Brain Infection by Group B Streptococcus Induces Inflammation and Affects Neurogenesis in the Adult Mouse Hippocampus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mouse Infection
2.3. Immunohistochemistry
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
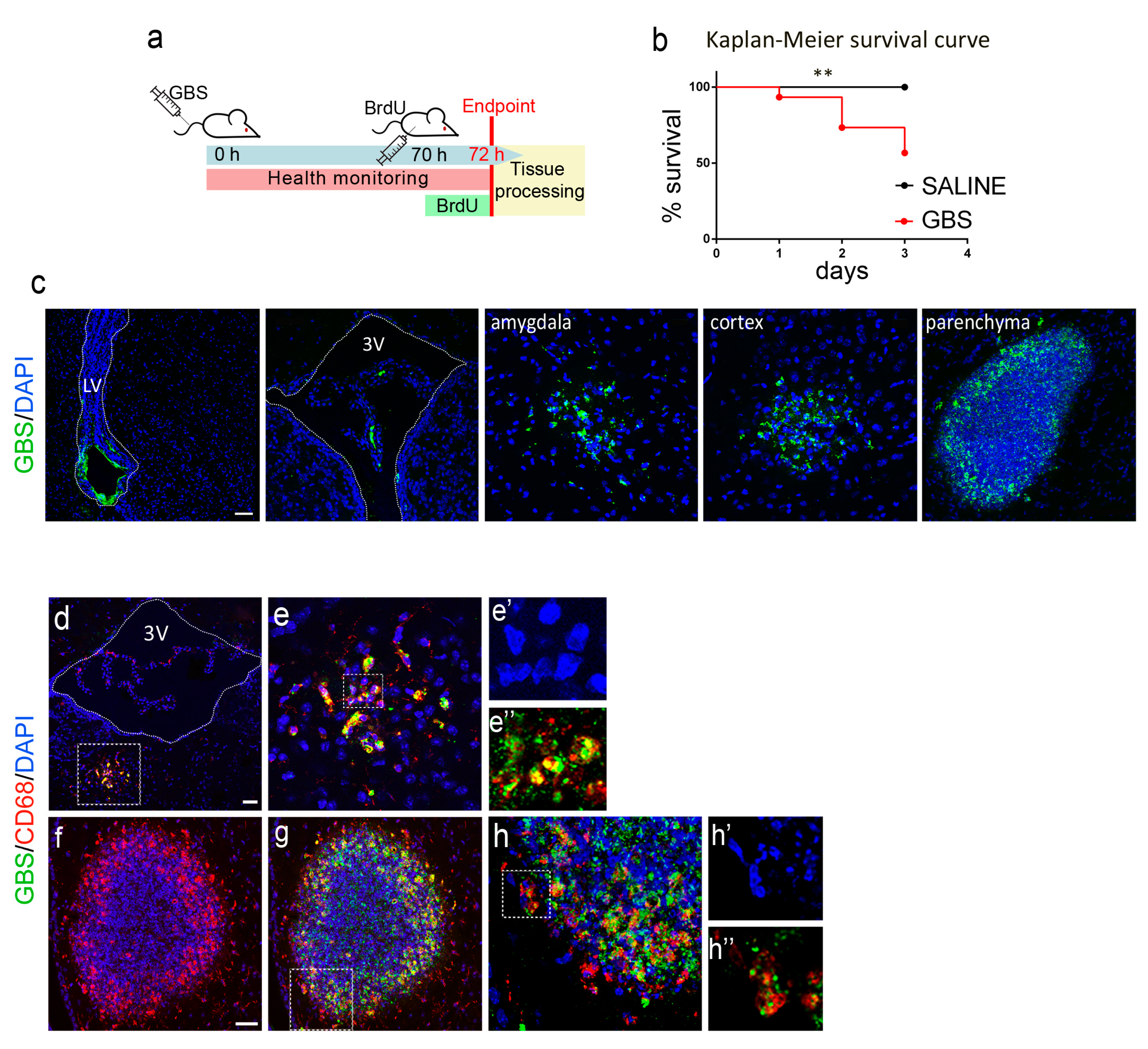
3.1. GBS Infection Elicits Neurological Deficits and Causes Death in Mice
3.2. GBS Infection Induces Inflammation and Peripheral Immune Cell Infiltration in the Mouse Brain
3.3. GBS Brain Infection Affects Adult Hippocampal Neurogenesis in the SGZ of the Dentate Gyrus
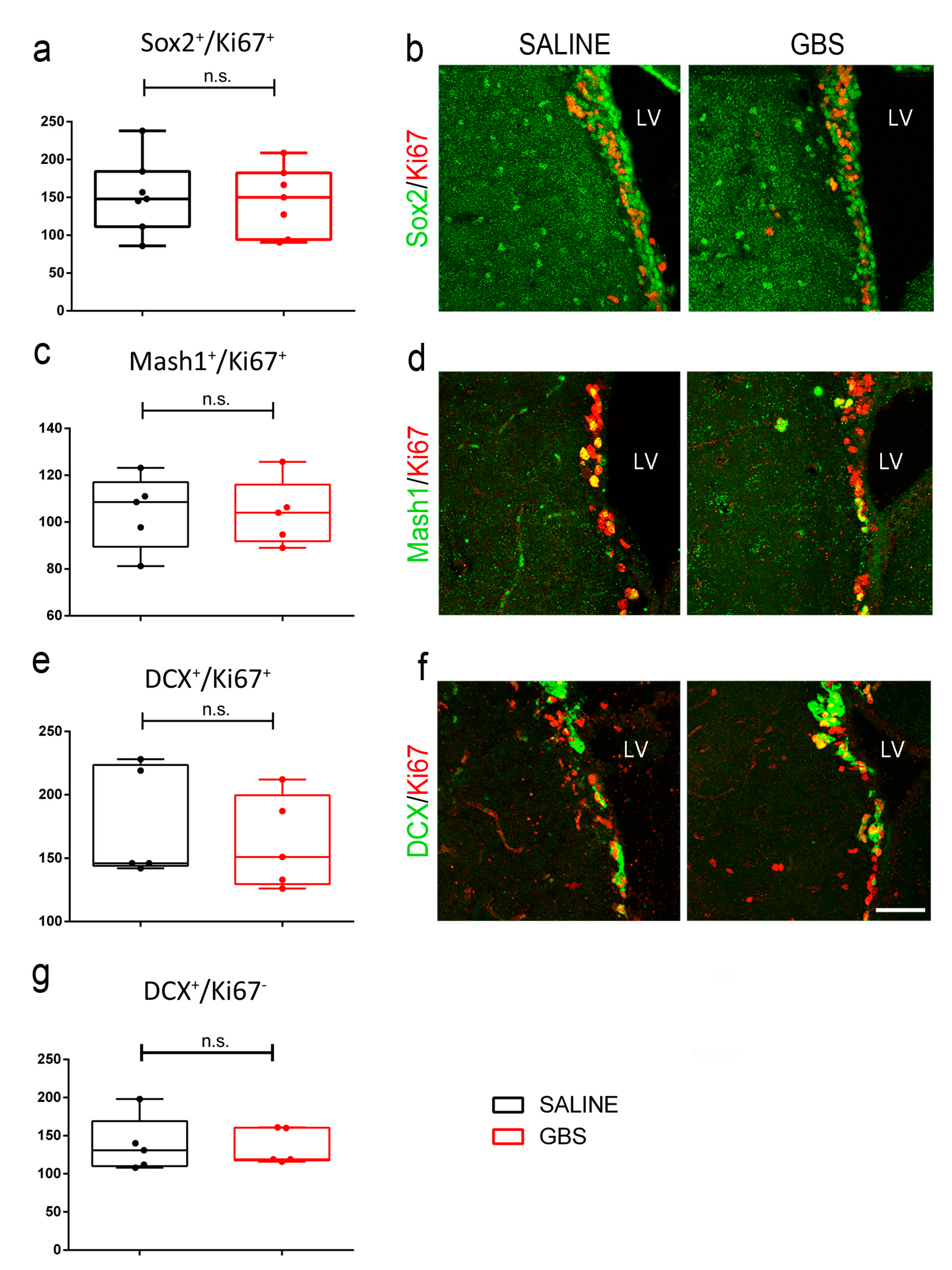
3.4. Adult SVZ Neurogenesis Is Not Affected upon GBS Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [PubMed]
- Liechti, F.D.; Grandgirard, D.; Leib, S.L. Bacterial meningitis: Insights into pathogenesis and evaluation of new treatment options: A perspective from experimental studies. Future Microbiol. 2015, 10, 1195–1213. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, D.; Leib, S.L. Meningitis in neonates: Bench to bedside. Clin. Perinatol. 2010, 37, 655–676. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Scheld, W.M.; Pfister, H.W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2002, 2, 721–736. [Google Scholar] [CrossRef]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef]
- Mizrahi, A.; Marvaud, J.C.; Pilmis, B.; Van, J.C.N.; Couzigou, C.; Bruel, C.; Engrand, N.; Le Monnier, A.; Lambert, T. Emergence of Ceftriaxone Resistance during a Case of Pneumococcal Meningitis with Fatal Evolution. Antimicrob. Agents Chemother. 2020, 64, e01958-19. [Google Scholar] [CrossRef]
- Andrade, E.B.; Magalhaes, A.; Puga, A.; Costa, M.; Bravo, J.; Portugal, C.C.; Ribeiro, A.; Correia-Neves, M.; Faustino, A.; Firon, A.; et al. A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat. Commun. 2018, 9, 3138. [Google Scholar] [CrossRef]
- Gianinazzi, C.; Grandgirard, D.; Imboden, H.; Egger, L.; Meli, D.N.; Bifrare, Y.D.; Joss, P.C.; Tauber, M.G.; Borner, C.; Leib, S.L. Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol. 2003, 105, 499–507. [Google Scholar] [CrossRef]
- Grandgirard, D.; Bifrare, Y.D.; Pleasure, S.J.; Kummer, J.; Leib, S.L.; Tauber, M.G. Pneumococcal meningitis induces apoptosis in recently postmitotic immature neurons in the dentate gyrus of neonatal rats. Dev. Neurosci. 2007, 29, 134–142. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Lucassen, P.J.; Korosi, A. The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Front. Mol. Neurosci. 2014, 7, 103. [Google Scholar] [CrossRef]
