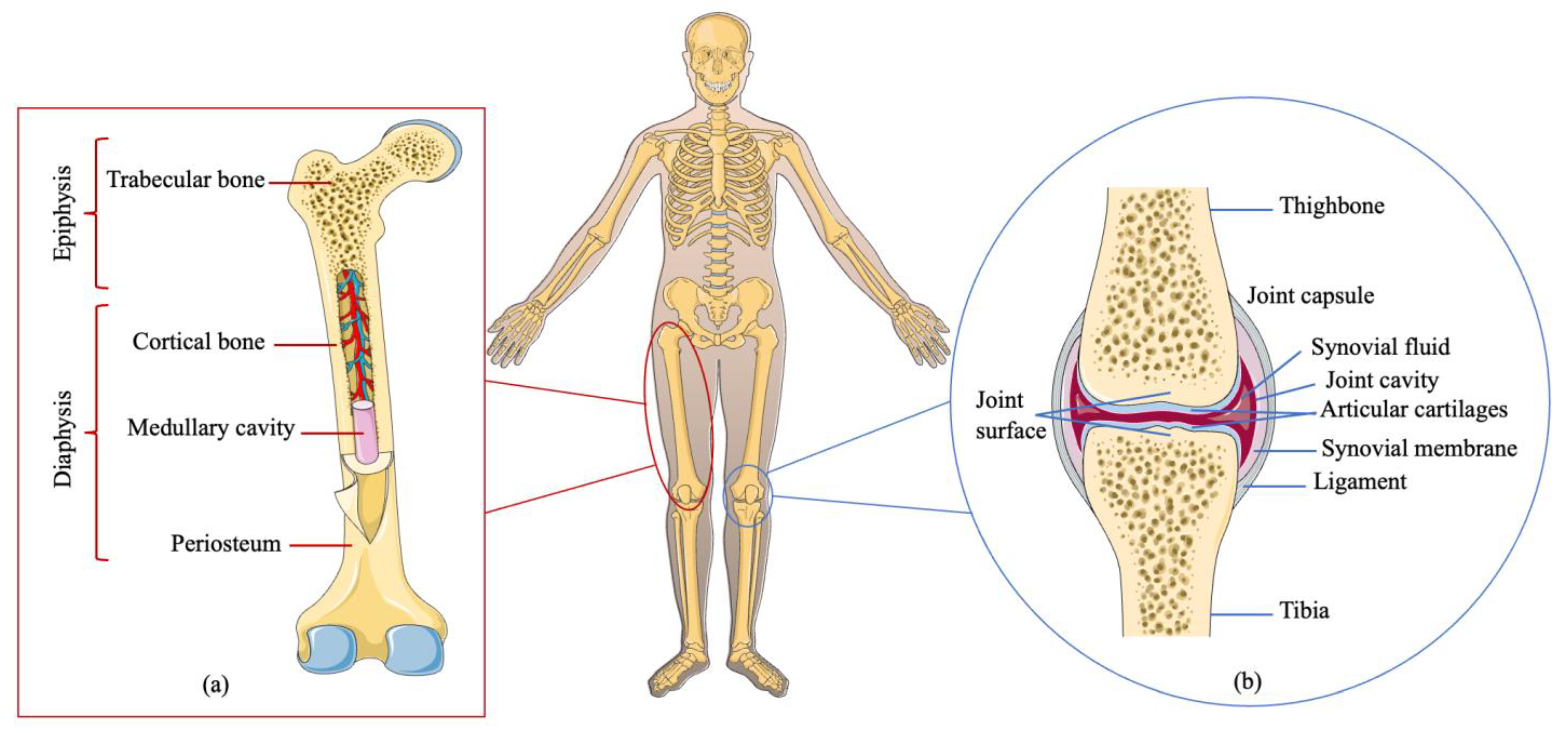
1.1. Bone and Joint Biology
1.2. Comparison of Research Models In Vitro
2. Organoid
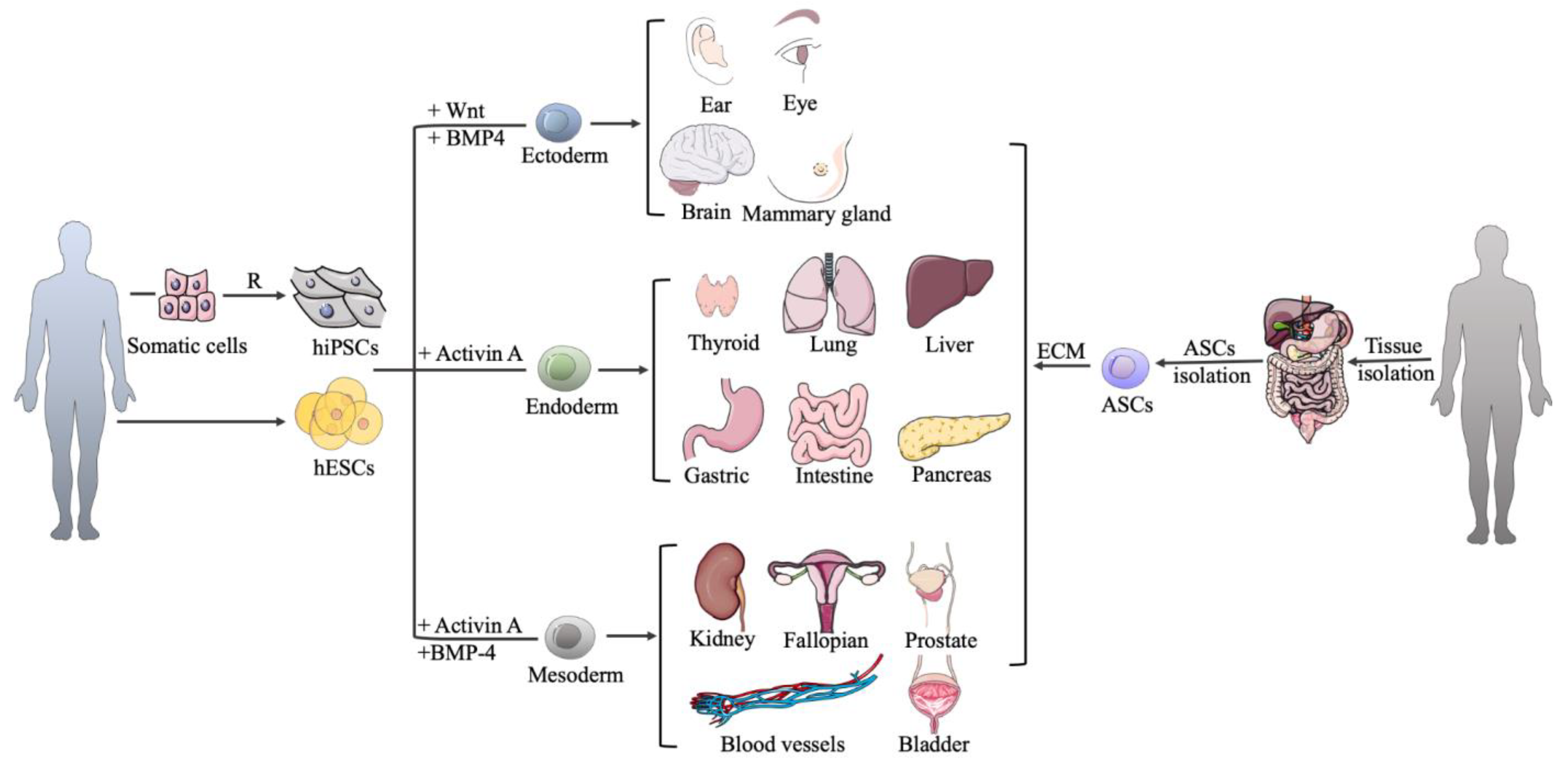
2.1. Cell Sources for Organoid Culture
2.2. Organoid Culture Techniques
2.3. Organoid Culture Techniques
| Organoid Type | Special Media Components # | Culture Techniques | References |
|---|---|---|---|
| The organoids derived from PSCs | |||
| Intestine | activin A, Wnt-3A, FGF-4, EGF, NOG, R-spondin 1, RA, CHIR99021 | Solid matrix; ALI | [42,53,54] |
| Gastric | activin A, Wnt-3A, FGF4, NOG, gastrin, nicotinamide, EGF, RA, CHIR99021, Y-27632 | Solid matrix | [33,55] |
| Liver | activin A, BMP-4, FGF-4, NOG, FGF-2, HGF, Y-27632, CHIR99021 | Solid matrix; ALI | [43,56,57] |
| Pancreas | activin A, BMP-4, FGF-4, Wnt-3A, NOG, Y-27632, CHIR99021, FGF-10, dorsomorphin, LDN193189, SANT-1, RA, EGF, Nicotinamide, KGF, MSC2530818, ZnSO4 | Solid matrix; ALI | [43,58] |
| Lung | Activin A, Wnt-3A, FGF-4, NOG, SB431542, SU5402, SANT-2, SAG, SHH, RA | Solid matrix | [59] |
| Mammary gland | hydrocortisone, insulin, FGF-10, HGF, pTHrP, FGF-2, heparin, prolactin | Suspension | [60] |
| Kidney | Activin A, BMP-4, BMP-7, FGF-2, FGF-8, FGF-9, HGF, IDE-1, JAG-1, NOG, Y-27632, CHIR99021, DAPT, IGF-1, Wnt-4, IWR-1, RA, SB431542 | Suspension; ALI | [61,62] |
| Fallopian | activin A, CHIR99021, Y-27632, BMP-4, Wnt-4, Wnt-3A, follistatin, estrogen, progesterone | Solid matrix | [63] |
| Inner ear | BMP-4, SB431542, FGF-2, LDN193189, Y-27632 | Solid matrix | [64] |
| Thyroid | Activin A, NOG, SB431542, Wnt-3A, KGF, FGF-10, BMP-4, EGF, FGF-2, HSS, IGF-1, insulin, Y-27632, dorsomorphin, CHIR99021, RA, TSH, R-spondin-1 | Solid matrix | [30,65] |
| Blood vessels | Wnt-3A, BMP-4, VEGF-A, CHIR99021, FGF-2, Y-27632, forskolin | Solid matrix | [27] |
| Optic cup | BMP-4, RA, Wnt-3A, Nodal, DAPT | Suspension | [45] |
| Brain | Forebrain: dorsomorphine, A83-01, Wnt-3A, CHIR99021, SB431542, BDNF, GDNF, TGF-β, c-AMP. Midbrain: LDN193189, SB431542, SHH, purmorphamine, FGF-8, CHIR99021, BDNF, GDNF, c-AMP. Hypothalamus: LDN193189, SB431542, 1-Thioglycerol, Wnt-3A, SHH, FGF-2 purmorphamine | Suspension | [47,66,67] |
| The organoids derived from ASCs | |||
| Intestine | EGF, NOG, R-spondin 1, Wnt-3A, JAG-1, Y-27632, CHIR99021, valproic acid | Solid matrix; ALI | [14,41,68] |
| Gastric | EGF, NOG, R-Spondin 1, Wnt-3A, FGF-10, Y-27632 | Solid matrix; ALI | [41,69] |
| Liver | EGF, NOG, R-Spondin 1, Wnt-3A, FGF-10, HGF, nicotinamide, gastrin | Solid matrix | [70] |
| Pancreas | EGF, NOG, R-Spondin 1, Wnt-3A, FGF-10, nicotinamide, Y-27632 | Solid matrix | [69] |
| Lung | Wnt3a, R-spondin 1, and NOG, Y-27632, FGF-7, FGF-10, SB202190, KGF, cAMP, monothioglycerol, CHIR99021, ascorbic acid, dexamethasone, IBMX | Solid matrix; ALI | [44,71] |
| Mammary gland | heparin, EGF, FGF2, insulin, hydrocortisone, cholera toxin, ciproflaxin, Nrg1, NOG, R-spondin 1 | Solid matrix | [72,73] |
| Kidney | EGF, FGF-10, Y-27632, SB431542, A83-01 | Solid matrix | [74] |
| Fallopian | Y-27632, EGF, NOG, FGF-10, nicotinamid, SB431542 | Solid matrix | [75] |
| Bladder | EGF, R-spondin 1, NOG, A83-01, FGF-10, FGF-2, SB202190 | Solid matrix; ALI | [25,76] |
| Prostate | EGF, R-spondin 1, NOG, A83-01, FGF-10, FGF-2, PGE2, SB202190, nicotinamide | Solid matrix | [77] |
| Special Media Components | Functions |
|---|---|
| Activin A, BMP-4 | As important members of the TGF-β superfamily, Activin A and BMP4 can induce the embryonic stem cells to differentiate into different germ layers. |
| Wnt-3A | Wnt-3A modulates embryonic development, cell growth, cell differentiation, and tumorigenesis via the canonical Wnt pathway. |
| Noggin | Noggin regulates the germ-layer-specific derivation of embryonic stem cells and acts as an antagonist of BMP during development. |
| Y-27632 | The ROCK inhibitor improves the survival of human ESC monolayers at the initiation of differentiation. |
