Pharmacogenomic Analyses Implicate B Cell Developmental Status and MKL1 as Determinants of Sensitivity toward Anti-CD20 Monoclonal Antibody Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Drugs
2.2. Drug Response Assays
2.3. Gene Expression Analyses
2.4. Genome-Wide Association Studies (GWAS)
2.5. MKL1 Knockdown
2.6. RNA Isolation and qPCR
2.7. Homotypic Aggregation
2.8. Western Blotting
2.9. Flow Cytometry
2.10. Ethidium Homodimer-1 (EthD-1) Dye Uptake
2.11. Isolation of Exosomes by Differential Centrifugation
3. Results
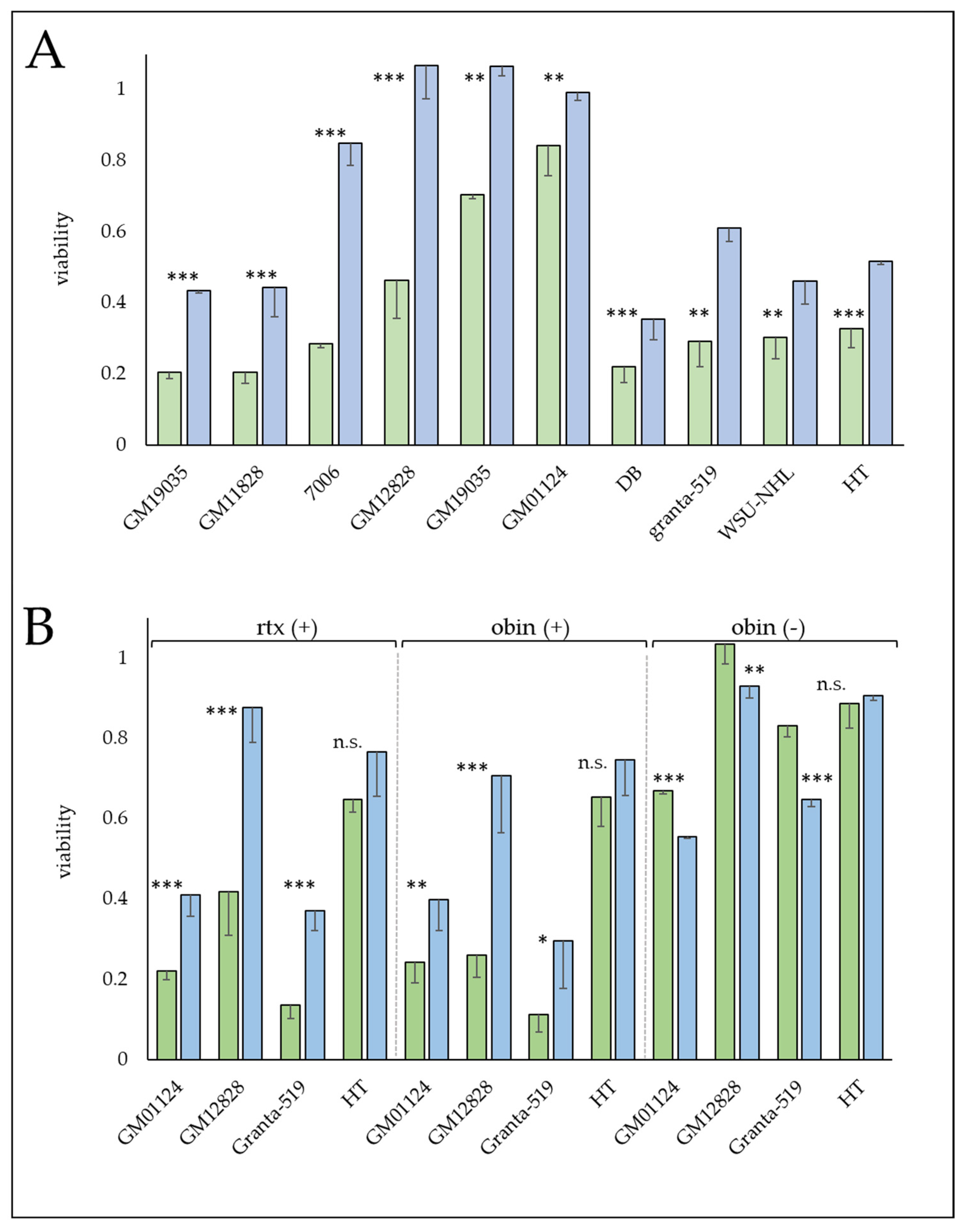
3.1. Lymphoblastoid Cell Line Drug Response Results
3.2. Genome-Wide Association Study (GWAS) Analysis
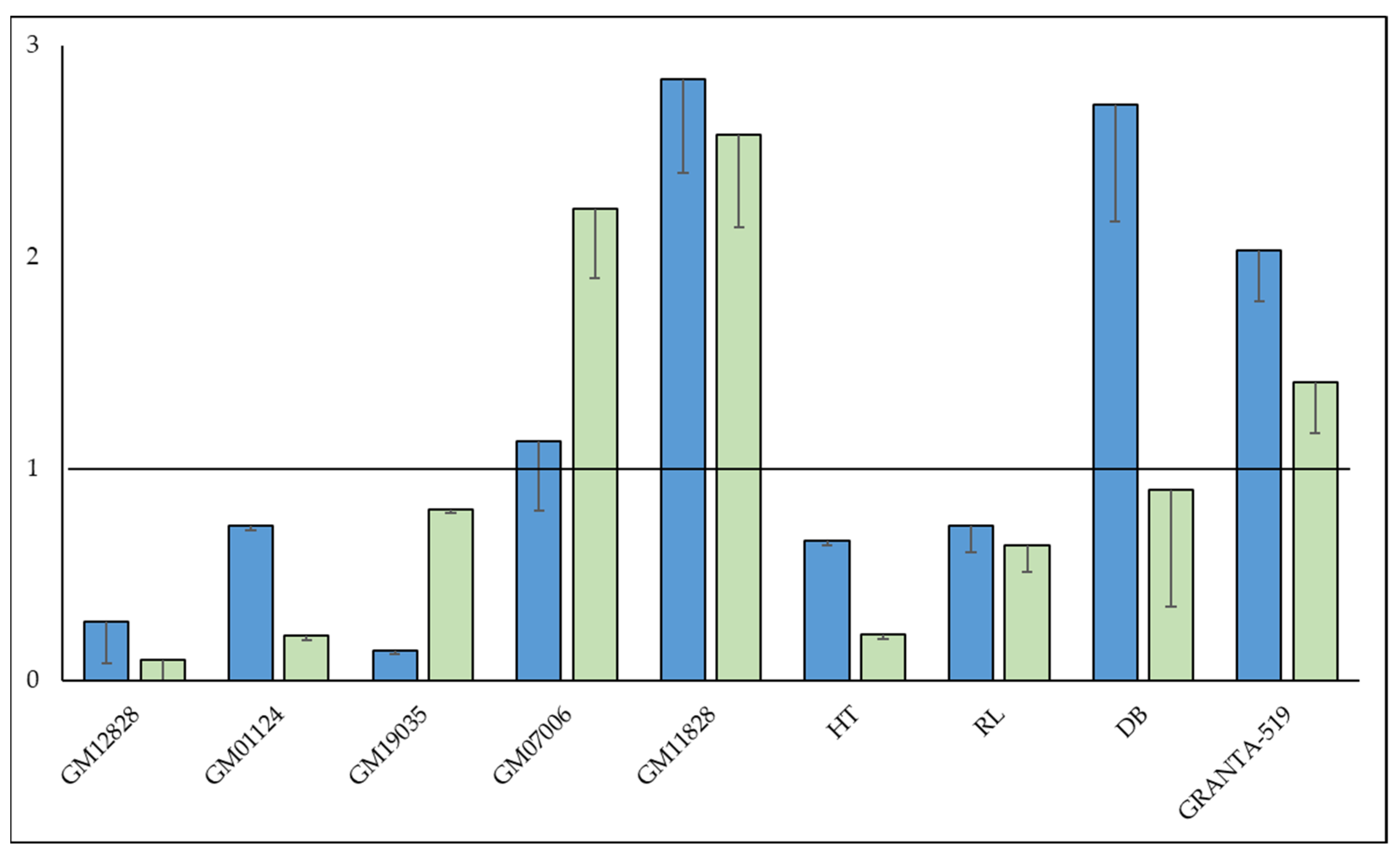
3.3. SNP, rs58600101 Affects MKL1 mRNA Abundance
3.4. MKL1 Knockdown Results in a More Resistant Phenotype
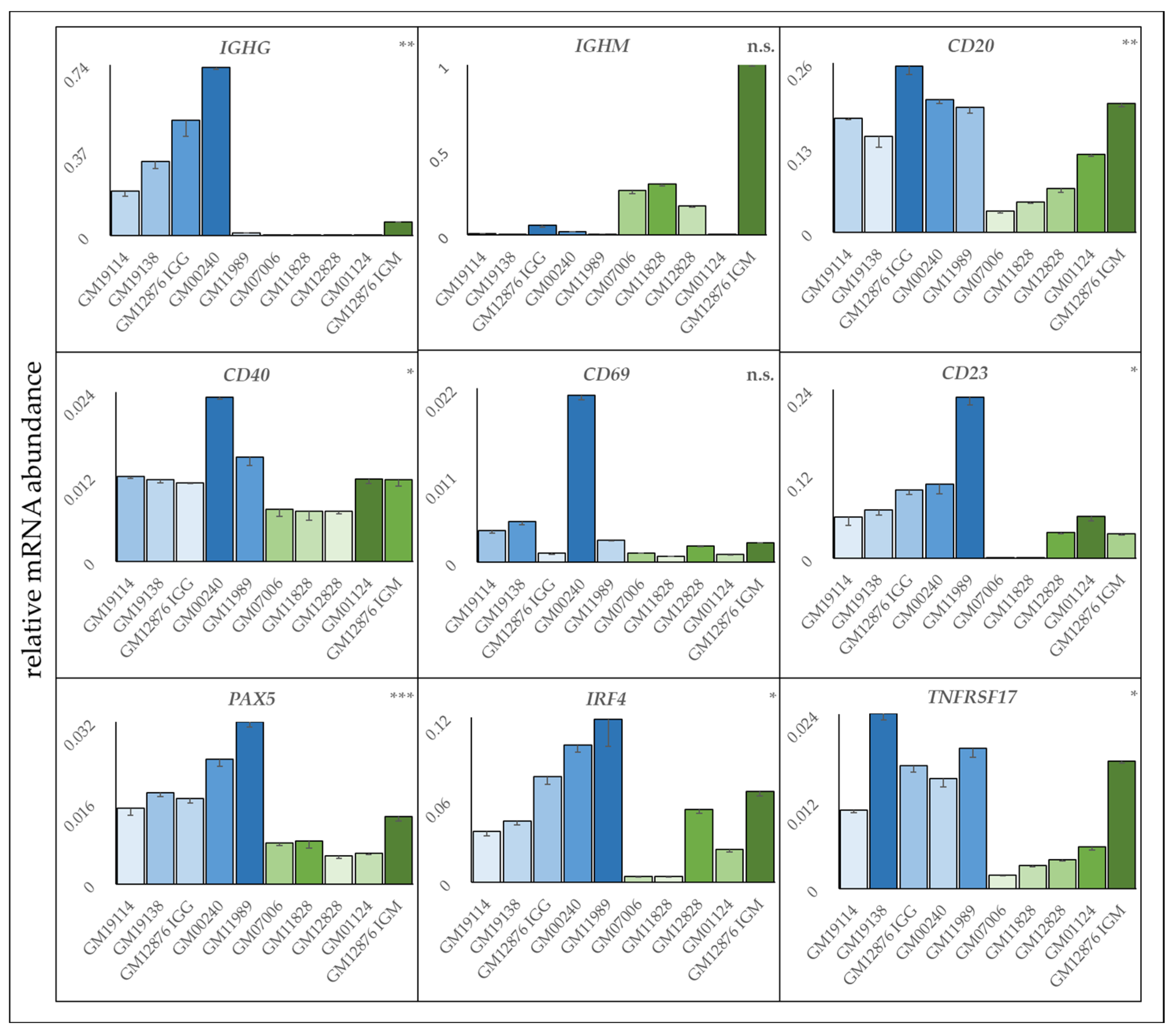
3.5. Gene Expression Analyses Results
3.6. Resistant Cell Lines Exhibit a More Activated B Cell Phenotype
3.7. The Glucocorticoid, Prednisolone Increases Sensitivity to CD20 mAbs
3.8. Protective Mechanisms Affecting Complement-Mediated Anti-CD20 mAb Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tedder, T.F.; Engel, P. CD20: A regulator of cell-cycle progression of B lymphocytes. Immunol. Today 1994, 15, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Nadler, L.M.; Hardy, R.; Schlossman, S.F. Characterization of a human B lymphocyte-specific antigen. J. Immunol. 1980, 125, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Barrett, M.; Foa, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deans, J.P.; Robbins, S.M.; Polyak, M.J.; Savage, J.A. Rapid redistribution of CD20 to a low density detergent-insoluble membrane compartment. J. Biol. Chem. 1998, 273, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.T.; Hughes, D.; French, R.R.; Tutt, A.L.; Walshe, C.A.; Teeling, J.L.; Glennie, M.J.; Cragg, M.S. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 2003, 63, 5480–5489. [Google Scholar]
- Cragg, M.S.; Morgan, S.M.; Chan, H.T.; Morgan, B.P.; Filatov, A.V.; Johnson, P.W.; French, R.R.; Glennie, M.J. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 2003, 101, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Cragg, M.S.; Glennie, M.J. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 2004, 103, 2738–2743. [Google Scholar] [CrossRef] [Green Version]