- Hoffmann, O.; Mahrhofer, C.; Rueter, N.; Freyer, D.; Bert, B.; Fink, H.; Weber, J.R. Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis. Infect. Immun. 2007, 75, 4289–4297. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, C.; Leib, S.L.; Rowland, I.; Brandt, C.T. Bacteremia causes hippocampal apoptosis in experimental pneumococcal meningitis. BMC Infect. Dis. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.P.; Bonfanti, L. Adult Neurogenesis in Mammals: Variations and Confusions. Brain Behav. Evol. 2016, 87, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef]
- Bergmann, O.; Spalding, K.L.; Frisen, J. Adult Neurogenesis in Humans. Cold Spring Harb. Perspect. Biol. 2015, 7, a018994. [Google Scholar] [CrossRef]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Bostrom, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Gandy, K.; Kim, S.; Sharp, C.; Dindo, L.; Maletic-Savatic, M.; Calarge, C. Pattern Separation: A Potential Marker of Impaired Hippocampal Adult Neurogenesis in Major Depressive Disorder. Front. Neurosci. 2017, 11, 571. [Google Scholar] [CrossRef]
- Seib, D.R.; Martin-Villalba, A. Neurogenesis in the Normal Ageing Hippocampus: A Mini-Review. Gerontology 2015, 61, 327–335. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Moreno-Jimenez, E.P.; Flor-Garcia, M.; Terreros-Roncal, J.; Rabano, A.; Cafini, F.; Pallas-Bazarra, N.; Avila, J.; Llorens-Martin, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Terreros-Roncal, J.; Flor-Garcia, M.; Moreno-Jimenez, E.P.; Rodriguez-Moreno, C.B.; Marquez-Valadez, B.; Gallardo-Caballero, M.; Rabano, A.; Llorens-Martin, M. Methods to study adult hippocampal neurogenesis in humans and across the phylogeny. Hippocampus 2022, 33, 271–306. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granger, J.; Alvargonzalez, J.C.; Berardi, A.; Berner, R.; Kunze, M.; Hufnagel, M.; Melin, P.; Decheva, A.; Orefici, G.; Poyart, C.; et al. Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.M.; Georgiou, S.; Xenakis, E.M.; Langer, O. Group B streptococcus infection rate unchanged by gestational diabetes. Obstet. Gynecol. 1999, 93, 292–296. [Google Scholar]
- Pitts, S.I.; Maruthur, N.M.; Langley, G.E.; Pondo, T.; Shutt, K.A.; Hollick, R.; Schrag, S.J.; Thomas, A.; Nichols, M.; Farley, M.; et al. Obesity, Diabetes, and the Risk of Invasive Group B Streptococcal Disease in Nonpregnant Adults in the United States. Open Forum Infect. Dis. 2018, 5, ofy030. [Google Scholar] [CrossRef]
- Bisno, A.L.; Stevens, D.L. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 1996, 334, 240–245. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, C.I.; Kim, J.; Joo, E.J.; Ha, Y.E.; Chung, D.R.; Lee, N.Y.; Peck, K.R.; Song, J.H. Association of liver cirrhosis with group B streptococcal bacteremia in non-pregnant adults. J. Infect. 2013, 67, 617–619. [Google Scholar] [CrossRef]
