Phospholipid Encapsulation of an Anti-Fibrotic Endopeptide to Enhance Cellular Uptake and Myocardial Retention
Abstract
1. Introduction
2. Methods
2.1. Ac-SDKP Liposome Preparation (L-Ac-SDKP), Purification and Characterization
Liposome Composition
2.2. Liposome Preparation
2.3. Liposome Purification
2.4. Liposome Characterization
2.5. Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.6. Fluorescence Confocal Microscopy and Flow Cytometry for Liposome Uptake In Vitro
2.7. Cell Proliferation and Toxicity Assay
2.8. L-Ac-SDKP in Lipopolysaccharide (LPS)-Induced Macrophage Activation
2.9. L-Ac-SDKP in TGFβ1-Induced Cardiac Fibroblast Activation
2.10. Animal Studies
2.11. Non-Invasive IVIS Spectrum Optical Imaging and Enzyme Immunoassay for Liposome Biodistribution
2.12. L-Ac-SDKP Biological Efficacy
2.13. Quantitative Real-Time PCR
2.14. Statistical Analyses
3. Results
3.1. Optimization and Characterization of Liposomes
3.2. Structural Homogeneity of Ac-SDKP-Containing Liposomes
3.3. Enhanced Uptake of L-Ac-SDKP by Cultured Cells
3.4. Effects of L-Ac-SDKP on Cell Survival and Gene Expression Profile in Cultured Macrophages and Cardiac Fibroblasts
3.5. Biodistribution and Bioavailability of Ac-SDKP after i.p. Administration
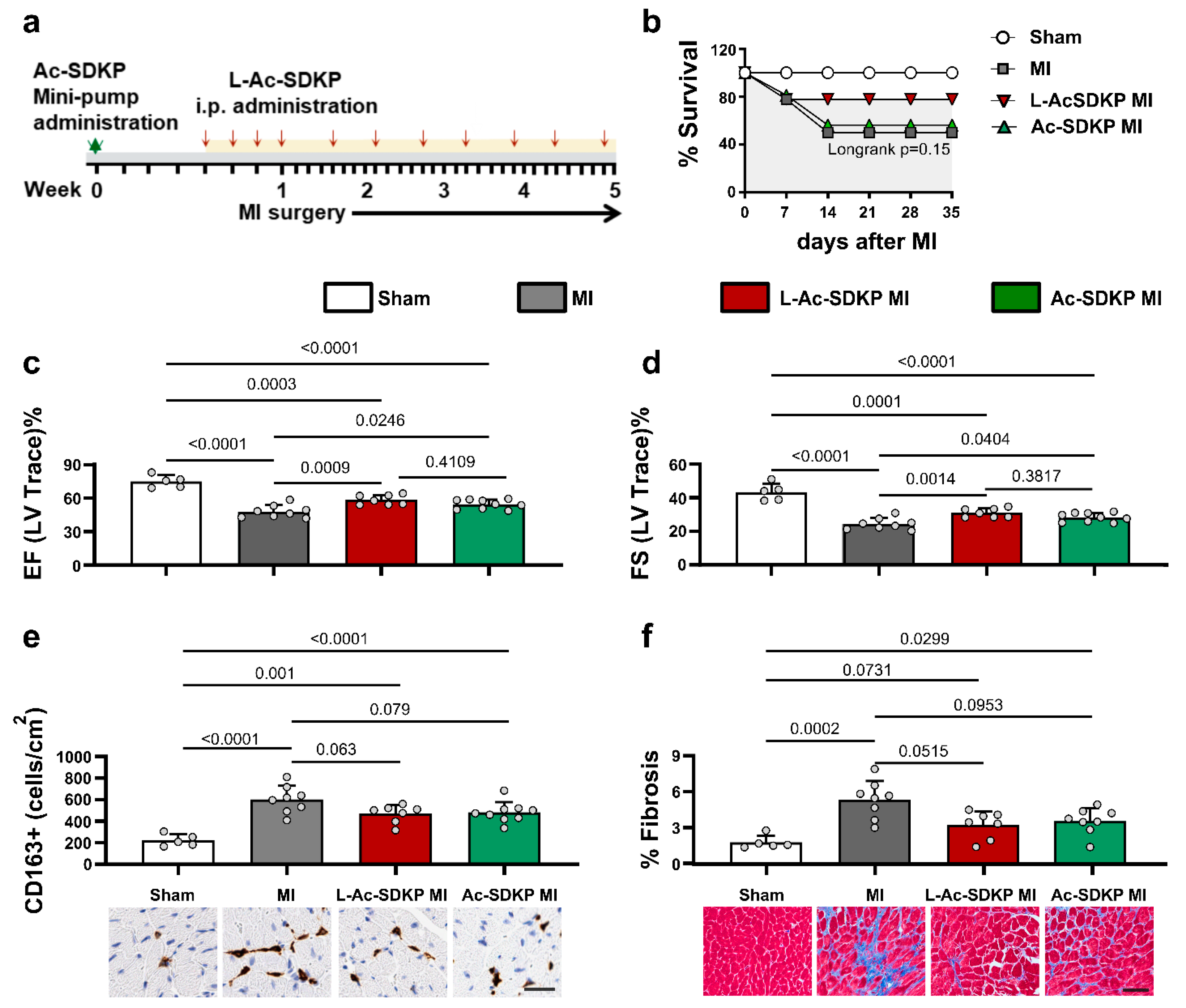
3.6. Effects of L-Ac-SDKP on Cardiac Function after Acute MI
3.7. Effects of L-Ac-SDKP on Myocardial Fibrosis and Macrophage Infiltration after Acute MI
3.8. Effects of L-Ac-SDKP on Fibroinflammatory Gene Expression Profile
4. Discussion
4.1. Liposomes as Vehicles for Stable Ac-SDKP Delivery and Cellular Uptake
4.2. Model of Radiation-Induced Cardiac Injury for L-Ac-SDKP Biodistribution
4.3. Biological Efficacy of L-Ac-SDKP in an Acute MI Model
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cleland, J.G.F.; Pellicori, P.; Gonzalez, A. A novel treatment for heart failure targets myocardial fibrosis. Nat. Med. 2021, 27, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef] [PubMed]
- DeBerge, M.; Shah, S.J.; Wilsbacher, L.; Thorp, E.B. Macrophages in Heart Failure with Reduced versus Preserved Ejection Fraction. Trends Mol. Med. 2019, 25, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Pinto, A.R.; Epelman, S.; Kopecky, B.J.; Clemente-Casares, X.; Godwin, J.; Rosenthal, N.; Kovacic, J.C. The Macrophage in Cardiac Homeostasis and Disease: JACC Macrophage in CVD Series (Part 4). J. Am. Coll. Cardiol. 2018, 72, 2213–2230. [Google Scholar] [CrossRef]
- Sweeney, M.; Corden, B.; Cook, S.A. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: Mirage or miracle? EMBO Mol. Med. 2020, 12, e10865. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Garcia-Banuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, J.; Sandoval-Rodriguez, A.; Monroy-Ramirez, H.C.; Vazquez-Del Mercado, M.; Santos-Garcia, A.; Armendariz-Borunda, J. Prolonged-release pirfenidone prevents obesity-induced cardiac steatosis and fibrosis in a mouse NASH model. Cardiovasc. Drugs Ther. 2021, 35, 927–938. [Google Scholar] [CrossRef]