- Teeling, J.L.; French, R.R.; Cragg, M.S.; van den Brakel, J.; Pluyter, M.; Huang, H.; Chan, C.; Parren, P.W.; Hack, C.E.; Dechant, M.; et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004, 104, 1793–1800. [Google Scholar] [CrossRef]
- Beers, S.A.; French, R.R.; Chan, H.T.; Lim, S.H.; Jarrett, T.C.; Vidal, R.M.; Wijayaweera, S.S.; Dixon, S.V.; Kim, H.; Cox, K.L.; et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: Implications for antibody selection. Blood 2010, 115, 5191–5201. [Google Scholar] [CrossRef] [Green Version]
- Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther. 2013, 12, 2031–2042. [Google Scholar] [CrossRef] [Green Version]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genomes Project, C.; Abecasis, G.R.; Auton, A.; Brooks, L.D.; DePristo, M.A.; Durbin, R.M.; Handsaker, R.E.; Kang, H.M.; Marth, G.T.; McVean, G.A. An integrated map of genetic variation from 1,092 human genomes. Nature 2012, 491, 56–65. [Google Scholar] [CrossRef] [Green Version]
- SoRelle, E.D.; Dai, J.; Bonglack, E.N.; Heckenberg, E.M.; Zhou, J.Y.; Giamberardino, S.N.; Bailey, J.A.; Gregory, S.G.; Chan, C.; Luftig, M.A. Single-cell RNA-seq reveals transcriptomic heterogeneity mediated by host-pathogen dynamics in lymphoblastoid cell lines. Elife 2021, 10, e62586. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.K. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2002, 2, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Petrie, R.J.; Deans, J.P. Colocalization of the B cell receptor and CD20 followed by activation-dependent dissociation in distinct lipid rafts. J. Immunol. 2002, 169, 2886–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyak, M.J.; Li, H.; Shariat, N.; Deans, J.P. CD20 homo-oligomers physically associate with the B cell antigen receptor. Dissociation upon receptor engagement and recruitment of phosphoproteins and calmodulin-binding proteins. J. Biol. Chem. 2008, 283, 18545–18552. [Google Scholar] [CrossRef] [Green Version]
- Bubien, J.K.; Zhou, L.J.; Bell, P.D.; Frizzell, R.A.; Tedder, T.F. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J. Cell Biol. 1993, 121, 1121–1132. [Google Scholar] [CrossRef]
- Li, H.; Ayer, L.M.; Lytton, J.; Deans, J.P. Store-operated cation entry mediated by CD20 in membrane rafts. J. Biol. Chem. 2003, 278, 42427–42434. [Google Scholar] [CrossRef] [Green Version]
