Kaempferol and Fisetin-Related Signaling Pathways Induce Apoptosis in Head and Neck Cancer Cells
Abstract
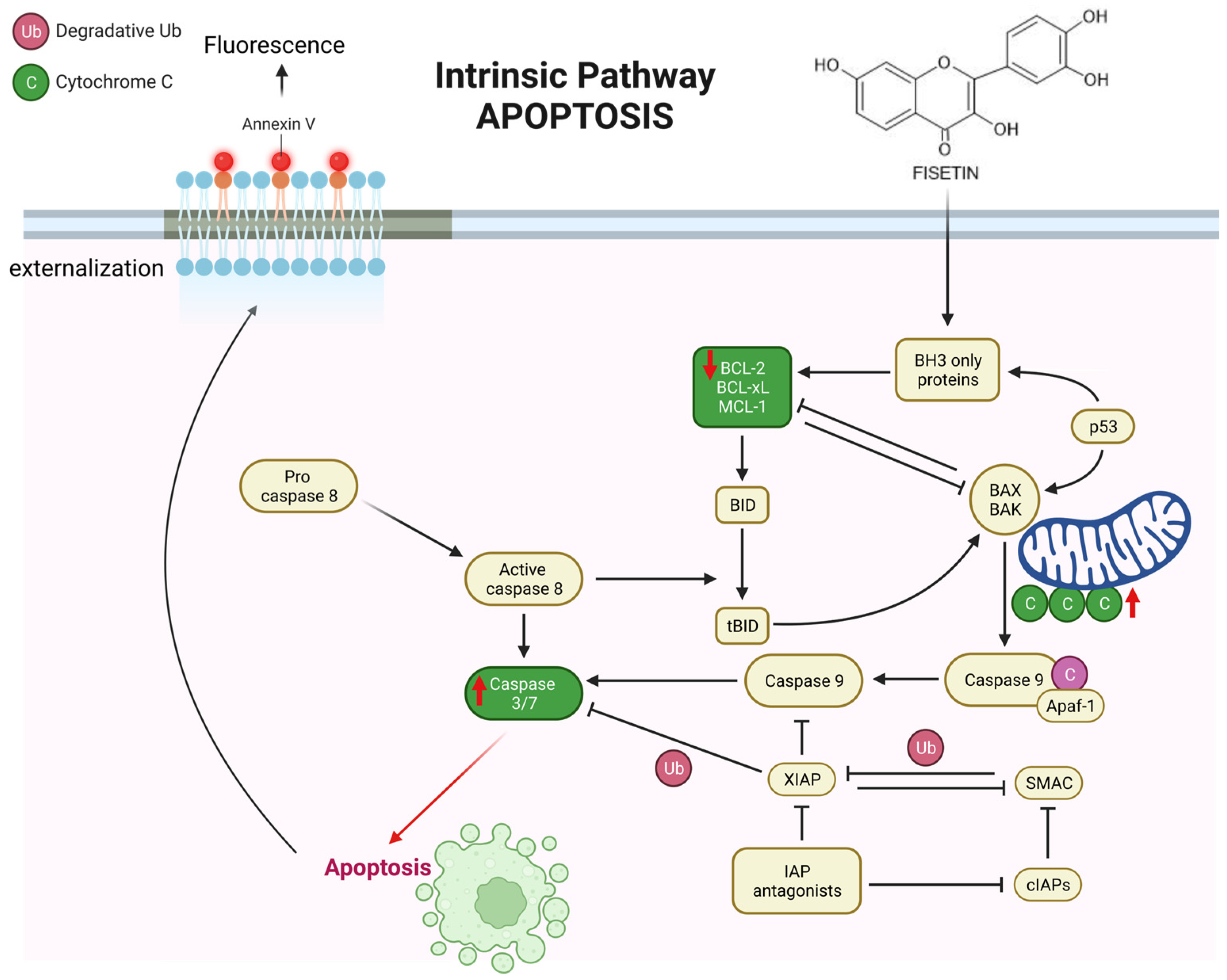
:1. Introduction
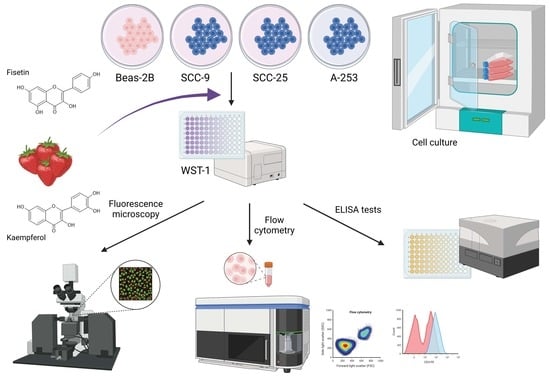
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Fisetin and Kaempferol Treatment