| FGF | The FGF family plays a central role during prenatal development, postnatal growth, and the regeneration of a variety of tissuesby promoting cellular proliferation and differentiation. |
| EGF | EGF is a protein that stimulates cell growth and differentiation by binding to its receptor. |
| HGF | HGF has a strong mitogenic ability to regulate cell growth and cell motility on hepatocytes and primary epithelial cells. |
| Gastrin | Gastrin acts as a growth factor in organoid culture and stimulates the proliferation of cells. |
| Retinoic acid | Retinoic acid helps to transform cell types from the proliferative profile to the maturation profile by inducing differentiation. |
| SB202190 | A p38 inhibitor induces cardiomyocyte differentiation from human embryonic stem cells. |
| R-spondin 1 | R-spondin proteins are a secreted agonist of the Wnt/β-catenin signaling pathway. |
| CHIR99021 | The small molecule is the GSK 3 inhibitor and the WNT activator, which can promote the differentiation of insulin-producing cells and cardiomyocytes from human PSCs. |
| Nicotinamide | A water-soluble vitamin is an active component of the coenzymes NAD and NADP and also act as an inhibitor of sirtuins. |
| SANT-1 | SANT-1 is a cell-permeable antagonist that binds directly to smoothened and inhibits the hedgehog signaling to promote β cell differentiation. |
| LDN193189 | A selective BMP signaling inhibitor inhibits the transcriptional activity of the BMP type I receptors ALK2 and ALK3. |
| Dorsomorphin | An inhibitor of the AMPK and BMP pathways is used to promote special cell differentiation. |
| KGF | KGF supports ductal specification by upregulating KRT19 and increasing culture homogeneity. |
| MSC2530818 | A WNT inhibitor increases expression. |
3. Bone-Related Organoid Culture
3.1. Bone-Related Organoids from PSCs
3.2. Bone-Related Organoids from ASCs
3.3. Bone-Related Organoids from Bone Precursor Cells
3.4. Bone-Related Organoids on a Chip
4. Bone Disease Organoid Models
5. Potential Applications of Bone Disease Organoid Models
5.1. Drug Screening
5.2. Precision Medicine
6. Perspectives and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.-L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, A.C.; Kearns, V.R.; Crawford, A.; Hatton, P.V. Skeletal tissue engineering using silk biomaterials. J. Tissue Eng. Regen. Med. 2008, 2, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Hagenmüller, H.; Koch, A.M.; Müller, R.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 2007, 28, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef]
- MacConaill, M.A. Joint; Encyclopedia Britannica: Chicago, IL, USA, 2020. [Google Scholar]
- Jiang, Y.; Tuan, R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef]
- Buckley, C.D.; Ospelt, C.; Gay, S.; Midwood, K.S. Location, location, location: How the tissue microenvironment affects inflammation in RA. Nat. Rev. Rheumatol. 2021, 17, 195–212. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef]
- Akkerman, N.; Defize, L.H. Dawn of the organoid era: 3D tissue and organ cultures revolutionize the study of development, disease, and regeneration. Bioessays 2017, 39, 1600244. [Google Scholar] [CrossRef]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef]
- Arora, N.; Alsous, J.I.; Guggenheim, J.W.; Mak, M.; Munera, J.; Wells, J.M.; Kamm, R.D.; Asada, H.H.; Shvartsman, S.Y.; Griffith, L.G. A process engineering approach to increase organoid yield. Development 2017, 144, 1128–1136. [Google Scholar] [CrossRef]
- Bredenoord, A.L.; Clevers, H.; Knoblich, J.A. Human tissues in a dish: The research and ethical implications of organoid technology. Science 2017, 355, eaaf9414. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Nishinakamura, R. Human kidney organoids: Progress and remaining challenges. Nat. Rev. Nephrol. 2019, 15, 613–624. [Google Scholar] [CrossRef]