- Merkenschlager, J.; Eksmond, U.; Danelli, L.; Attig, J.; Young, G.R.; Nowosad, C.; Tolar, P.; Kassiotis, G. MHC class II cell-autonomously regulates self-renewal and differentiation of normal and malignant B cells. Blood 2019, 133, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, M.; Li, T.; Pihkala, J.P.; Koni, P.A. Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response. J. Immunol. 2006, 176, 2122–2133. [Google Scholar] [CrossRef] [Green Version]
- Record, J.; Sendel, A.; Kritikou, J.S.; Kuznetsov, N.V.; Brauner, H.; He, M.; Nagy, N.; Oliveira, M.M.S.; Griseti, E.; Haase, C.B.; et al. An intronic deletion in megakaryoblastic leukemia 1 is associated with hyperproliferation of B cells in triplets with Hodgkin lymphoma. Haematologica 2020, 105, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, E.J.; Motsinger-Reif, A.; Havener, T.M.; Everitt, L.; Hardison, N.E.; Watson, V.G.; Wagner, M.; Richards, K.L.; Province, M.A.; McLeod, H.L. Pharmacogenomic characterization of US FDA-approved cytotoxic drugs. Pharmacogenomics 2011, 12, 1407–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtari, F.S.; Green, A.J.; Small, G.W.; Havener, T.M.; House, J.S.; Roell, K.R.; Reif, D.M.; McLeod, H.L.; Wiltshire, T.; Motsinger-Reif, A.A. High-throughput screening and genome-wide analyses of 44 anticancer drugs in the 1000 Genomes cell lines reveals an association of the NQO1 gene with the response of multiple anticancer drugs. PLoS Genet. 2021, 17, e1009732. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, T.; Sammeth, M.; Friedlander, M.R.; ‘t Hoen, P.A.; Monlong, J.; Rivas, M.A.; Gonzalez-Porta, M.; Kurbatova, N.; Griebel, T.; Ferreira, P.G.; et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 2013, 501, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [Green Version]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 2 April 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Brown, C.C.; Havener, T.M.; Medina, M.W.; Jack, J.R.; Krauss, R.M.; McLeod, H.L.; Motsinger-Reif, A.A. Genome-wide association and pharmacological profiling of 29 anticancer agents using lymphoblastoid cell lines. Pharmacogenomics 2014, 15, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdo, N.; Xia, M.; Brown, C.C.; Kosyk, O.; Huang, R.; Sakamuru, S.; Zhou, Y.H.; Jack, J.R.; Gallins, P.; Xia, K.; et al. Population-based in vitro hazard and concentration-response assessment of chemicals: The 1000 genomes high-throughput screening study. Environ. Health Perspect. 