- Zandbergen, H.R.; Sharma, U.C.; Gupta, S.; Verjans, J.W.; van den Borne, S.; Pokharel, S.; van Brakel, T.; Duijvestijn, A.; van Rooijen, N.; Maessen, J.G.; et al. Macrophage depletion in hypertensive rats accelerates development of cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 68–75. [Google Scholar] [CrossRef]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 821–829. [Google Scholar] [CrossRef]
- Marijic, P.; Schwarzkopf, L.; Schwettmann, L.; Ruhnke, T.; Trudzinski, F.; Kreuter, M. Pirfenidone vs. nintedanib in patients with idiopathic pulmonary fibrosis: A retrospective cohort study. Respir. Res. 2021, 22, 268. [Google Scholar] [CrossRef]
- Richeldi, L.; Fernandez Perez, E.R.; Costabel, U.; Albera, C.; Lederer, D.J.; Flaherty, K.R.; Ettinger, N.; Perez, R.; Scholand, M.B.; Goldin, J.; et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2020, 8, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-beta Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Murphy, A.J.; Dart, A.M. A Clinical Perspective of Anti-Fibrotic Therapies for Cardiovascular Disease. Front. Pharmacol. 2017, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Parichatikanond, W.; Luangmonkong, T.; Mangmool, S.; Kurose, H. Therapeutic Targets for the Treatment of Cardiac Fibrosis and Cancer: Focusing on TGF-beta Signaling. Front. Cardiovasc. Med. 2020, 7, 34. [Google Scholar] [CrossRef]
- Tan, S.M.; Zhang, Y.; Connelly, K.A.; Gilbert, R.E.; Kelly, D.J. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1415–H1425. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Selhub, J.; Jacques, P.; Paynter, N.P.; MacFadyen, J.G.; Glynn, R.J.; Ridker, P.M.; Solomon, D.H. Adverse effects related to methotrexate polyglutamate levels: Adjudicated results from the cardiovascular inflammation reduction trial. Rheumatology 2021, 60, 2963–2968. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Bulkley, B.H.; Roberts, W.C. Steroid therapy during acute myocardial infarction. A cause of delayed healing and of ventricular aneurysm. Am. J. Med. 1974, 56, 244–250. [Google Scholar] [CrossRef]
- Sharma, U.C.; Sonkawade, S.D.; Spernyak, J.A.; Sexton, S.; Nguyen, J.; Dahal, S.; Attwood, K.M.; Singh, A.K.; van Berlo, J.H.; Pokharel, S. A Small Peptide Ac-SDKP Inhibits Radiation-Induced Cardiomyopathy. Circ. Heart Fail. 2018, 11, e004867. [Google Scholar] [CrossRef]
- Pokharel, S.; van Geel, P.P.; Sharma, U.C.; Cleutjens, J.P.; Bohnemeier, H.; Tian, X.L.; Schunkert, H.; Crijns, H.J.; Paul, M.; Pinto, Y.M. Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin-converting enzyme is related to enhanced breakdown of N-acetyl-Ser-Asp-Lys-Pro and increased phosphorylation of Smad2/3. Circulation 2004, 110, 3129–3135. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, P.; Liu, Y.; Liao, T.D.; Chen, X.; Gonzalez, G.E.; Bobbitt, K.R.; Smolarek, D.; Peterson, E.L.; Kedl, R.; Yang, X.P.; et al. Treatment with N-acetyl-seryl-aspartyl-lysyl-proline prevents experimental autoimmune myocarditis in rats. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1114–H1127. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, M.A.; Rhaleb, N.E.; Yang, X.P.; Carretero, O.A. Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 2004, 43, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Carretero, O.A.; Vuljaj, N.; Liao, T.D.; Motivala, A.; Peterson, E.L.; Rhaleb, N.E. Angiotensin-converting enzyme inhibitors: A new mechanism of action. Circulation 2005, 112, 2436–2445. [Google Scholar] [CrossRef]