- Farley, M.M. Group B streptococcal disease in nonpregnant adults. Clin. Infect. Dis. 2001, 33, 556–561. [Google Scholar] [CrossRef]
- Farley, M.M.; Harvey, R.C.; Stull, T.; Smith, J.D.; Schuchat, A.; Wenger, J.D.; Stephens, D.S. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N. Engl. J. Med. 1993, 328, 1807–1811. [Google Scholar] [CrossRef]
- Kohli-Lynch, M.; Russell, N.J.; Seale, A.C.; Dangor, Z.; Tann, C.J.; Baker, C.J.; Bartlett, L.; Cutland, C.; Gravett, M.G.; Heath, P.T.; et al. Neurodevelopmental Impairment in Children After Group B Streptococcal Disease Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65, S190–S199. [Google Scholar] [CrossRef]
- Libster, R.; Edwards, K.M.; Levent, F.; Edwards, M.S.; Rench, M.A.; Castagnini, L.A.; Cooper, T.; Sparks, R.C.; Baker, C.J.; Shah, P.E. Long-term outcomes of group B streptococcal meningitis. Pediatrics 2012, 130, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Skoff, T.H.; Farley, M.M.; Petit, S.; Craig, A.S.; Schaffner, W.; Gershman, K.; Harrison, L.H.; Lynfield, R.; Mohle-Boetani, J.; Zansky, S.; et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 2009, 49, 85–92. [Google Scholar] [CrossRef]
- Tibussek, D.; Sinclair, A.; Yau, I.; Teatero, S.; Fittipaldi, N.; Richardson, S.E.; Mayatepek, E.; Jahn, P.; Askalan, R. Late-onset group B streptococcal meningitis has cerebrovascular complications. J. Pediatr. 2015, 166, 1187–1192.e1. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Sun, J.; Fukahori, K.; Ando, N.; Wu, M.; Schwarz, F.; Siddiqui, S.S.; Varki, A.; Marth, J.D.; Nizet, V. Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing. Proc. Natl. Acad. Sci. USA 2019, 116, 7465–7470. [Google Scholar] [CrossRef] [PubMed]
- Levent, F.; Baker, C.J.; Rench, M.A.; Edwards, M.S. Early outcomes of group B streptococcal meningitis in the 21st century. Pediatr. Infect. Dis. J. 2010, 29, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Maisey, H.C.; Doran, K.S.; Nizet, V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev. Mol. Med. 2008, 10, e27. [Google Scholar] [CrossRef]
- Melin, P.; Efstratiou, A. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine 2013, 31 (Suppl. 4), D31–D42. [Google Scholar] [CrossRef]
- Shabayek, S.; Spellerberg, B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef]
- Chacko, A.; Delbaz, A.; Choudhury, I.N.; Eindorf, T.; Shah, M.; Godfrey, C.; Sullivan, M.J.; St John, J.A.; Ulett, G.C.; Ekberg, J.A.K. Streptococcus agalactiae Infects Glial Cells and Invades the Central Nervous System via the Olfactory and Trigeminal Nerves. Front. Cell Infect. Microbiol. 2022, 12, 793416. [Google Scholar] [CrossRef]
