Non-Vesicular Release of Alarmin Prothymosin α Complex Associated with Annexin-2 Flop-Out
Abstract
:1. Introduction
2. Various Modes of Extracellular Release
2.1. Classical Release Modes for Biologically Active Molecules
2.2. Non-Classical Constitutive Vesicular Release
2.3. Non-Classical and Non-Vesicular Release Mediated via GSDMD and MLKL Pore Formation
3. New Type of Non-Classical and Non-Vesicular Release
3.1. Identification of Prothymosin α Causing Cell Death Mode Switch
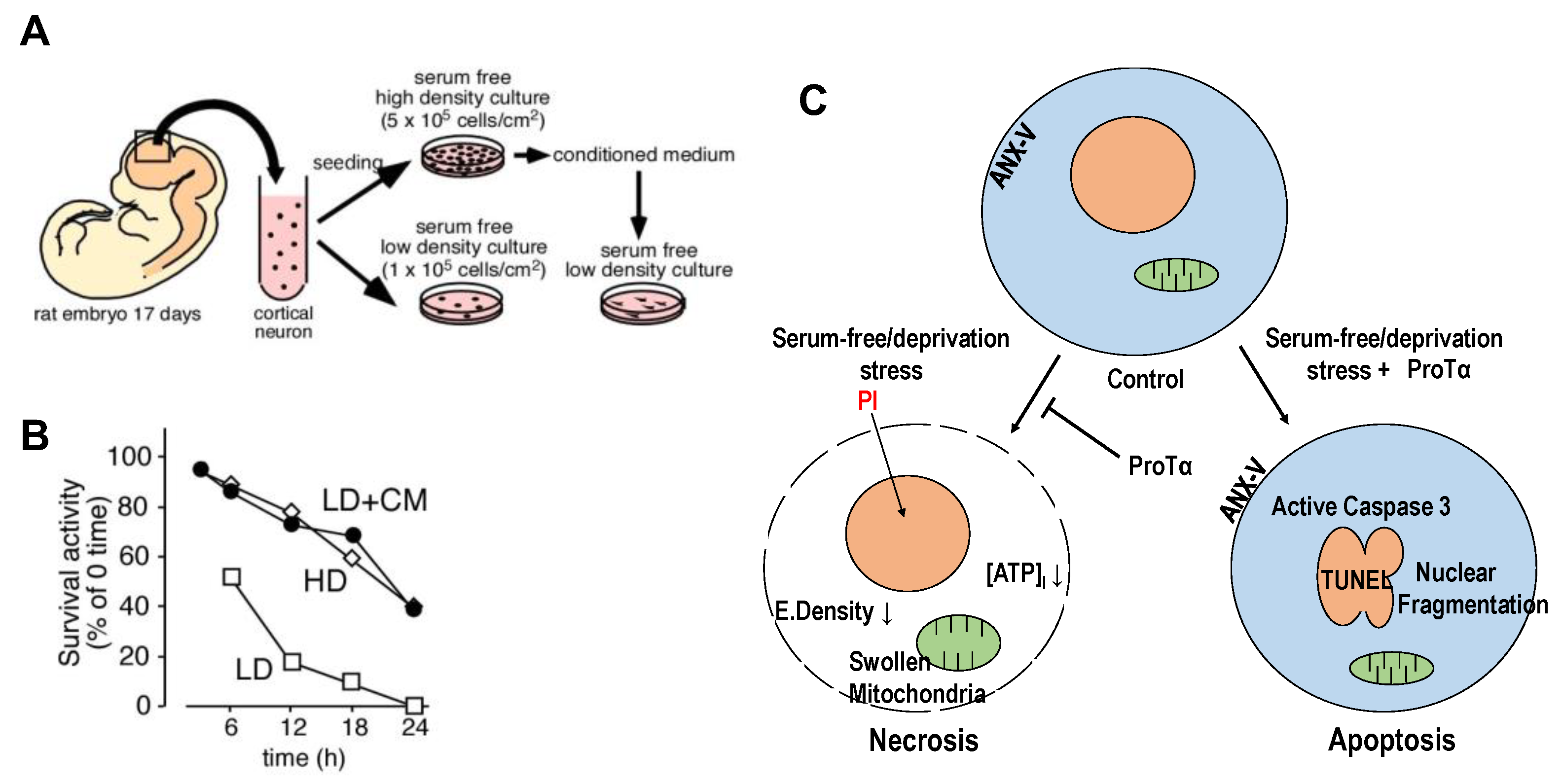
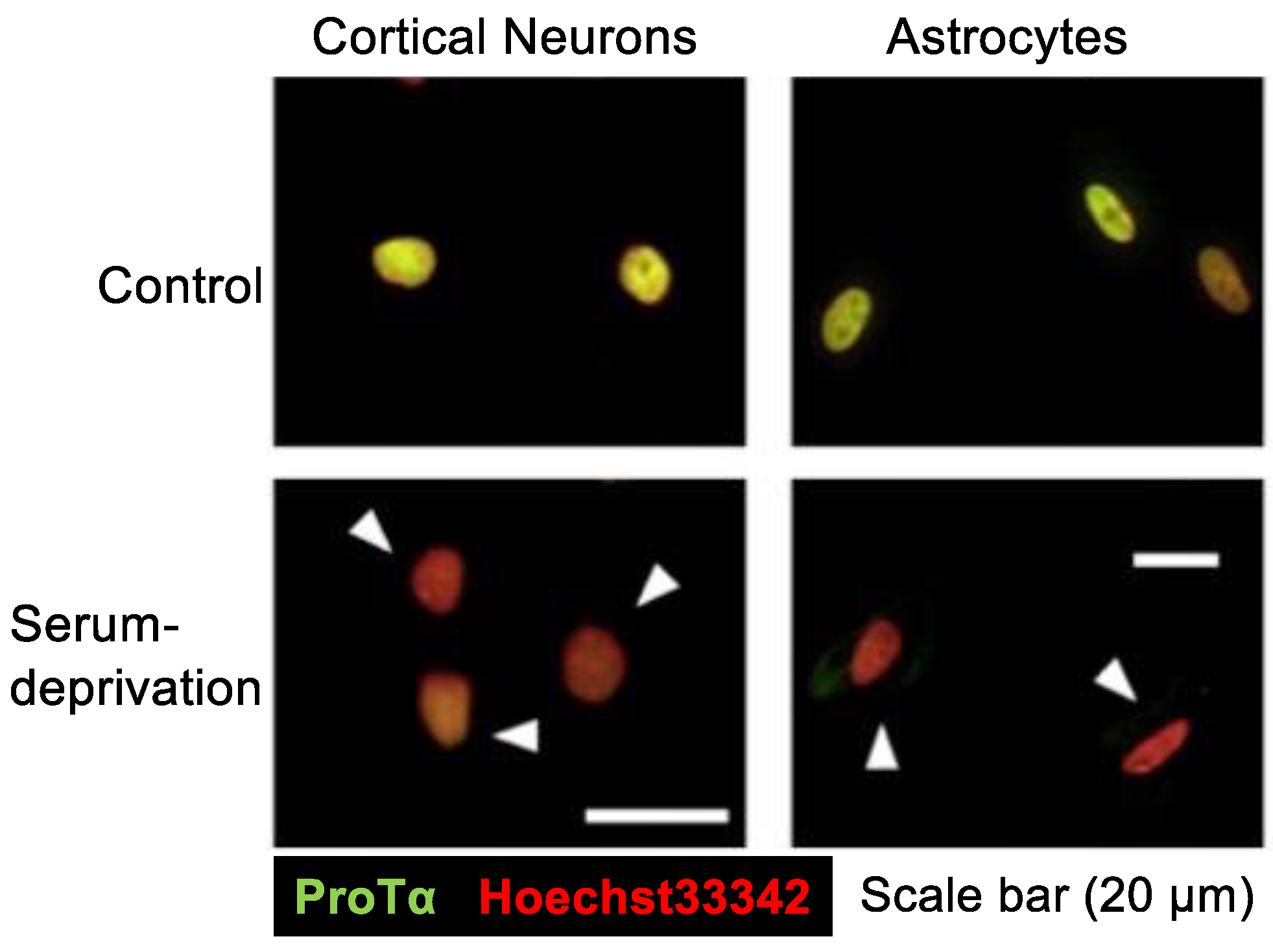
3.2. Serum-Free Starvation-Induced Extracellular Release of ProTα
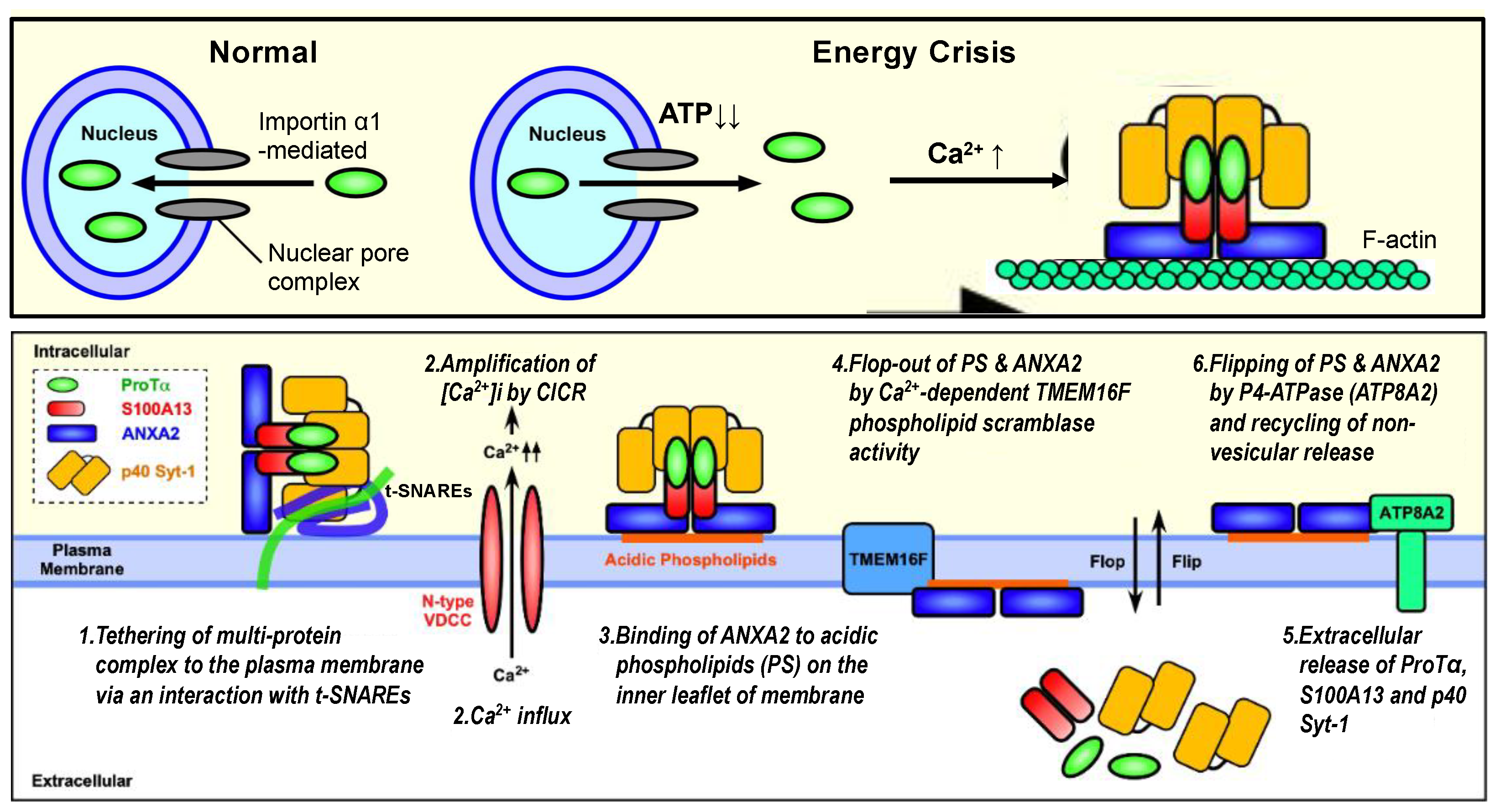
3.2.1. Ischemic ATP Loss-Induced ProTα Release from the Nucleus
3.2.2. Ca2+-Dependent ProTα–S100A13 Interaction
3.2.3. Anti-Apoptosis Action of Cytosol ProTα
3.2.4. Ca2+-Dependent Interaction between S100A13 and p40 Syt-1
3.2.5. Syt-1 Involved in the Release of ProTα and S100A13
3.2.6. Interaction between p40 Syt-1 and Target SNARE Syntaxin-1
3.2.7. p40-Syt-1-Stimulation of S100A13 Interaction with Annexin A2
3.2.8. Annexin A2 Flop-Out
3.2.9. Possible Machineries of ANXA2 Flop-Out
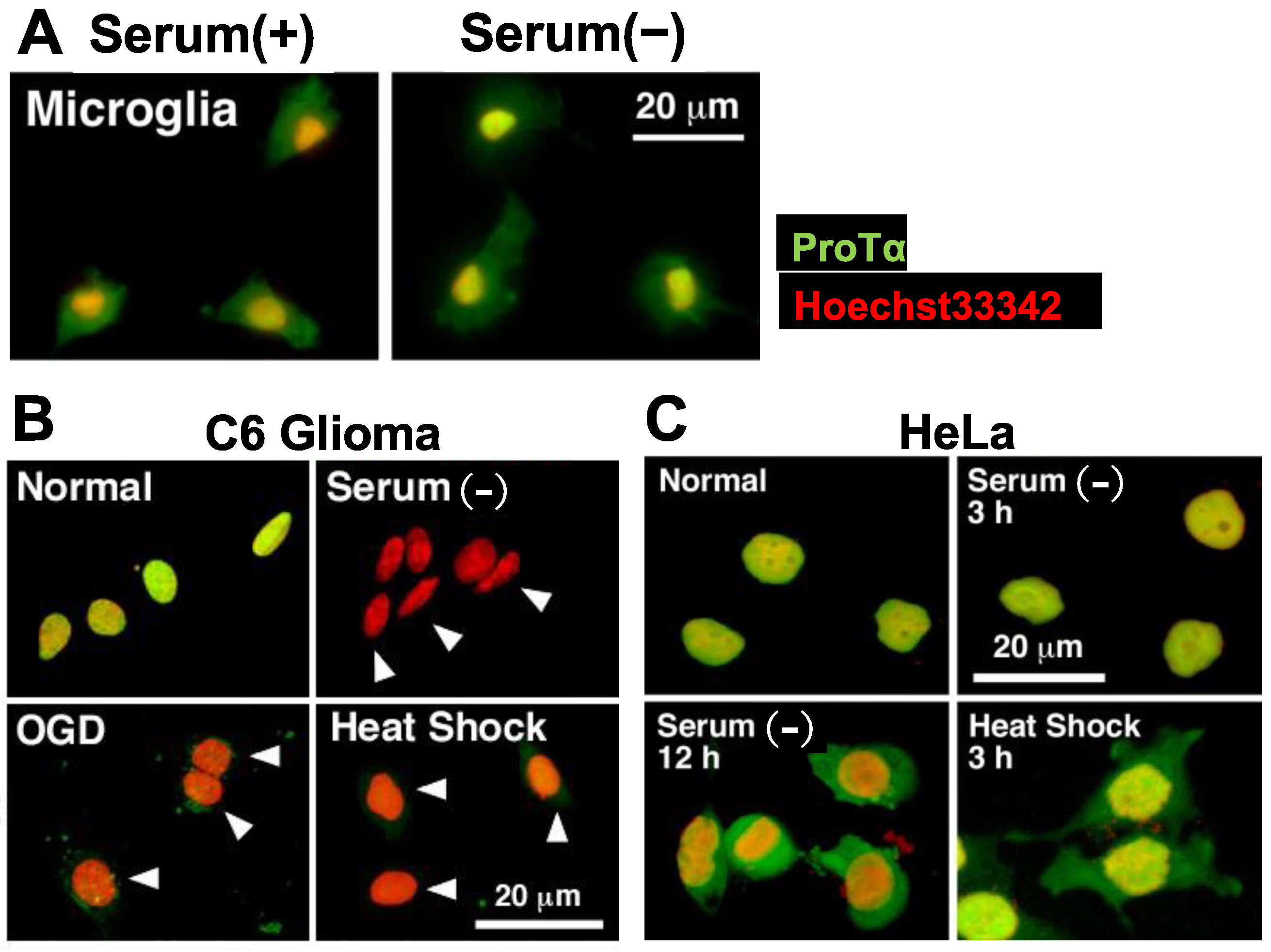
3.2.10. Cell-Type-Specific Non-Classical and Non-Vesicular Release
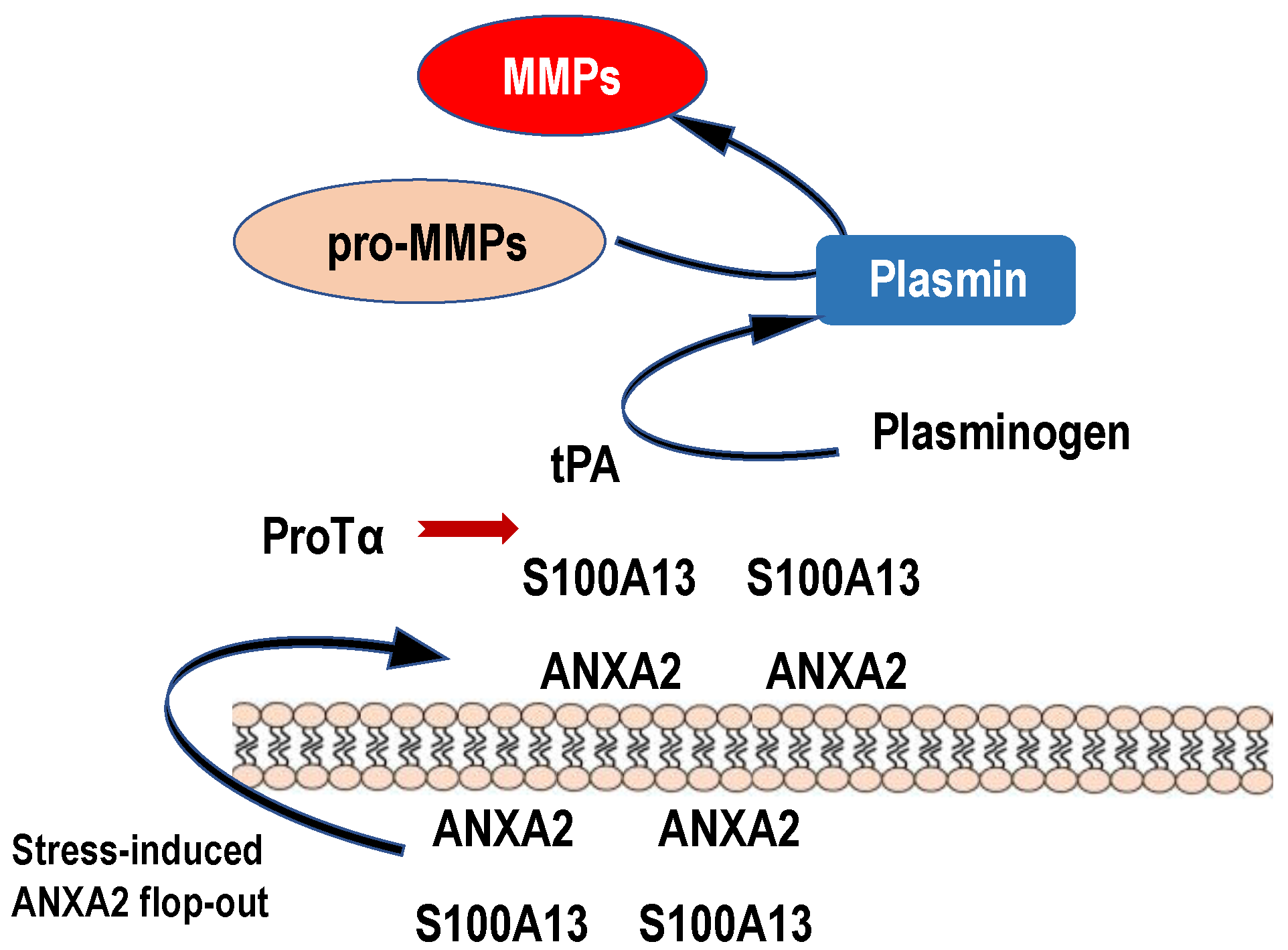
3.3. ANXA2 Flop System Underlying Anti-Stroke Actions of ProTα
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, H.; Itoh, K.; Kaneko, Y.; Kishishita, M.; Yoshida, O.; Fujita, J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J. Cell Biol. 1997, 137, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, H.; Zhao, L. PAMPs and DAMPs as the Bridge Between Periodontitis and Atherosclerosis: The Potential Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 856118. [Google Scholar] [CrossRef]