- Rousseau, A.; Michaud, A.; Chauvet, M.T.; Lenfant, M.; Corvol, P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J. Biol. Chem. 1995, 270, 3656–3661. [Google Scholar] [CrossRef]
- Wang, J.; Qian, Y.; Gao, X.; Mao, N.; Geng, Y.; Lin, G.; Zhang, G.; Li, H.; Yang, F.; Xu, H. Synthesis and Identification of a Novel Peptide, Ac-SDK (Biotin) Proline, That Can Elicit Anti-Fibrosis Effects in Rats Suffering from Silicosis. Drug Des. Devel. Ther. 2020, 14, 4315–4326. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, Y.; Zhang, Z.; Zhang, Y.; Li, M. An analog of Ac-SDKP improves heart functions after myocardial infarction by suppressing alternative activation (M2) of macrophages. Int. J. Cardiol. 2014, 175, 376–378. [Google Scholar] [CrossRef]
- Miranda, D.; Carter, K.; Luo, D.; Shao, S.; Geng, J.; Li, C.; Chitgupi, U.; Turowski, S.G.; Li, N.; Atilla-Gokcumen, G.E.; et al. Multifunctional Liposomes for Image-Guided Intratumoral Chemo-Phototherapy. Adv. Healthc. Mater. 2017, 6, 1700253. [Google Scholar] [CrossRef]
- Kilian, H.I.; Pradhan, A.J.; Jahagirdar, D.; Ortega, J.; Atilla-Gokcumen, G.E.; Lovell, J.F. Light-Triggered Release of Large Biomacromolecules from Porphyrin-Phospholipid Liposomes. Langmuir 2021, 37, 10859–10865. [Google Scholar] [CrossRef]
- Carter, K.A.; Luo, D.; Razi, A.; Geng, J.; Shao, S.; Ortega, J.; Lovell, J.F. Sphingomyelin Liposomes Containing Porphyrin-phospholipid for Irinotecan Chemophototherapy. Theranostics 2016, 6, 2329–2336. [Google Scholar] [CrossRef]
- Azizi, M.; Ezan, E.; Nicolet, L.; Grognet, J.M.; Menard, J. High plasma level of N-acetyl-seryl-aspartyl-lysyl-proline: A new marker of chronic angiotensin-converting enzyme inhibition. Hypertension 1997, 30, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.C.; Sonkawade, S.D.; Baird, A.; Chen, M.; Xu, S.; Sexton, S.; Singh, A.K.; Groman, A.; Turowski, S.G.; Spernyak, J.A.; et al. Effects of a novel peptide Ac-SDKP in radiation-induced coronary endothelial damage and resting myocardial blood flow. Cardiooncology 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xu, J.; Yang, X.P.; Kassem, K.M.; Rhaleb, I.A.; Peterson, E.; Rhaleb, N.E. N-acetyl-seryl-aspartyl-lysyl-proline treatment protects heart against excessive myocardial injury and heart failure in mice. Can. J. Physiol. Pharmacol. 2019, 97, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Hassanabad, A.; Belke, D.D.; Turnbull, J.; Dundas, J.A.; Vasanthan, V.; Teng, G.; Fedak, P.W.M.; Deniset, J.F. An Intact Pericardium Ischemic Rodent Model. J. Vis. Exp. 2021, 175, e62720. [Google Scholar] [CrossRef]
- Mosleh, W.; Chaudhari, M.R.; Sonkawade, S.; Mahajan, S.; Khalil, C.; Frodey, K.; Shah, T.; Dahal, S.; Karki, R.; Katkar, R.; et al. The Therapeutic Potential of Blocking Galectin-3 Expression in Acute Myocardial Infarction and Mitigating Inflammation of Infarct Region: A Clinical Outcome-Based Translational Study. Biomark. Insights 2018, 13, 1177271918771969. [Google Scholar] [CrossRef]