- Glaser, P.; Rusniok, C.; Buchrieser, C.; Chevalier, F.; Frangeul, L.; Msadek, T.; Zouine, M.; Couve, E.; Lalioui, L.; Poyart, C.; et al. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 2002, 45, 1499–1513. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.A.; Beveridge, C.J.; McCormick, D.; Aten, E.; Jones, N.; Snyder, L.A.; Saunders, N.J. Genetic islands of Streptococcus agalactiae strains NEM316 and 2603VR and their presence in other Group B streptococcal strains. BMC Microbiol. 2005, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Buscetta, M.; Papasergi, S.; Firon, A.; Pietrocola, G.; Biondo, C.; Mancuso, G.; Midiri, A.; Romeo, L.; Teti, G.; Speziale, P.; et al. FbsC, a novel fibrinogen-binding protein, promotes Streptococcus agalactiae-host cell interactions. J. Biol. Chem. 2014, 289, 21003–21015. [Google Scholar] [CrossRef] [PubMed]
- Doran, K.S.; Engelson, E.J.; Khosravi, A.; Maisey, H.C.; Fedtke, I.; Equils, O.; Michelsen, K.S.; Arditi, M.; Peschel, A.; Nizet, V. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Investig. 2005, 115, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Doran, K.S.; Liu, G.Y.; Nizet, V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Investig. 2003, 112, 736–744. [Google Scholar] [CrossRef]
- Henneke, P.; Dramsi, S.; Mancuso, G.; Chraibi, K.; Pellegrini, E.; Theilacker, C.; Hubner, J.; Santos-Sierra, S.; Teti, G.; Golenbock, D.T.; et al. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 2008, 180, 6149–6158. [Google Scholar] [CrossRef]
- Mu, R.; Kim, B.J.; Paco, C.; Del Rosario, Y.; Courtney, H.S.; Doran, K.S. Identification of a group B streptococcal fibronectin binding protein, SfbA, that contributes to invasion of brain endothelium and development of meningitis. Infect. Immun. 2014, 82, 2276–2286. [Google Scholar] [CrossRef]
- Benmimoun, B.; Papastefanaki, F.; Perichon, B.; Segklia, K.; Roby, N.; Miriagou, V.; Schmitt, C.; Dramsi, S.; Matsas, R.; Speder, P. An original infection model identifies host lipoprotein import as a route for blood-brain barrier crossing. Nat. Commun. 2020, 11, 6106. [Google Scholar] [CrossRef]
- Gerber, J.; Bottcher, T.; Bering, J.; Bunkowski, S.; Bruck, W.; Kuhnt, U.; Nau, R. Increased neurogenesis after experimental Streptococcus pneumoniae meningitis. J. Neurosci. Res. 2003, 73, 441–446. [Google Scholar] [CrossRef]
- Tauber, S.C.; Stadelmann, C.; Spreer, A.; Bruck, W.; Nau, R.; Gerber, J. Increased expression of BDNF and proliferation of dentate granule cells after bacterial meningitis. J. Neuropathol. Exp. Neurol. 2005, 64, 806–815. [Google Scholar] [CrossRef]