- Batulan, Z.; Pulakazhi Venu, V.K.; Li, Y.; Koumbadinga, G.; Alvarez-Olmedo, D.G.; Shi, C.; O’Brien, E.R. Extracellular Release and Signaling by Heat Shock Protein 27: Role in Modifying Vascular Inflammation. Front. Immunol. 2016, 7, 285. [Google Scholar] [CrossRef] [Green Version]
- Young, B.D.; Cook, M.E.; Costabile, B.K.; Samanta, R.; Zhuang, X.; Sevdalis, S.E.; Varney, K.M.; Mancia, F.; Matysiak, S.; Lattman, E.; et al. Binding and Functional Folding (BFF): A Physiological Framework for Studying Biomolecular Interactions and Allostery. J. Mol. Biol. 2022, 434, 167872. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R. HMGB1 is a promising therapeutic target for asthma. Cytokine 2023, 165, 156171. [Google Scholar] [CrossRef]
- Zhong, P.; Zhou, M.; Zhang, J.; Peng, J.; Zeng, G.; Huang, H. The role of Cold-Inducible RNA-binding protein in respiratory diseases. J. Cell. Mol. Med. 2022, 26, 957–965. [Google Scholar] [CrossRef]
- Chauvin, C.; Retnakumar, S.V.; Bayry, J. Gasdermin D as a cellular switch to orientate immune responses via IL-33 or IL-1beta. Cell. Mol. Immunol. 2023, 20, 8–10. [Google Scholar] [CrossRef]
- Tonnus, W.; Linkermann, A. The in vivo evidence for regulated necrosis. Immunol. Rev. 2017, 277, 128–149. [Google Scholar] [CrossRef]
- Matsunaga, H.; Halder, S.K.; Ueda, H. Annexin A2 Flop-Out Mediates the Non-Vesicular Release of DAMPs/Alarmins from C6 Glioma Cells Induced by Serum-Free Conditions. Cells 2021, 10, 567. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Tsien, R.W. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 2009, 323, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Seino, S.; Shibasaki, T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 2005, 85, 1303–1342. [Google Scholar] [CrossRef] [Green Version]