2015, 123, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, J.; Small, G.W.; Brown, C.C.; Havener, T.M.; McLeod, H.L.; Motsinger-Reif, A.A.; Richards, K.L. Gene expression and linkage analysis implicate CBLB as a mediator of rituximab resistance. Pharm. J. 2018, 18, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; McLeod, H.L.; Richards, K.L. Analysis of innate and acquired resistance to anti-CD20 antibodies in malignant and nonmalignant B cells. PeerJ 2013, 1, e31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, F.; Larsuel, S.T.; Mayday, M.Y.; Scanlon, V.; Krause, D.S. MRTFA: A critical protein in normal and malignant hematopoiesis and beyond. J. Biol. Chem. 2021, 296, 100543. [Google Scholar] [CrossRef]
- Cao, L.; Yoshino, T.; Nishiuchi, R.; Yamadori, I.; Akagi, T. Homotypic cell aggregation via conformational change of CD44 molecule induced by anti-CD44 monoclonal antibodies. Immunobiology 1995, 193, 1–14. [Google Scholar] [CrossRef]
- Barrett, T.B.; Shu, G.; Clark, E.A. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J. Immunol. 1991, 146, 1722–1729. [Google Scholar] [CrossRef]
- Kassambara, A.; Reme, T.; Jourdan, M.; Fest, T.; Hose, D.; Tarte, K.; Klein, B. GenomicScape: An easy-to-use web tool for gene expression data analysis. Application to investigate the molecular events in the differentiation of B cells into plasma cells. PLoS Comput. Biol. 2015, 11, e1004077. [Google Scholar] [CrossRef]
- Hatzoglou, A.; Roussel, J.; Bourgeade, M.F.; Rogier, E.; Madry, C.; Inoue, J.; Devergne, O.; Tsapis, A. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J. Immunol. 2000, 165, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Thorley-Lawson, D.A.; Gross, A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 2004, 350, 1328–1337. [Google Scholar] [CrossRef]
- Katira, A.; Knox, K.A.; Finney, M.; Michell, R.H.; Wakelam, M.; Gordon, J. Inhibition by glucocorticoid and staurosporine of IL-4-dependent CD23 production in B lymphocytes is reversed on engaging CD40. Clin. Exp. Immunol. 1993, 92, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Vaughan, A.T.; Ashton-Key, M.; Williams, E.L.; Dixon, S.V.; Chan, H.T.; Beers, S.A.; French, R.R.; Cox, K.L.; Davies, A.J.; et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011, 118, 2530–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beum, P.V.; Kennedy, A.D.; Williams, M.E.; Lindorfer, M.A.; Taylor, R.P. The shaving reaction: Rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J. Immunol. 