- Barichello, T.; Fagundes, G.D.; Generoso, J.S.; Dagostin, C.S.; Simoes, L.R.; Vilela, M.C.; Comim, C.M.; Petronilho, F.; Quevedo, J.; Teixeira, A.L. Environmental enrichment restores cognitive deficits induced by experimental childhood meningitis. Braz. J. Psychiatry 2014, 36, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; He, D.; Wu, J.; Liu, Y.; Zhu, M.; Sun, J.; Chen, F.; Li, L. Exogenous BDNF increases neurogenesis in the hippocampus in experimental Streptococcus pneumoniae meningitis. J. Neuroimmunol. 2016, 294, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.C.; Harms, K.; Falkenburger, B.; Weis, J.; Sellhaus, B.; Nau, R.; Schulz, J.B.; Reich, A. Modulation of hippocampal neuroplasticity by Fas/CD95 regulatory protein 2 (Faim2) in the course of bacterial meningitis. J. Neuropathol. Exp. Neurol. 2014, 73, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Song, J.; Sun, J.Q.; Moss, J.; Wen, Z.X.; Sun, G.J.; Hsu, D.; Zhong, C.; Davoudi, H.; Christian, K.M.; Toni, N.; et al. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 2013, 16, 1728–1730. [Google Scholar] [CrossRef]
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.Y.; Enikolopov, G.; et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489, 150–154. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Andreska, T.; Rauskolb, S.; Schukraft, N.; Luningschror, P.; Sasi, M.; Signoret-Genest, J.; Behringer, M.; Blum, R.; Sauer, M.; Tovote, P.; et al. Induction of BDNF Expression in Layer II/III and Layer V Neurons of the Motor Cortex Is Essential for Motor Learning. J. Neurosci. 2020, 40, 6289–6308. [Google Scholar] [CrossRef]
- De Bruyckere, E.; Simon, R.; Nestel, S.; Heimrich, B.; Katzel, D.; Egorov, A.V.; Liu, P.; Jenkins, N.A.; Copeland, N.G.; Schwegler, H.; et al. Stability and Function of Hippocampal Mossy Fiber Synapses Depend on Bcl11b/Ctip2. Front. Mol. Neurosci. 2018, 11, 103. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Deng, L.; Spencer, B.L.; Holmes, J.A.; Mu, R.; Rego, S.; Weston, T.A.; Hu, Y.; Sanches, G.F.; Yoon, S.; Park, N.; et al. The Group B Streptococcal surface antigen I/II protein, BspC, interacts with host vimentin to promote adherence to brain endothelium and inflammation during the pathogenesis of meningitis. PLoS Pathog. 2019, 15, e1007848. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Belarmino, E.; Comim, C.M.; Cipriano, A.L.; Generoso, J.S.; Savi, G.D.; Stertz, L.; Kapczinski, F.; Quevedo, J. Correlation between behavioral deficits and decreased brain-derived neurotrofic factor in neonatal meningitis. J. Neuroimmunol. 2010, 223, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Widmann, C.N.; Okulla, T.; Urbach, H.; Kaiser, M.; Widman, G.; Mormann, F.; Weide, J.; Fliessbach, K.; Hoeft, A.; et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J. Neurol. Neurosurg. Psychiatry 2013, 84, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.R.; Ye, S.; Tang, F.; Sun, D.; Zhang, H.; Mei, L.; Xiong, W.C. Increased Microglial Activity, Impaired Adult Hippocampal Neurogenesis, and Depressive-like Behavior in Microglial VPS35-Depleted Mice. J. Neurosci. 2018, 38, 5949–5968. [Google Scholar] [CrossRef]