- Grenier, K.; Kao, J.; Diamandis, P. Three-dimensional modeling of human neurodegeneration: Brain organoids coming of age. Mol. Psychiatry 2019, 25, 254–274. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid Cultures Derived from Patients with Advanced Prostate Cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Brassard, J.A.; Lutolf, M.P. Engineering Stem Cell Self-organization to Build Better Organoids. Cell Stem Cell 2019, 24, 860–876. [Google Scholar] [CrossRef]
- Aurora, M.; Spence, J.R. hPSC-derived lung and intestinal organoids as models of human fetal tissue. Dev. Biol. 2016, 420, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Thacker, V.V.; Dhar, N.; Cabrer, M.C.; Dubois, A.; Signorino-Gelo, F.; Mullenders, J.; Knott, G.W.; Clevers, H.; McKinney, J.D. Early invasion of the bladder wall by solitary bacteria protects UPEC from antibiotics and neutrophil swarms in an organoid model. Cell Rep. 2021, 36, 109351. [Google Scholar] [CrossRef]
- Drost, J.; Karthaus, W.R.; Gao, D.; Driehuis, E.; Sawyers, C.L.; Chen, Y.; Clevers, H. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 2016, 11, 347–358. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef]
- Otani, T.; Marchetto, M.C.; Gage, F.H.; Simons, B.D.; Livesey, F.J. 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size. Cell Stem Cell 2016, 18, 467–480. [Google Scholar] [CrossRef]
- Capowski, E.E.; Samimi, K.; Mayerl, S.J.; Phillips, M.J.; Pinilla, I.; Howden, S.E.; Saha, J.; Jansen, A.D.; Edwards, K.L.; Jager, L.D.; et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 2019, 146, dev.171686. [Google Scholar] [CrossRef]
- Kurmann, A.A.; Serra, M.; Hawkins, F.; Rankin, S.A.; Mori, M.; Astapova, I.; Ullas, S.; Lin, S.; Bilodeau, M.; Rossant, J.; et al. Faculty Opinions recommendation of Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 2015, 17, 527–542. [Google Scholar] [CrossRef]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 1977, 145, 204–220. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Faculty Opinions recommendation of Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.-H.; Mayhew, C.; Spence, J.R.; Zavros, Y.; et al. Modelling Human Development and Disease in Pluripotent Stem-Cell-Derived Gastric Organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- Simian, M.; Hirai, Y.; Navre, M.; Werb, Z.; Lochter, A.; Bissell, M.J. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001, 128, 3117–3131. [Google Scholar] [CrossRef]
- Linnemann, J.R.; Miura, H.; Meixner, L.K.; Irmler, M.; Kloos, U.J.; Hirschi, B.; Bartsch, H.S.; Sass, S.; Beckers, J.; Theis, F.J.; et al. Quantification of regenerative potential in primary human mammary epithelial cells. Development 2015, 142, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Tsukamoto, Y.; Kujala, P.; Peters, P.J.; Clevers, H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development 2017, 144, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, B.A.; Brekke, J.H.; Vegoe, A.L.; Ulrich, C.B.; Haider, K.T.; Subramaniam, S.; Venhuizen, S.L.; Eide, C.R.; Orchard, P.J.; Chen, W.; et al. Rapid Induction of Cerebral Organoids from Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium. Stem Cells Transl. Med. 2016, 5, 970–979. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Becroft, M.; Vanslambrouck, J.M.; Stanley, E.G.; Elefanty, A.G.; Little, M.H. Faculty Opinions recommendation of Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 2015, 16, 118–126. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Li, X.; Ootani, A.; Kuo, C. An Air–Liquid Interface Culture System for 3D Organoid Culture of Diverse Primary Gastrointestinal Tissues. Methods Mol. Biol. 2016, 1422, 33–40. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Kimura, M.; Kodaka, A.; Nishii, S.; Thompson, W.L.; Takebe, T. Engineering human hepato-biliary-pancreatic organoids from pluripotent stem cells. Nat. Protoc. 2021, 16, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Tindle, C.; Fuller, M.; Fonseca, A.; Taheri, S.; Ibeawuchi, S.R.; Beutler, N.; Katkar, G.D.; Claire, A.; Castillo, V.; Hernandez, M.; et al. Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19. eLife 2021, 10, e66417. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-Organization of Polarized Cerebellar Tissue in 3D Culture of Human Pluripotent Stem Cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 2015, 6, 8896. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Tam, W.L.; Mendes, L.F.; Chen, X.; Lesage, R.; Van Hoven, I.; Leysen, E.; Kerckhofs, G.; Bosmans, K.; Chai, Y.C.; Yamashita, A.; et al. Human pluripotent stem cell-derived cartilaginous organoids promote scaffold-free healing of critical size long bone defects. Stem Cell Res. Ther. 2021, 12, 513. [Google Scholar] [CrossRef]