2.4. Cell Proliferation Assay (WST-1 Test)
2.5. Lactate Dehydrogenase Release Test
2.6. Evaluation of Cell Morphology via H&E Cytological Staining
2.7. Evaluation of Apoptosis and Necrosis Using Fluorescence Microscopy
2.8. Flow Cytometry Analysis
2.8.1. FITC Annexin V Apoptosis Detection Kit II
2.8.2. Cell Cycle Analysis—PI/RNase Staining Buffer
2.8.3. Staining Cells with JC-1
2.8.4. PE Active Caspase-3 Apoptosis Kit
2.8.5. PE Anti-Human Bcl-2 Kit
2.9. Cell Migration Test
2.10. Cytochrome c Release Assay
2.11. Statistical Analysis
3. Results
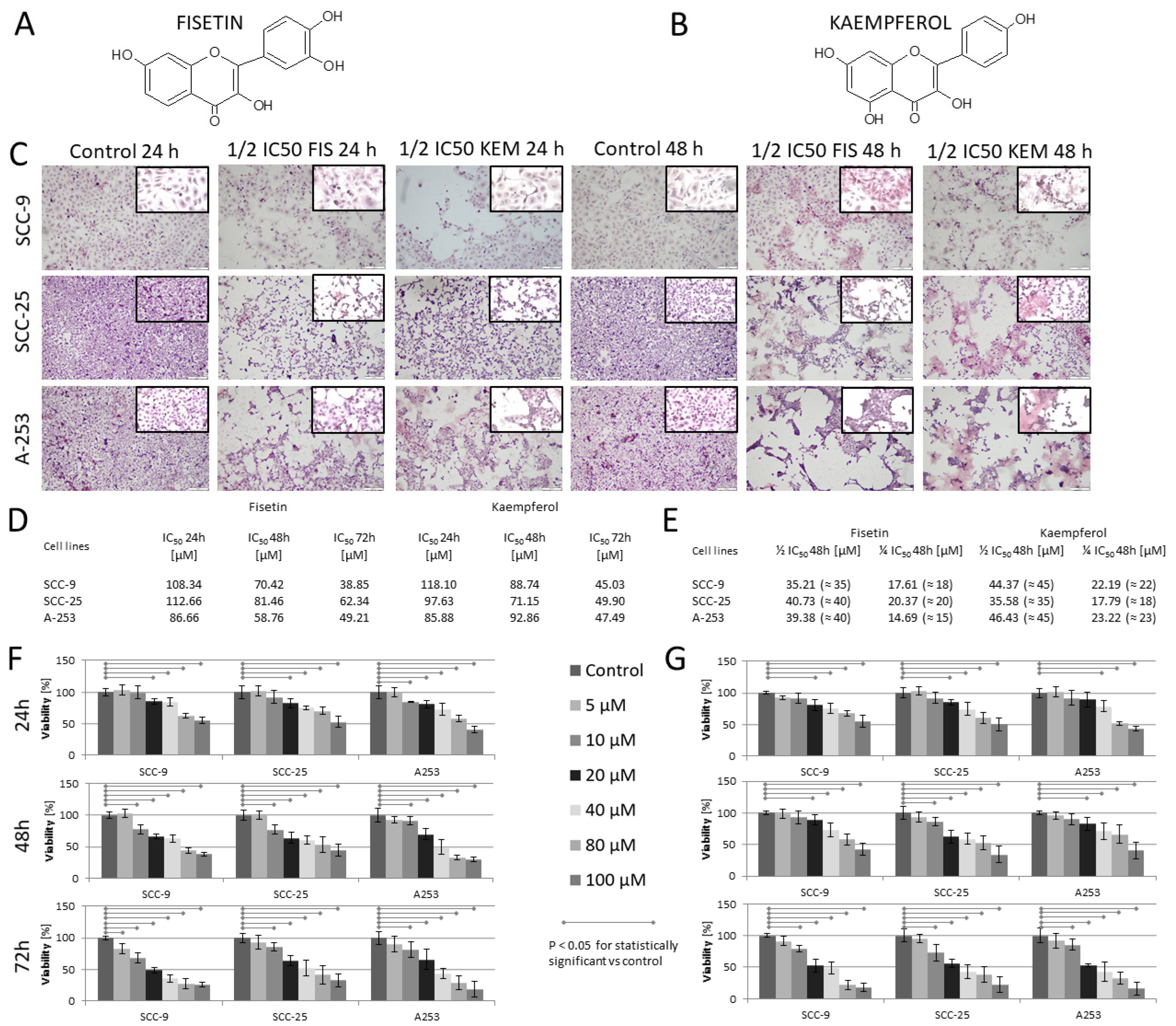
3.1. Kaempferol and Fisetin Inhibit HNCs Cell Proliferation
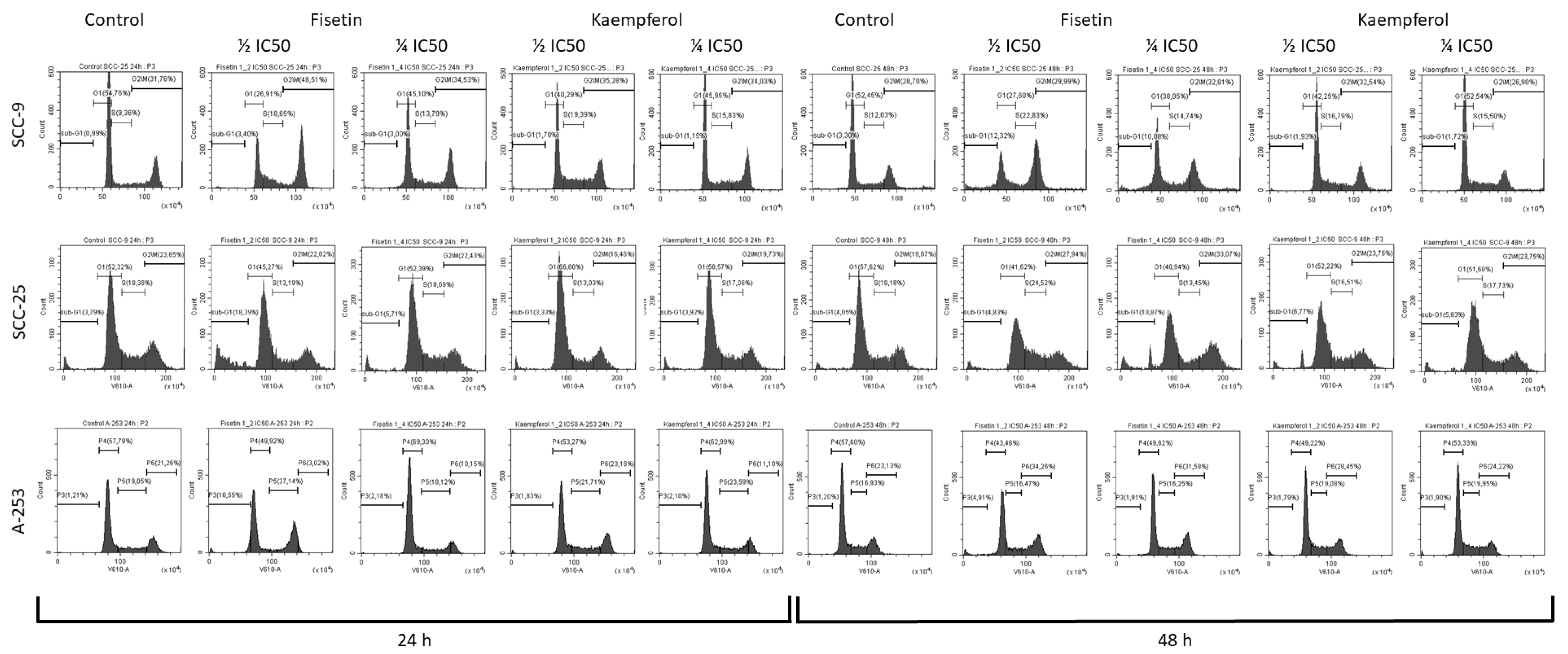
3.2. Kaempferol and Fisetin Induce Cell Cycle Arrest in SCC-25, SCC-9, and A-253 HNC Cell Lines