- Sierra, A.; Beccari, S.; Diaz-Aparicio, I.; Encinas, J.M.; Comeau, S.; Tremblay, M.E. Surveillance, phagocytosis, and inflammation: How never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014, 2014, 610343. [Google Scholar] [CrossRef]
- Stefani, J.; Tschesnokowa, O.; Parrilla, M.; Robaye, B.; Boeynaems, J.M.; Acker-Palmer, A.; Zimmermann, H.; Gampe, K. Disruption of the Microglial ADP Receptor P2Y13 Enhances Adult Hippocampal Neurogenesis. Front. Cell Neurosci. 2018, 12, 134. [Google Scholar] [CrossRef]
- Perez-Rodriguez, D.R.; Blanco-Luquin, I.; Mendioroz, M. The Participation of Microglia in Neurogenesis: A Review. Brain Sci. 2021, 11, 658. [Google Scholar] [CrossRef]
- Wadhwa, M.; Prabhakar, A.; Ray, K.; Roy, K.; Kumari, P.; Jha, P.K.; Kishore, K.; Kumar, S.; Panjwani, U. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J. Neuroinflamm. 2017, 14, 222. [Google Scholar] [CrossRef]
- Zhang, J.Q.; He, H.; Qiao, Y.; Zhou, T.; He, H.L.; Yi, S.N.; Zhang, L.J.; Mo, L.; Li, Y.H.; Jiang, W.K.; et al. Priming of microglia with IFN-gamma impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Goldman, D.; Song, X.; Kitai, R.; Casadevall, A.; Zhao, M.L.; Lee, S.C. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: Role of specific antibody and soluble capsular polysaccharide. Infect. Immun. 2001, 69, 1808–1815. [Google Scholar] [CrossRef]
- Linker, R.A.; Maurer, M.; Gaupp, S.; Martini, R.; Holtmann, B.; Giess, R.; Rieckmann, P.; Lassmann, H.; Toyka, K.V.; Sendtner, M.; et al. CNTF is a major protective factor in demyelinating CNS disease: A neurotrophic cytokine as modulator in neuroinflammation. Nat. Med. 2002, 8, 620–624. [Google Scholar] [CrossRef]
- Liesz, A.; Zhou, W.; Mracsko, E.; Karcher, S.; Bauer, H.; Schwarting, S.; Sun, L.; Bruder, D.; Stegemann, S.; Cerwenka, A.; et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 2011, 134, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Kerschensteiner, M.; Meinl, E.; Hohlfeld, R. Neuro-immune crosstalk in CNS diseases. Neuroscience 2009, 158, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Djukic, M.; Mildner, A.; Schmidt, H.; Czesnik, D.; Bruck, W.; Priller, J.; Nau, R.; Prinz, M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain 2006, 129, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Soto, A.; Bruck, W. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J. Neuropathol. Exp. Neurol. 1999, 58, 265–274. [Google Scholar] [CrossRef]