- Akiva, A.; Melke, J.; Ansari, S.; Liv, N.; van der Meijden, R.; van Erp, M.; Zhao, F.; Stout, M.; Nijhuis, W.H.; de Heus, C.; et al. An Organoid for Woven Bone. Adv. Funct. Mater. 2021, 31, 2010524. [Google Scholar] [CrossRef]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.-F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2016, 23, 49–59. [Google Scholar] [CrossRef]
- McCracken, K.W.; Howell, J.C.; Wells, J.M.; Spence, J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011, 6, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.A.; Ninomiya, N.; Sekine, M.; Komazaki, S.; Wang, P.C.; Asashima, M.; Kurisaki, A. Faculty Opinions recommendation of Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 2015, 17, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.J.; Ryu, J.-S.; Lee, M.-O.; Son, Y.S.; Oh, S.J.; Cho, H.-S.; Son, M.-Y.; Kim, D.-S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.-R.; Dunn, A.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell–Derived Organoids. Gastroenterology 2021, 160, 831–846.e10. [Google Scholar] [CrossRef]
- Breunig, M.; Merkle, J.; Wagner, M.; Melzer, M.K.; Barth, T.F.E.; Engleitner, T.; Krumm, J.; Wiedenmann, S.; Cohrs, C.M.; Perkhofer, L.; et al. Modeling plasticity and dysplasia of pancreatic ductal organoids derived from human pluripotent stem cells. Cell Stem Cell 2021, 28, 1105–1124.e19. [Google Scholar] [CrossRef]
- Miller, A.J.; Dye, B.R.; Ferrer-Torres, D.; Hill, D.R.; Overeem, A.W.; Shea, L.D.; Spence, J.R. Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 2019, 14, 518–540. [Google Scholar] [CrossRef]
- Qu, Y.; Han, B.; Gao, B.; Bose, S.; Gong, Y.; Wawrowsky, K.; Giuliano, A.E.; Sareen, D.; Cui, X. Differentiation of Human Induced Pluripotent Stem Cells to Mammary-like Organoids. Stem Cell Rep. 2017, 8, 205–215. [Google Scholar] [CrossRef]
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valerius, M.T.; Bonventre, J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016, 11, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Yucer, N.; Holzapfel, M.; Vogel, T.J.; Lenaeus, L.; Ornelas, L.; Laury, A.; Sareen, D.; Barrett, R.; Karlan, B.Y.; Svendsen, C.N. Directed Differentiation of Human Induced Pluripotent Stem Cells into Fallopian Tube Epithelium. Sci. Rep. 2017, 7, 10741. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Hashino, E. Generation of Inner Ear Organoids from Human Pluripotent Stem Cells; Academic Press: Cambridge, MA, USA, 2020; Volume 159, pp. 303–321. [Google Scholar] [CrossRef]
- Saito, Y.; Onishi, N.; Takami, H.; Seishima, R.; Inoue, H.; Hirata, Y.; Kameyama, K.; Tsuchihashi, K.; Sugihara, E.; Uchino, S.; et al. Development of a functional thyroid model based on an organoid culture system. Biochem. Biophys. Res. Commun. 2018, 497, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Yin, X.; Farin, H.F.; van Es, J.H.; Clevers, H.; Langer, R.; Karp, J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; Van De Wetering, M.; Snippert, H.J.; Van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Lee, J.-H.; Bhang, D.H.; Beede, A.; Huang, T.L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.-H.; Ryeom, S.; Kim, C.F. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell 2014, 156, 440–455. [Google Scholar] [CrossRef]
- Dravis, C.; Spike, B.T.; Harrell, J.C.; Johns, C.; Trejo, C.L.; Southard-Smith, E.M.; Perou, C.M.; Wahl, G.M. Sox10 Regulates Stem/Progenitor and Mesenchymal Cell States in Mammary Epithelial Cells. Cell Rep. 2015, 12, 2035–2048. [Google Scholar] [CrossRef]