2006, 176, 2600–2609. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, A.; Prywes, R. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol. Biol. 2004, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.Z.; Li, S.; Hockemeyer, D.; Sutherland, L.; Wang, Z.; Schratt, G.; Richardson, J.A.; Nordheim, A.; Olson, E.N. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. USA 2002, 99, 14855–14860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.C.; Teixeira, A.M.; Chen, R.C.; Wang, L.; Gao, Y.; Hahn, K.L.; Krause, D.S. Induction of megakaryocyte differentiation drives nuclear accumulation and transcriptional function of MKL1 via actin polymerization and RhoA activation. Blood 2013, 121, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Bros, M.; Haas, K.; Moll, L.; Grabbe, S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 2019, 8, 733. [Google Scholar] [CrossRef] [Green Version]
- Alon, R.; Ley, K. Cells on the run: Shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr. Opin. Cell Biol. 2008, 20, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Sandbo, N.; Kregel, S.; Taurin, S.; Bhorade, S.; Dulin, N.O. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am. J. Respir. Cell Mol. Biol. 2009, 41, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.Z.; Bialik, J.F.; Speight, P.; Dan, Q.; Yeung, T.; Szaszi, K.; Pedersen, S.F.; Kapus, A. TGF-beta1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J. Biol. Chem. 2017, 292, 14902–14920. [Google Scholar] [CrossRef] [Green Version]
- Elberg, G.; Chen, L.; Elberg, D.; Chan, M.D.; Logan, C.J.; Turman, M.A. MKL1 mediates TGF-beta1-induced alpha-smooth muscle actin expression in human renal epithelial cells. Am. J. Physiol. Renal Physiol. 2008, 294, F1116–F1128. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J. Cardiovasc. Transl. Res. 2012, 5, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Fleige, A.; Alberti, S.; Grobe, L.; Frischmann, U.; Geffers, R.; Muller, W.; Nordheim, A.; Schippers, A. Serum response factor contributes selectively to lymphocyte development. J. Biol. Chem. 2007, 282, 24320–24328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehmann, J.A.; LeBien, T.W. Transforming growth factor-beta regulates normal human pre-B cell differentiation. Int. Immunol. 1994, 6, 315–322. [Google Scholar] [CrossRef]