- Sonkawade, S.D.; Pokharel, S.; Karthikeyan, B.; Kim, M.; Xu, S.; Kc, K.; Sexton, S.; Catalfamo, K.; Spernyak, J.A.; Sharma, U.C. Small Endogeneous Peptide Mitigates Myocardial Remodeling in a Mouse Model of Cardioselective Galectin-3 Overexpression. Circ. Heart Fail. 2021, 14, e008510. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Klibanov, A.L.; Huang, L.; O’Donnell, S.; Nossiff, N.D.; Khaw, B.A. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium. FASEB J. 1992, 6, 2716–2719. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Martin, F.J.; Working, P.; Volberding, P.A.; Russell, J.; Newman, M.; Amantea, M.A.; Kaplan, L.D. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: Pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J. Clin. Pharmacol. 1996, 36, 55–63. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Gabizon, A.; Peretz, T.; Sulkes, A.; Amselem, S.; Ben-Yosef, R.; Ben-Baruch, N.; Catane, R.; Biran, S.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Gabizon, A.; Mayhew, E.; Papahadjopoulos, D.; Nir, S.; Goren, D.; Zalipsky, S.; Cohen, R.; Barenholz, Y.; Catane, R. Pharmacokinetic and imaging studies in patients receiving a formulation of liposome-associated adriamycin. Br. J. Cancer 1991, 64, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Wu, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kirchmeier, M.J.; Moase, E.H.; Zalipsky, S.; Allen, T.M. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim. Biophys. Acta 2001, 1515, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Xu, Z.P.; Niebert, M.; Porazik, K.; Walker, T.L.; Cooper, H.M.; Middelberg, A.P.; Gray, P.P.; Bartlett, P.F.; Lu, G.Q. Subcellular compartment targeting of layered double hydroxide nanoparticles. J. Control. Release 2008, 130, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Cauzzo, J.; Nystad, M.; Holsaeter, A.M.; Basnet, P.; Skalko-Basnet, N. Following the Fate of Dye-Containing Liposomes In Vitro. Int. J. Mol. Sci. 2020, 21, 4847. [Google Scholar] [CrossRef] [PubMed]
- Snipstad, S.; Hak, S.; Baghirov, H.; Sulheim, E.; Morch, Y.; Lelu, S.; von Haartman, E.; Back, M.; Nilsson, K.P.R.; Klymchenko, A.S.; et al. Labeling nanoparticles: Dye leakage and altered cellular uptake. Cytom. Part A 2017, 91, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.H.; Yang, X.P.; Xu, J.; Kapke, A.; Carretero, O.A. Myocardial infarction and cardiac remodelling in mice. Exp. Physiol. 2002, 87, 547–555. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Han, W.; Li, J.; Xiang, Y.; Liu, F.; Ma, X.; Zhang, J.; Fu, Z.; Su, Y.D.; et al. Age-related differences in postinfarct left ventricular rupture and remodeling. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1815–H1822. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Rhaleb, N.E.; Pokharel, S.; Harding, P.; Rasoul, S.; Peng, H.; Carretero, O.A. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1226–H1232. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Zhao, M.; Zhang, X.; Chi, J.; Liu, Y.; Lin, F.; Fu, Y.; Ma, D.; Yin, X. Activation in M1 but not M2 Macrophages Contributes to Cardiac Remodeling after Myocardial Infarction in Rats: A Critical Role of the Calcium Sensing Receptor/NRLP3 Inflammasome. Cell. Physiol. Biochem. 2015, 35, 2483–2500. [Google Scholar] [CrossRef]