- Centonze, A.; Lin, S.; Tika, E.; Sifrim, A.; Fioramonti, M.; Malfait, M.; Song, Y.; Wuidart, A.; Van Herck, J.; Dannau, A.; et al. Heterotypic cell-cell communication regulates glandular stem cell multipotency. Nature 2020, 584, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, F.; Rookmaaker, M.B.; Margaritis, T.; Rios, A.; Ammerlaan, C.; Jansen, J.; Gijzen, L.; Vormann, M.; Vonk, A.; Viveen, M.; et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 2019, 37, 303–313. [Google Scholar] [CrossRef]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.-J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef]
- Shin, K.; Lee, J.; Guo, N.; Kim, J.; Lim, A.; Qu, L.; Mysorekar, I.U.; Beachy, P.A. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 2011, 472, 110–114. [Google Scholar] [CrossRef]
- Karthaus, W.R.; Iaquinta, P.J.; Drost, J.; Gracanin, A.; van Boxtel, R.; Wongvipat, J.; Dowling, C.M.; Gao, D.; Begthel, H.; Sachs, N.; et al. Identification of Multipotent Luminal Progenitor Cells in Human Prostate Organoid Cultures. Cell 2014, 159, 163–175. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.K.; Katz, M.D.; Oswald, S.J.; Groneck, L.; Guilak, F. Formation of Osteochondral Organoids from Murine Induced Pluripotent Stem Cells. Tissue Eng. Part A 2021, 27, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Biermann, S.; Edwards, C.; Tarnowski, C.; Morris, M.; Long, M.W. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat. Biotechnol. 2000, 18, 954–958. [Google Scholar] [CrossRef]
- Park, Y.; Cheong, E.; Kwak, J.-G.; Carpenter, R.; Shim, J.-H.; Lee, J. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 2021, 7, eabd6495. [Google Scholar] [CrossRef]
- Iordachescu, A.; Hughes, E.A.B.; Joseph, S.; Hill, E.J.; Grover, L.M.; Metcalfe, A.D. Trabecular bone organoids: A micron-scale ‘humanised’ prototype designed to study the effects of microgravity and degeneration. npj Microgravity 2021, 7, 17. [Google Scholar] [CrossRef]
- Torisawa, Y.S.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Faculty Opinions recommendation of Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2021, 11, 663–669. [Google Scholar] [CrossRef]
- Serafini, M.; Sacchetti, B.; Pievani, A.; Redaelli, D.; Remoli, C.; Biondi, A.; Riminucci, M.; Bianco, P. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 2014, 12, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Pievani, A.; Sacchetti, B.; Corsi, A.; Rambaldi, B.; Donsante, S.; Scagliotti, V.; Vergani, P.; Remoli, C.; Biondi, A.; Robey, P.G.; et al. Human umbilical cord blood-borne fibroblasts contain marrow niche precursors that form a bone/marrow organoid in vivo. Development 2017, 144, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, A.; Scherberich, A.; Güven, S.; Theilgaard, N.; Crooijmans, H.; Santini, F.; Scheffler, K.; Zallone, A.; Martin, I. A 3D in vitro bone organ model using human progenitor cells. Eur. Cells Mater. 2011, 21, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.N.; Mendes, L.F.; Gklava, C.; Geris, L.; Luyten, F.P.; Papantoniou, I. Developmentally Engineered Callus Organoid Bioassemblies Exhibit Predictive In Vivo Long Bone Healing. Adv. Sci. 2019, 7, 1902295. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Buttery, L.D.K.; Bourne, S.; Xynos, J.D.; Wood, H.; Hughes, F.J.; Hughes, S.P.F.; Episkopou, V.; Polak, J.M. Differentiation of Osteoblasts and in Vitro Bone Formation from Murine Embryonic Stem Cells. Tissue Eng. 2001, 7, 89–99. [Google Scholar] [CrossRef]
- Bielby, R.C.; Boccaccini, A.R.; Polak, J.M.; Buttery, L.D. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004, 10, 1518–1525. [Google Scholar] [CrossRef]
- Li, Z.A.; Shang, J.; Xiang, S.; Li, E.N.; Yagi, H.; Riewruja, K.; Lin, H.; Tuan, R.S. Articular Tissue-Mimicking Organoids Derived from Mesenchymal Stem Cells and Induced Pluripotent Stem Cells. Organoids 2022, 1, 135–148. [Google Scholar] [CrossRef]