- Kehrl, J.H.; Roberts, A.B.; Wakefield, L.M.; Jakowlew, S.; Sporn, M.B.; Fauci, A.S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J. Immunol. 1986, 137, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Kehrl, J.H.; Thevenin, C.; Rieckmann, P.; Fauci, A.S. Transforming growth factor-beta suppresses human B lymphocyte Ig production by inhibiting synthesis and the switch from the membrane form to the secreted form of Ig mRNA. J. Immunol. 1991, 146, 4016–4023. [Google Scholar] [CrossRef]
- Thomas, S.M.; Hagel, M.; Turner, C.E. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 1999, 112 Pt 2, 181–190. [Google Scholar] [CrossRef]
- Matsuya, M.; Sasaki, H.; Aoto, H.; Mitaka, T.; Nagura, K.; Ohba, T.; Ishino, M.; Takahashi, S.; Suzuki, R.; Sasaki, T. Cell adhesion kinase beta forms a complex with a new member, Hic-5, of proteins localized at focal adhesions. J. Biol. Chem. 1998, 273, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Chodankar, R.; Wu, D.Y.; Schiller, B.J.; Yamamoto, K.R.; Stallcup, M.R. Hic-5 is a transcription coregulator that acts before and/or after glucocorticoid receptor genome occupancy in a gene-selective manner. Proc. Natl. Acad. Sci. USA 2014, 111, 4007–4012. [Google Scholar] [CrossRef] [Green Version]
- Lutz-Nicoladoni, C.; Wolf, D.; Sopper, S. Modulation of Immune Cell Functions by the E3 Ligase Cbl-b. Front. Oncol. 2015, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Qu, X.; Ma, Y.; Liu, Y.; Hu, X. Cbl-b promotes cell detachment via ubiquitination of focal adhesion kinase. Oncol. Lett. 2016, 12, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.E.; Kales, S.C.; Yadavalli, R.; Nau, M.M.; Zhang, H.; Lipkowitz, S. Cbl-c ubiquitin ligase activity is increased via the interaction of its RING finger domain with a LIM domain of the paxillin homolog, Hic 5. PLoS ONE 2012, 7, e49428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, T.; Hinterleitner, R.; Hermann-Kleiter, N.; Meisel, M.; Kleiter, I.; Wang, C.M.; Viola, A.; Pfeifhofer-Obermair, C.; Baier, G. Cbl-b mediates TGFbeta sensitivity by downregulating inhibitory SMAD7 in primary T cells. J. Mol. Cell Biol. 2013, 5, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogan, I.; Bertocci, B.; Vilmont, V.; Delbos, F.; Megret, J.; Storck, S.; Reynaud, C.A.; Weill, J.C. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009, 10, 1292–1299. [Google Scholar] [CrossRef]