- Shiraishi, M.; Shintani, Y.; Shintani, Y.; Ishida, H.; Saba, R.; Yamaguchi, A.; Adachi, H.; Yashiro, K.; Suzuki, K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Investig. 2016, 126, 2151–2166. [Google Scholar] [CrossRef]
- Nakagawa, P.; Romero, C.A.; Jiang, X.; D’Ambrosio, M.; Bordcoch, G.; Peterson, E.L.; Harding, P.; Yang, X.P.; Carretero, O.A. Ac-SDKP decreases mortality and cardiac rupture after acute myocardial infarction. PLoS ONE 2018, 13, e0190300. [Google Scholar] [CrossRef]
- Turner, N.A.; Das, A.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Porter, K.E. Interleukin-1alpha stimulates proinflammatory cytokine expression in human cardiac myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1117–H1127. [Google Scholar] [CrossRef]
- Zymek, P.; Nah, D.Y.; Bujak, M.; Ren, G.; Koerting, A.; Leucker, T.; Huebener, P.; Taffet, G.; Entman, M.; Frangogiannis, N.G. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc. Res. 2007, 74, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Schwimmbeck, P.L.; Tschope, C.; Leschka, S.; Husmann, L.; Rutschow, S.; Reichenbach, F.; Noutsias, M.; Kobalz, U.; Poller, W.; et al. Collagen degradation in a murine myocarditis model: Relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc. Res. 2002, 56, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Colucci, W.S. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail. Rev. 2004, 9, 43–51. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonkawade, S.D.; Xu, S.; Kim, M.; Nepali, S.; Karambizi, V.-G.; Sexton, S.; Turowski, S.G.; Li, K.; Spernyak, J.A.; Lovell, J.F.; et al. Phospholipid Encapsulation of an Anti-Fibrotic Endopeptide to Enhance Cellular Uptake and Myocardial Retention. Cells 2023, 12, 1589. https://doi.org/10.3390/cells12121589
Sonkawade SD, Xu S, Kim M, Nepali S, Karambizi V-G, Sexton S, Turowski SG, Li K, Spernyak JA, Lovell JF, et al. Phospholipid Encapsulation of an Anti-Fibrotic Endopeptide to Enhance Cellular Uptake and Myocardial Retention. Cells. 2023; 12(12):1589. https://doi.org/10.3390/cells12121589
Chicago/Turabian StyleSonkawade, Swati D., Shirley Xu, Minhyung Kim, Sarmila Nepali, Victoire-Grace Karambizi, Sandra Sexton, Steven G. Turowski, Kunpeng Li, Joseph A. Spernyak, Jonathan F. Lovell, and et al. 2023. "Phospholipid Encapsulation of an Anti-Fibrotic Endopeptide to Enhance Cellular Uptake and Myocardial Retention" Cells 12, no. 12: 1589. https://doi.org/10.3390/cells12121589
APA StyleSonkawade, S. D., Xu, S., Kim, M., Nepali, S., Karambizi, V.-G., Sexton, S., Turowski, S. G., Li, K., Spernyak, J. A., Lovell, J. F., George, A., Suwal, S., Sharma, U. C., & Pokharel, S. (2023). Phospholipid Encapsulation of an Anti-Fibrotic Endopeptide to Enhance Cellular Uptake and Myocardial Retention. Cells, 12(12), 1589. https://doi.org/10.3390/cells12121589