- Alkire, K.; Collingwood, J. Physiology of blood and bone marrow. Semin. Oncol. Nurs. 1990, 6, 99–108. [Google Scholar] [CrossRef]
- Khan, A.O.; Rodriguez-Romera, A.; Reyat, J.S.; Olijnik, A.-A.; Colombo, M.; Wang, G.; Wen, W.X.; Sousos, N.; Murphy, L.C.; Grygielska, B.; et al. Human Bone Marrow Organoids for Disease Modeling, Discovery, and Validation of Therapeutic Targets in Hematologic Malignancies. Cancer Discov. 2023, 13, 364–385. [Google Scholar] [CrossRef]
- Khan, A.; Rodriguez-Romera, A.; Colombo, M.; Reyat, J.S.; Wang, G.; Wen, W.X.; Murphy, L.C.; Ling, R.; Elliott, N.; Sousos, N.; et al. Human Bone Marrow Organoids Enable the Study of Hematopoietic Cell-Stromal Interactions and Support the Survival of Malignant Cells from Patients. Blood 2022, 140, 1679–1681. [Google Scholar] [CrossRef]
- Frenz, S.; Goek, I.; Buser, M.; Salewskij, K.; Fairley, S.; Conca, R.; Drexler, N.; Jonsson, G.; Thomas, M.; Mizoguchi, Y.; et al. Generation of Human Induced Pluripotent Stem Cell-Derived Bone Marrow Organoids. Blood 2022, 140, 1682–1683. [Google Scholar] [CrossRef]
- Giger, S.; Hofer, M.; Miljkovic-Licina, M.; Hoehnel, S.; Brandenberg, N.; Guiet, R.; Ehrbar, M.; Kleiner, E.; Gegenschatz-Schmid, K.; Matthes, T.; et al. Microarrayed human bone marrow organoids for modeling blood stem cell dynamics. APL Bioeng. 2022, 6, 036101. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.J.; Kolaja, K.; Cottrell, J.A. A functional three-dimensional microphysiological human model of myeloma bone disease. J. Bone Miner. Res. 2021, 36, 1914–1930. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Docheva, D.; Knothe, U.R.; Tate, M.L.K. Arthritic Periosteal Tissue from Joint Replacement Surgery: A Novel, Autologous Source of Stem Cells. Stem Cells Transl. Med. 2014, 3, 308–317. [Google Scholar] [CrossRef]
- Han, Y.; Feng, H.; Sun, J.; Liang, X.; Wang, Z.; Xing, W.; Dai, Q.; Yang, Y.; Han, A.; Wei, Z.; et al. Lkb1 deletion in periosteal mesenchymal progenitors induces osteogenic tumors through mTORC1 activation. J. Clin. Investig. 2019, 129, 1895–1909. [Google Scholar] [CrossRef]
- Xie, C.; Liang, R.; Ye, J.; Peng, Z.; Sun, H.; Zhu, Q.; Shen, X.; Hong, Y.; Wu, H.; Sun, W.; et al. High-efficient engineering of osteo-callus organoids for rapid bone regeneration within one month. Biomaterials 2022, 288, 121741. [Google Scholar] [CrossRef]
- Gamblin, A.L.; Renaud, A.; Charrier, C.; Hulin, P.; Louarn, G.; Heymann, D.; Trichet, V.; Layrolle, P. Osteoblastic and osteoclastic differentiation of human mesenchymal stem cells and monocytes in a miniaturized three-dimensional culture with mineral granules. Acta Biomater. 2014, 10, 5139–5147. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Middleton, K.; Al-Dujaili, S.; Mei, X.; Günther, A.; You, L. Microfluidic co-culture platform for investigating osteocyte-osteoclast signalling during fluid shear stress mechanostimulation. J. Biomech. 2017, 59, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.E.; Curtis, M.B.; Sariano, P.A.; Rollins, Z.A.; Shergill, B.S.; Anand, A.; Deely, A.M.; Shirure, V.S.; Anderson, L.; Lowen, J.M.; et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials 2021, 280, 121245. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Ding, J.; Li, Z. Bone-on-a-chip platforms and integrated biosensors: Towards advanced in vitro bone models with real-time biosensing. Biosens. Bioelectron. 2023, 219, 114798. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, C.; Simon, M. In Vitro Human Joint Models Combining Advanced 3D Cell Culture and Cutting-Edge 3D Bioprinting Technologies. Cells 2021, 10, 596. [Google Scholar] [CrossRef]
- Paggi, C.; Venzac, B.; Leijten, J.; Leijten, L.M.T.; Le Gac, S.; Karperien, M. Cartilage-on-chip: A multi-modal platform to study human chondrocyte’s response to mechanical stimuli. Osteoarthr. Cartil. 2020, 28, S176–S177. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Wang, S.; Cao, L.; Zhou, F.; Jing, Y.; Su, J. Bone/cartilage organoid on-chip: Construction strategy and application. Bioact. Mater. 2023, 25, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.A.; Sant, S.; Cho, S.K.; Goodman, S.B.; Bunnell, B.A.; Tuan, R.S.; Gold, M.S.; Lin, H. Synovial joint-on-a-chip for modeling arthritis: Progress, pitfalls, and potential. Trends Biotechnol. 2022, 41, 511–527. [Google Scholar] [CrossRef]