- Machamer, C.E. Accommodation of large cargo within Golgi cisternae. Histochem. Cell Biol. 2013, 140, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ito, Y.; Chappel, J.; Andrews, N.W.; Teitelbaum, S.L.; Ross, F.P. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell 2008, 14, 914–925. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Henne, W.M.; Stenmark, H.; Emr, S.D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 2013, 5, a016766. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Heilig, R.; Dick, M.S.; Sborgi, L.; Meunier, E.; Hiller, S.; Broz, P. The Gasdermin-D pore acts as a conduit for IL-1beta secretion in mice. Eur. J. Immunol. 2018, 48, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Brough, D.; Rothwell, N.J. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J. Cell Sci. 2007, 120, 772–781. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.J. Cell death and inflammation: The case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016, 283, 2599–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickard, J.A.; O’Donnell, J.A.; Evans, J.M.; Lalaoui, N.; Poh, A.R.; Rogers, T.; Vince, J.E.; Lawlor, K.E.; Ninnis, R.L.; Anderton, H.; et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 2014, 157, 1175–1188. [Google Scholar] [CrossRef] [Green Version]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Murai, S.; Moriwaki, K. Regulation of the release of damage-associated molecular patterns from necroptotic cells. Biochem. J. 2022, 479, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.S. Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Ueda, H.; Fujita, R.; Yoshida, A.; Matsunaga, H.; Ueda, M. Identification of prothymosin-alpha1, the necrosis-apoptosis switch molecule in cortical neuronal cultures. J. Cell Biol. 2007, 176, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, H.; Ueda, H. Stress-induced non-vesicular release of prothymosin-alpha initiated by an interaction with S100A13, and its blockade by caspase-3 cleavage. Cell Death Differ. 2010, 17, 1760–1772. [Google Scholar] [CrossRef] [Green Version]