- Engelen-Lee, J.Y.; Brouwer, M.C.; Aronica, E.; van de Beek, D. Pneumococcal meningitis: Clinical-pathological correlations (MeninGene-Path). Acta Neuropathol. Commun. 2016, 4, 26. [Google Scholar] [CrossRef]
- Bifrare, Y.D.; Gianinazzi, C.; Imboden, H.; Leib, S.L.; Tauber, M.G. Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus 2003, 13, 481–488. [Google Scholar] [CrossRef]
- Bifrare, Y.D.; Kummer, J.; Joss, P.; Tauber, M.G.; Leib, S.L. Brain-derived neurotrophic factor protects against multiple forms of brain injury in bacterial meningitis. J. Infect. Dis. 2005, 191, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.N.; Zhang, Z.J.; Xu, D.F.; Wang, Y.F.; Li, L. Selective Loss of Brain-Derived Neurotrophic Factor Exacerbates Brain Injury by Enhancing Neuroinflammation in Experimental Streptococcus pneumoniae Meningitis. Front. Immunol. 2020, 11, 1357. [Google Scholar] [CrossRef]
- Chiaretti, A.; Antonelli, A.; Piastra, M.; Genovese, O.; Polidori, G.; Aloe, L. Expression of neurotrophic factors in cerebrospinal fluid and plasma of children with viral and bacterial meningoencephalitis. Acta Paediatr. 2004, 93, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Kizawa-Ueda, M.; Ueda, A.; Kawamura, N.; Ishikawa, T.; Mutoh, E.; Fukuda, Y.; Shiroki, R.; Hoshinaga, K.; Ito, S.; Asakura, K.; et al. Neurotrophin levels in cerebrospinal fluid of adult patients with meningitis and encephalitis. Eur. Neurol. 2011, 65, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.; Tauber, S.C.; Armbrecht, I.; Schmidt, H.; Bruck, W.; Nau, R. Increased neuronal proliferation in human bacterial meningitis. Neurology 2009, 73, 1026–1032. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segklia, K.; Matsas, R.; Papastefanaki, F. Brain Infection by Group B Streptococcus Induces Inflammation and Affects Neurogenesis in the Adult Mouse Hippocampus. Cells 2023, 12, 1570. https://doi.org/10.3390/cells12121570
Segklia K, Matsas R, Papastefanaki F. Brain Infection by Group B Streptococcus Induces Inflammation and Affects Neurogenesis in the Adult Mouse Hippocampus. Cells. 2023; 12(12):1570. https://doi.org/10.3390/cells12121570
Chicago/Turabian StyleSegklia, Katerina, Rebecca Matsas, and Florentia Papastefanaki. 2023. "Brain Infection by Group B Streptococcus Induces Inflammation and Affects Neurogenesis in the Adult Mouse Hippocampus" Cells 12, no. 12: 1570. https://doi.org/10.3390/cells12121570
APA StyleSegklia, K., Matsas, R., & Papastefanaki, F. (2023). Brain Infection by Group B Streptococcus Induces Inflammation and Affects Neurogenesis in the Adult Mouse Hippocampus. Cells, 12(12), 1570. https://doi.org/10.3390/cells12121570