- Lutz, J.; Dittmann, K.; Bosl, M.R.; Winkler, T.H.; Wienands, J.; Engels, N. Reactivation of IgG-switched memory B cells by BCR-intrinsic signal amplification promotes IgG antibody production. Nat. Commun. 2015, 6, 8575. [Google Scholar] [CrossRef] [Green Version]
- Reth, M. Antigen Receptors on B Lymphocytes. Annu. Rev. Immunol. 1992, 10, 97–121. [Google Scholar] [CrossRef]
- Martin, S.W.; Goodnow, C.C. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat. Immunol. 2002, 3, 182–188. [Google Scholar] [CrossRef]
- Horikawa, K.; Martin, S.W.; Pogue, S.L.; Silver, K.; Peng, K.; Takatsu, K.; Goodnow, C.C. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J. Exp. Med. 2007, 204, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Meckel, T.; Tolar, P.; Sohn, H.W.; Pierce, S.K. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity 2010, 32, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Chen, X.; Chen, H.; Ji, Q.; Chen, Y.; Wang, J.; Cao, Y.; Wang, F.; Lou, J.; Tang, Z.; et al. The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. Elife 2015, 4, e06925. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Wan, Z.; Sun, X.; Lu, Y.; Huang, J.; Wang, F.; Zeng, Y.; Chen, Y.H.; Shi, Y.; et al. Profiling the origin, dynamics, and function of traction force in B cell activation. Sci. Signal. 2018, 11, eaai9192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casan, J.M.L.; Wong, J.; Northcott, M.J.; Opat, S. Anti-CD20 monoclonal antibodies: Reviewing a revolution. Hum. Vaccin Immunother. 2018, 14, 2820–2841. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.J.E.; Stopforth, R.J.; Cragg, M.S. Therapeutic Antibodies: What Have We Learnt from Targeting CD20 and Where Are We Going? Front. Immunol. 2017, 8, 1245. [Google Scholar] [CrossRef] [Green Version]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front Oncol 2018, 8, 163. [Google Scholar] [CrossRef] [PubMed]


| Complement Present | Drug (mAb) | Chr | SNP | p-Value | Consequence | Host Gene Symbol | Host Gene Ensembl ID |
|---|---|---|---|---|---|---|---|
| + | ofatumumab | 22 | rs58600101 | 4.654 × 10−8 | intron_variant, regulatory_region_variant | MKL1 (MRTFA) | ENSG00000196588 |
| + | ofatumumab | 17 | rs115172145 | 5.369 × 10−8 | intron_variant | PRPSAP1 | ENSG00000161542 |
| + | rituximab | 13 | rs9542996 | 1.272 × 10−7 | intergenic_variant | - | - |
| + | ofatumumab | 5 | rs111502792 | 1.291 × 10−7 | intron_variant | KCTD16 | ENSG00000183775 |
| + | obinutuzumab | 13 | rs9540041 | 1.797 × 10−7 | intergenic_variant | - | - |