- Fujita, R.; Ueda, H. Prothymosin-alpha1 prevents necrosis and apoptosis following stroke. Cell Death Differ. 2007, 14, 1839–1842. [Google Scholar] [CrossRef] [Green Version]
- Fujita, R.; Ueda, M.; Fujiwara, K.; Ueda, H. Prothymosin-alpha plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition. Cell Death Differ. 2009, 16, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H. Prothymosin alpha Plays Role as a Brain Guardian through Ecto-F(1) ATPase-P2Y(12) Complex and TLR4/MD2. Cells 2023, 12, 496. [Google Scholar] [CrossRef]
- Nebenfuhr, A.; Ritzenthaler, C.; Robinson, D.G. Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002, 130, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, Y.; Miyamoto, Y.; Saiwaki, T.; Yoneda, Y. Mechanism of the stress-induced collapse of the Ran distribution. Exp. Cell Res. 2006, 312, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Shishibori, T.; Oyama, Y.; Matsushita, O.; Yamashita, K.; Furuichi, H.; Okabe, A.; Maeta, H.; Hata, Y.; Kobayashi, R. Three distinct anti-allergic drugs, amlexanox, cromolyn and tranilast, bind to S100A12 and S100A13 of the S100 protein family. Biochem. J. 1999, 338, 583–589. [Google Scholar] [CrossRef]
- Matsunaga, H.; Ueda, H. Evidence for serum-deprivation-induced co-release of FGF-1 and S100A13 from astrocytes. Neurochem. Int. 2006, 49, 294–303. [Google Scholar] [CrossRef]
- Landriscina, M.; Soldi, R.; Bagala, C.; Micucci, I.; Bellum, S.; Tarantini, F.; Prudovsky, I.; Maciag, T. S100A13 participates in the release of fibroblast growth factor 1 in response to heat shock in vitro. J. Biol. Chem. 2001, 276, 22544–22552. [Google Scholar] [CrossRef] [Green Version]