- Wei, X.; Calvo-Vidal, M.N.; Chen, S.; Wu, G.; Revuelta, M.V.; Sun, J.; Zhang, J.; Walsh, M.F.; Nichols, K.E.; Joseph, V.; et al. Germline Lysine-Specific Demethylase 1 (LSD1/KDM1A) Mutations Confer Susceptibility to Multiple Myeloma. Cancer Res. 2018, 78, 2747–2759. [Google Scholar] [CrossRef]
- He, A.; Huang, Y.; Cheng, W.; Zhang, D.; He, W.; Bai, Y.; Gu, C.; Ma, Z.; He, Z.; Si, G.; et al. Organoid culture system for patient-derived lung metastatic osteosarcoma. Med. Oncol. 2020, 37, 105. [Google Scholar] [CrossRef]
- Subramaniam, D.; Angulo, P.; Ponnurangam, S.; Dandawate, P.; Ramamoorthy, P.; Srinivasan, P.; Iwakuma, T.; Weir, S.J.; Chastain, K.; Anant, S. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020, 11, 149. [Google Scholar] [CrossRef]
- Toni, R.; Di Conza, G.; Barbaro, F.; Zini, N.; Consolini, E.; Dallatana, D.; Antoniel, M.; Quarantini, E.; Quarantini, M.; Maioli, S.; et al. Microtopography of Immune Cells in Osteoporosis and Bone Lesions by Endocrine Disruptors. Front. Immunol. 2020, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Guarch-Pérez, C.; Riool, M.; Zaat, S. Current osteomyelitis mouse models, a systematic review. Eur. Cells Mater. 2021, 42, 334–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wijenayaka, A.R.; Solomon, L.B.; Pederson, S.M.; Findlay, D.M.; Kidd, S.P.; Atkins, G.J. Novel Insights into Staphylococcus aureus Deep Bone Infections: The Involvement of Osteocytes. mBio 2018, 9, e00415-18. [Google Scholar] [CrossRef] [PubMed]
- Karve, S.S.; Pradhan, S.; Ward, D.V.; Weiss, A.A. Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS ONE 2017, 12, e0178966. [Google Scholar] [CrossRef]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection. Gastroenterology 2015, 148, 126–136.e6. [Google Scholar] [CrossRef]
- Haisma, E.M.; Rietveld, M.H.; De Breij, A.; Van Dissel, J.T.; El Ghalbzouri, A.; Nibbering, P.H. Inflammatory and Antimicrobial Responses to Methicillin-Resistant Staphylococcus aureus in an In Vitro Wound Infection Model. PLoS ONE 2013, 8, e82800. [Google Scholar] [CrossRef]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D printing of bacteria into functional complex materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef]
- Han, H.S.; Kim, T.W.; Lee, M.C.; Bae, H.C. Direct coculture of human chondrocytes and synovium-derived stem cells enhances in vitro chondrogenesis. Cell J. 2018, 20, 53–60. [Google Scholar] [CrossRef]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.-A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef]
- Muraglia, A.; Corsi, A.; Riminucci, M.; Mastrogiacomo, M.; Cancedda, R.; Bianco, P.; Quarto, R. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J. Cell Sci. 2003, 116, 2949–2955. [Google Scholar] [CrossRef]
- Abraham, D.M.; Herman, C.; Witek, L.; Cronstein, B.N.; Flores, R.L.; Coelho, P.G. Self-assembling human skeletal organoids for disease modeling and drug testing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Del Nery, E.; Ebner, D.; Montoya, M.C.; Östling, P.; Pietiäinen, V.; Price, L.S.; et al. Screening out irrelevant cell-based models of disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Liu, J.R.; Patel, B.; Solomon, D.E.; Vaidya, B.; Gupta, V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng. Transl. Med. 2016, 1, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.N.; Siu, H.C.; Law, S.; Ho, S.L.; Yue, S.S.K.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Ma, S.; Lam, K.O.; et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell 2018, 23, 882–897.e811. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Zilbauer, M. Biobanking of human gut organoids for translational research. Exp. Mol. Med. 2021, 53, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Woo, F.W.; Castro, C.S.; Cohen, M.A.; Karanxha, J.; Mittal, J.; Chhibber, T.; Jhaveri, V.M. Organ-on-chip models: Implications in drug discovery and clinical applications. J. Cell. Physiol. 2019, 234, 8352–8380. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Gordon, R.; Zhang, L.; Bergan, R.; Keene, D.R.; Jones, J.M.; Xie, H.; Chen, Z.; Tao, J.; et al. Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat. Commun. 2019, 10, 3520. [Google Scholar] [CrossRef]