| + | ofatumumab | 13 | rs114465368 | 8.115 × 10−7 | intergenic_variant | - | - |
| + | ofatumumab | 13 | rs116833413 | 8.125 × 10−7 | intergenic_variant | - | - |
| − | obinutuzumab | 9 | rs3904461 | 1.174 × 10−8 | intergenic_variant | - | - |
| − | obinutuzumab | 15 | rs77545126 | 3.218 × 10−7 | intron_variant | SCAMP5 | ENSG00000198794 |
| − | obinutuzumab | 14 | rs75671053 | 3.254 × 10−7 | intron_variant | AKAP6 | ENSG00000151320 |
| − | obinutuzumab | 4 | rs58361570 | 3.516 × 10−7 | intergenic_variant | - | - |
| − | obinutuzumab | 15 | rs60910940 | 3.582 × 10−7 | synonymous_variant | SCAMP5 | ENSG00000198794 |
| − | ofatumumab | 7 | rs79213207 | 7.742 × 10−7 | intergenic_variant | - | - |
| − | obinutuzumab | 13 | rs80101498 | 7.908 × 10−7 | intergenic_variant | - | - |
| − | obinutuzumab | 11 | rs6579002 | 8.263 × 10−7 | intergenic_variant | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Small, G.W.; Akhtari, F.S.; Green, A.J.; Havener, T.M.; Sikes, M.; Quintanhila, J.; Gonzalez, R.D.; Reif, D.M.; Motsinger-Reif, A.A.; McLeod, H.L.; et al. Pharmacogenomic Analyses Implicate B Cell Developmental Status and MKL1 as Determinants of Sensitivity toward Anti-CD20 Monoclonal Antibody Therapy. Cells 2023, 12, 1574. https://doi.org/10.3390/cells12121574
Small GW, Akhtari FS, Green AJ, Havener TM, Sikes M, Quintanhila J, Gonzalez RD, Reif DM, Motsinger-Reif AA, McLeod HL, et al. Pharmacogenomic Analyses Implicate B Cell Developmental Status and MKL1 as Determinants of Sensitivity toward Anti-CD20 Monoclonal Antibody Therapy. Cells. 2023; 12(12):1574. https://doi.org/10.3390/cells12121574
Chicago/Turabian StyleSmall, George W., Farida S. Akhtari, Adrian J. Green, Tammy M. Havener, Michael Sikes, Julia Quintanhila, Ricardo D. Gonzalez, David M. Reif, Alison A. Motsinger-Reif, Howard L. McLeod, and et al. 2023. "Pharmacogenomic Analyses Implicate B Cell Developmental Status and MKL1 as Determinants of Sensitivity toward Anti-CD20 Monoclonal Antibody Therapy" Cells 12, no. 12: 1574. https://doi.org/10.3390/cells12121574
APA StyleSmall, G. W., Akhtari, F. S., Green, A. J., Havener, T. M., Sikes, M., Quintanhila, J., Gonzalez, R. D., Reif, D. M., Motsinger-Reif, A. A., McLeod, H. L., & Wiltshire, T. (2023). Pharmacogenomic Analyses Implicate B Cell Developmental Status and MKL1 as Determinants of Sensitivity toward Anti-CD20 Monoclonal Antibody Therapy. Cells, 12(12), 1574. https://doi.org/10.3390/cells12121574