- Mandinova, A.; Soldi, R.; Graziani, I.; Bagala, C.; Bellum, S.; Landriscina, M.; Tarantini, F.; Prudovsky, I.; Maciag, T. S100A13 mediates the copper-dependent stress-induced release of IL-1alpha from both human U937 and murine NIH 3T3 cells. J. Cell Sci. 2003, 116, 2687–2696. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, H.; Ueda, H. Voltage-dependent N-type Ca2+ channel activity regulates the interaction between FGF-1 and S100A13 for stress-induced non-vesicular release. Cell. Mol. Neurobiol. 2006, 26, 237–246. [Google Scholar] [CrossRef]
- Matsunaga, H.; Ueda, H. Synergistic Ca2+ and Cu2+ requirements of the FGF1-S100A13 interaction measured by quartz crystal microbalance: An initial step in amlexanox-reversible non-classical release of FGF1. Neurochem. Int. 2008, 52, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Mouta Carreira, C.; LaVallee, T.M.; Tarantini, F.; Jackson, A.; Lathrop, J.T.; Hampton, B.; Burgess, W.H.; Maciag, T. S100A13 is involved in the regulation of fibroblast growth factor-1 and p40 synaptotagmin-1 release in vitro. J. Biol. Chem. 1998, 273, 22224–22231. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Kim, H.E.; Shu, H.; Zhao, Y.; Zhang, H.; Kofron, J.; Donnelly, J.; Burns, D.; Ng, S.C.; Rosenberg, S.; et al. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science 2003, 299, 223–226. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ma, C. Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion. Elife 2016, 5, e14211. [Google Scholar] [CrossRef]
- Matsunaga, H.; Halder, S.K.; Ueda, H. Involvement of SNARE Protein Interaction for Non-classical Release of DAMPs/Alarmins Proteins, Prothymosin Alpha and S100A13. Cell. Mol. Neurobiol. 2021, 41, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Endo, M. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 2009, 89, 1153–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, N.; Petrenko, A.G.; Sudhof, T.C.; Jahn, R. Synaptotagmin: A calcium sensor on the synaptic vesicle surface. Science 1992, 256, 1021–1025. [Google Scholar] [CrossRef]