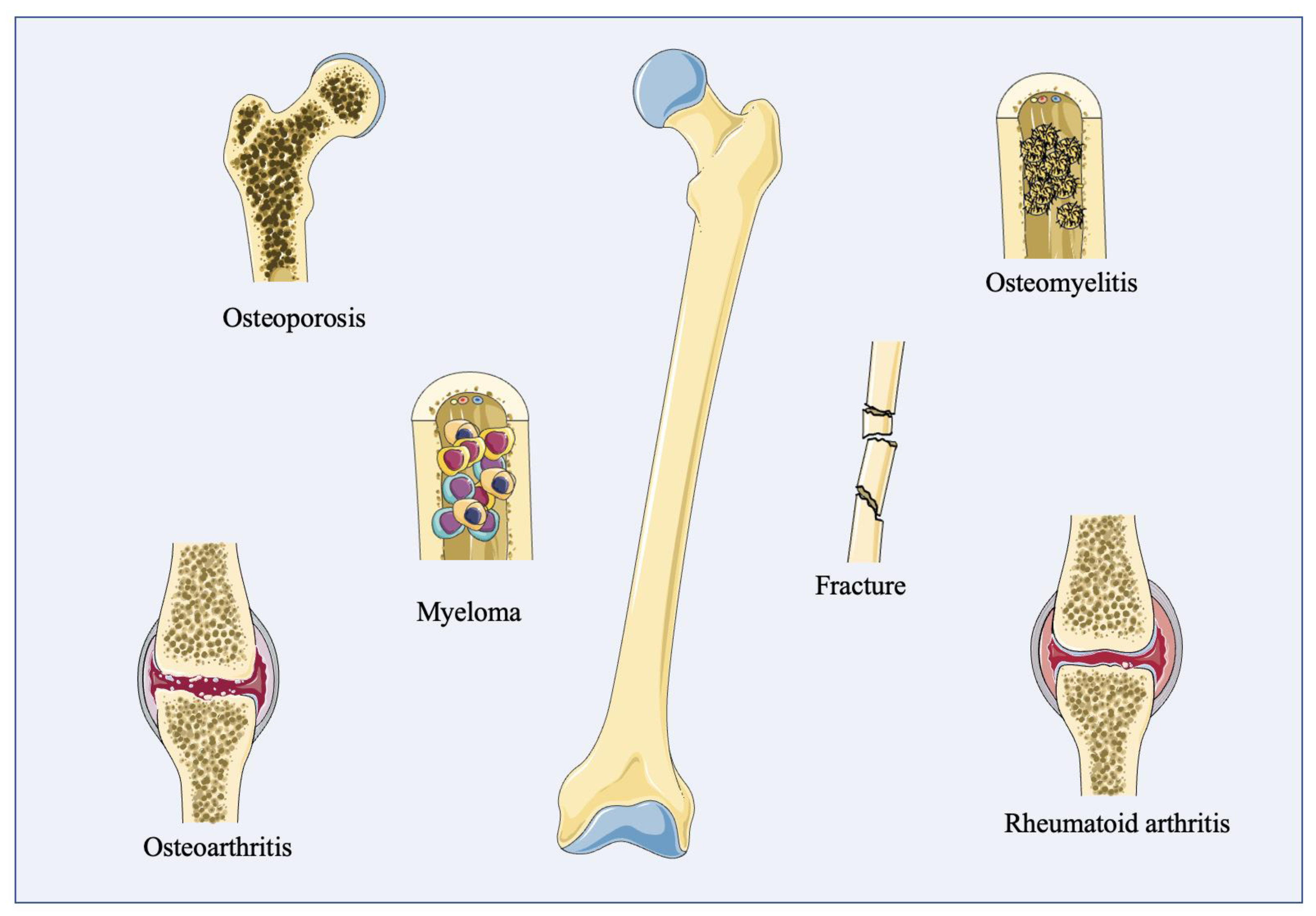
| Disease Types | Features | |
|---|---|---|
| Bone disorders | ||
| Fracture | a break in the bone caused by stress | |
| Osteoporosis | a degenerative disease, characterized by the porosity and brittleness of bone in the aged | |
| Osteomyelitis | an infectious disease caused by the infectious organism Staphylococcus aureus (S. aureus) | |
| Osteomalacia | rickets in adults is caused by the inadequate mineralization of the bone | |
| Osteitis fibrosa cystica | a disease in which bone is replaced by fibrous tissue | |
| Osteosarcoma | a disorder in which bone tissue grows uncontrollably (either malignant or benign) | |
| Multiple myeloma | one of the broadest hematologic cancers, characterized by anemia, hypercalcemia, malignant bone infiltration, increased infectious susceptibility, and kidney failure | |
| Joint disorders | ||
| Osteoarthritis | a ubiquitous noninflammatory degenerative joint disease, characterized by the progressive deterioration of the whole joint | |
| Bursitis | an inflammation of the lubricating sac located around joints, the synovial bursa, or between tendons and muscles or bones | |
| Infectious arthritis | a set of arthritis caused by exposure to certain microorganisms | |
| Rheumatoid arthritis | a chronic, frequently progressive autoinflammatory disease in which inflammation and the thickening of the synovial membranes cause irreversible damage to the joint capsule | |
| Several other types | psoriatic arthritis | resembles rheumatoid arthritis, but lacks rheumatoid factors in the blood |
| ankylosing spondylitis | ||
| 3D Self-Organizing Structures or Organoids | Cells Source | Special Media Components | Culture Techniques | References |
|---|---|---|---|---|
| Human cartilaginous organoids | Human iPSCs or ESCs | CHIR99021, FGF-2, RA, AA, β-Mercaptoethanol, TGF-β1, FGF-2, BMP-2, GDF5 | Solid matrix | [51] |
| Murine osteochondral organoids | murine iPSC | TGF-β3, BMP-2, AA, β-mercaptoethanol, β-glycerophosphate, dexamethasone | Solid matrix | [78] |
| Bone spheroids | Osteoblasts | TGF-β1, ITS+ | - | [79] |
| Woven bone organoids | Human BMSCs | AA, dexamethasone, and β-glycerophosphate | Solid matrix | [52] |
| Trabecular bone organoids | Human osteoblasts and osteoclasts | VD3, PGE2, AA, RANKL, β-glycerophosphate, M-CSF | Solid matrix | [80,81] |
| Bone marrow organoids | human BMSCs or human CB-BFs | 2-phosphate–ascorbic acid, dexamethasone, and TGF-β1 or TGF-β3 | in vivo model | [82,83,84] |
| Bone organoids | Osteoblasts, osteoclasts, endothelial cells | M-CSF, RANKL | Solid matrix | [85] |
| Callus organoid | PDCs | ascorbate-2 phosphate, dexamethasone, proline, Y27632, BMP-2, GDF5, TGF- β1, BMP-6, FGF-2 | Solid matrix | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, L.; Lu, A.; Liang, C. Organoids as Innovative Models for Bone and Joint Diseases. Cells 2023, 12, 1590. https://doi.org/10.3390/cells12121590
Huang J, Zhang L, Lu A, Liang C. Organoids as Innovative Models for Bone and Joint Diseases. Cells. 2023; 12(12):1590. https://doi.org/10.3390/cells12121590
Chicago/Turabian StyleHuang, Jie, Lingqiang Zhang, Aiping Lu, and Chao Liang. 2023. "Organoids as Innovative Models for Bone and Joint Diseases" Cells 12, no. 12: 1590. https://doi.org/10.3390/cells12121590
APA StyleHuang, J., Zhang, L., Lu, A., & Liang, C. (2023). Organoids as Innovative Models for Bone and Joint Diseases. Cells, 12(12), 1590. https://doi.org/10.3390/cells12121590