- Weber, T.; Zemelman, B.V.; McNew, J.A.; Westermann, B.; Gmachl, M.; Parlati, F.; Sollner, T.H.; Rothman, J.E. SNAREpins: Minimal machinery for membrane fusion. Cell 1998, 92, 759–772. [Google Scholar] [CrossRef] [Green Version]
- Mion, D.; Bunel, L.; Heo, P.; Pincet, F. The beginning and the end of SNARE-induced membrane fusion. FEBS Open Bio 2022, 12, 1958–1979. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, B.K.; Choi, M.G.; Kim, S.A.; Lai, Y.; Shin, Y.K.; Lee, N.K. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 2012, 31, 2144–2155. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, V.V.; Yoshino, K.; Jahnz, M.; Dorries, C.; Bade, S.; Nauenburg, S.; Niemann, H.; Binz, T. Proteolysis of SNAP-25 isoforms by botulinum neurotoxin types A, C, and E: Domains and amino acid residues controlling the formation of enzyme-substrate complexes and cleavage. J. Neurochem. 1999, 72, 327–337. [Google Scholar] [CrossRef]
- Creutz, C.E. The annexins and exocytosis. Science 1992, 258, 924–931. [Google Scholar] [CrossRef]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Gabel, M.; Chasserot-Golaz, S. Annexin A2, an essential partner of the exocytotic process in chromaffin cells. J. Neurochem. 2016, 137, 890–896. [Google Scholar] [CrossRef] [Green Version]
- Gabel, M.; Royer, C.; Thahouly, T.; Calco, V.; Gasman, S.; Bader, M.F.; Vitale, N.; Chasserot-Golaz, S. Annexin A2 Egress during Calcium-Regulated Exocytosis in Neuroendocrine Cells. Cells 2020, 9, 2059. [Google Scholar] [CrossRef] [PubMed]
- Umbrecht-Jenck, E.; Demais, V.; Calco, V.; Bailly, Y.; Bader, M.F.; Chasserot-Golaz, S. S100A10-mediated translocation of annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic 2010, 11, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Shor, E.; Wang, Y.; Perlin, D.S.; Xue, C. Cryptococcus flips its lid—Membrane phospholipid asymmetry modulates antifungal drug resistance and virulence. Microb. Cell 2016, 3, 358–360. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, H.; Li, X.; Wu, J.; Xue, T.; Wu, J.; Shen, H.; Li, X.; Shen, M.; Chen, G. TMEM16F Aggravates Neuronal Loss by Mediating Microglial Phagocytosis of Neurons in a Rat Experimental Cerebral Ischemia and Reperfusion Model. Front. Immunol. 2020, 11, 1144. [Google Scholar] [CrossRef]
- Stewart, S.E.; Ashkenazi, A.; Williamson, A.; Rubinsztein, D.C.; Moreau, K. Transbilayer phospholipid movement facilitates the translocation of annexin across membranes. J. Cell Sci. 2018, 131, jcs217034. [Google Scholar] [CrossRef] [Green Version]
- Menke, M.; Gerke, V.; Steinem, C. Phosphatidylserine membrane domain clustering induced by annexin A2/S100A10 heterotetramer. Biochemistry 2005, 44, 15296–15303. [Google Scholar] [CrossRef]
- Halder, S.K.; Matsunaga, H.; Ueda, H. Prothymosin alpha and its mimetic hexapeptide improve delayed tissue plasminogen activator-induced brain damage following cerebral ischemia. J. Neurochem. 2020, 153, 772–789. [Google Scholar] [CrossRef]
- Seo, J.S.; Svenningsson, P. Modulation of Ion Channels and Receptors by p11 (S100A10). Trends Pharmacol. Sci. 2020, 41, 487–497. [Google Scholar] [CrossRef]
- Pedemonte, N.; Galietta, L.J. Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 2014, 94, 419–459. [Google Scholar] [CrossRef] [Green Version]
- Morozova, K.; Sridhar, S.; Zolla, V.; Clement, C.C.; Scharf, B.; Verzani, Z.; Diaz, A.; Larocca, J.N.; Hajjar, K.A.; Cuervo, A.M.; et al. Annexin A2 promotes phagophore assembly by enhancing Atg16L+ vesicle biogenesis and homotypic fusion. Nat. Commun. 2015, 6, 5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaVallee, T.M.; Tarantini, F.; Gamble, S.; Mouta Carreira, C.; Jackson, A.; Maciag, T. Synaptotagmin-1 is required for fibroblast growth factor-1 release. J. Biol. Chem. 1998, 273, 22217–22223. [Google Scholar] [CrossRef] [Green Version]
- Tarantini, F.; Micucci, I.; Bellum, S.; Landriscina, M.; Garfinkel, S.; Prudovsky, I.; Maciag, T. The precursor but not the mature form of IL1alpha blocks the release of FGF1 in response to heat shock. J. Biol. Chem. 2001, 276, 5147–5151. [Google Scholar] [CrossRef] [Green Version]
- Prudovsky, I.; Mandinova, A.; Soldi, R.; Bagala, C.; Graziani, I.; Landriscina, M.; Tarantini, F.; Duarte, M.; Bellum, S.; Doherty, H.; et al. The non-classical export routes: FGF1 and IL-1alpha point the way. J. Cell Sci. 2003, 116, 4871–4881. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, K.; Hosojima, S.; Hara, H.; Kushiyama, H.; Mahib, M.R.; Kinoshita, T.; Suda, T. Gasdermin D mediates the maturation and release of IL-1alpha downstream of inflammasomes. Cell Rep. 2021, 34, 108887. [Google Scholar] [CrossRef]
- Voss, O.H.; Cobb, J.; Gaytan, H.; Rivera Diaz, N.; Sanchez, R.; DeTolla, L.; Rahman, M.S.; Azad, A.F. Pathogenic, but Not Nonpathogenic, Rickettsia spp. Evade Inflammasome-Dependent IL-1 Responses to Establish an Intracytosolic Replication Niche. mBio 2021, 13, e0291821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, H. Non-Vesicular Release of Alarmin Prothymosin α Complex Associated with Annexin-2 Flop-Out. Cells 2023, 12, 1569. https://doi.org/10.3390/cells12121569
Ueda H. Non-Vesicular Release of Alarmin Prothymosin α Complex Associated with Annexin-2 Flop-Out. Cells. 2023; 12(12):1569. https://doi.org/10.3390/cells12121569
Chicago/Turabian StyleUeda, Hiroshi. 2023. "Non-Vesicular Release of Alarmin Prothymosin α Complex Associated with Annexin-2 Flop-Out" Cells 12, no. 12: 1569. https://doi.org/10.3390/cells12121569