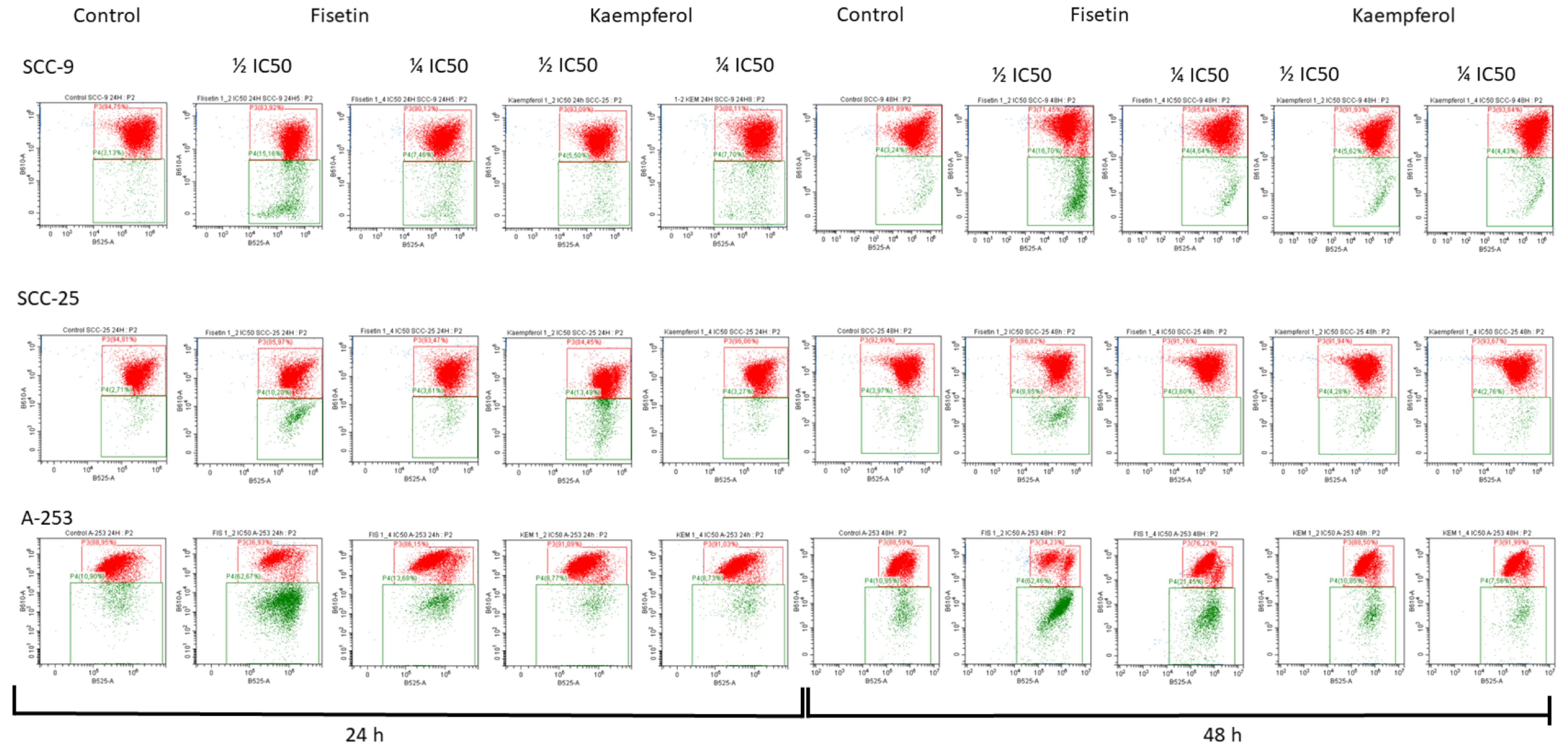
3.3. Kaempferol and Fisetin Induce Apoptosis in HNC Cells
3.4. Kaempferol and Fisetin Change the Potential of the Mitochondrial Membrane
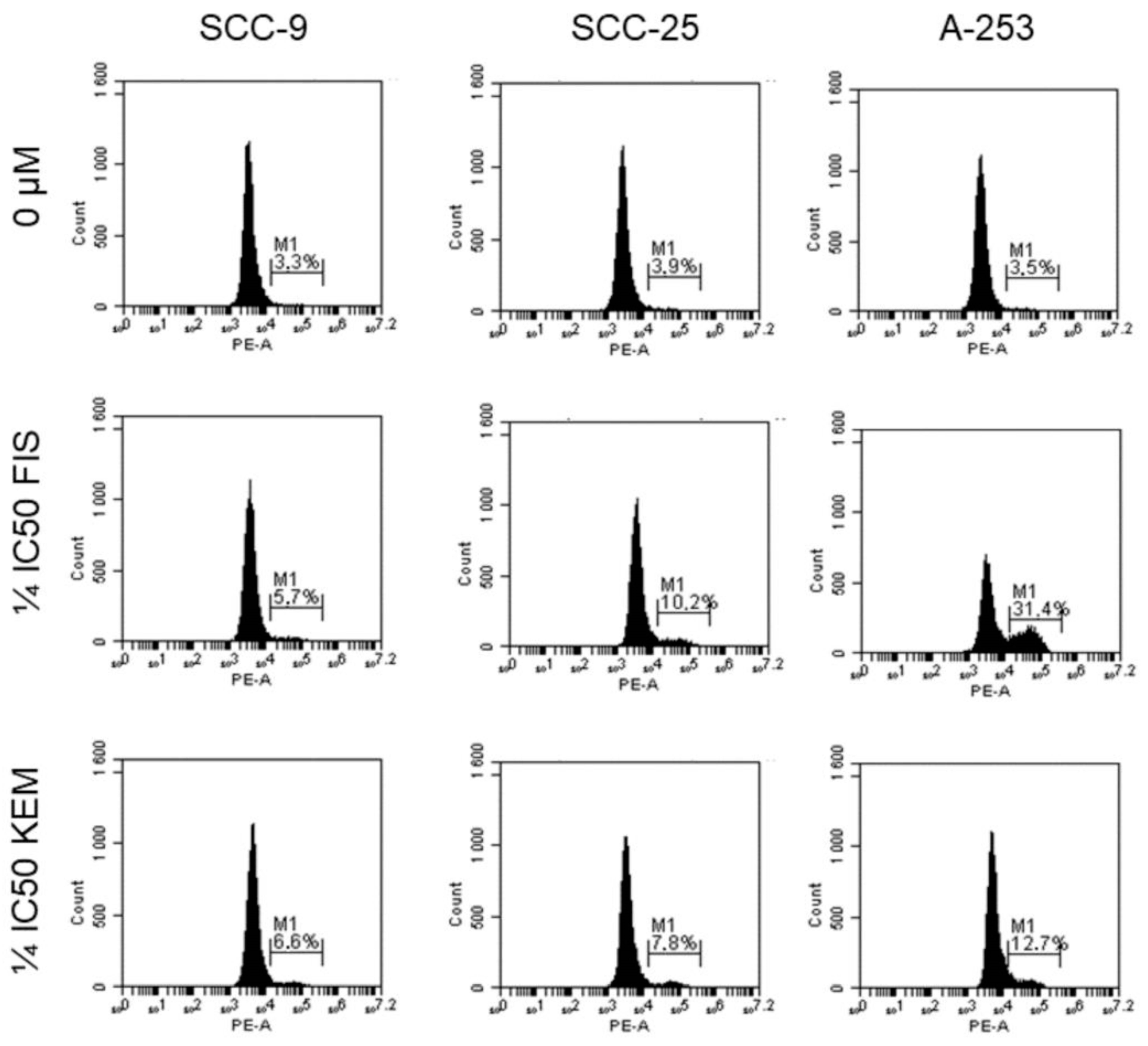
3.5. Kaempferol and Fisetin Induce Apoptosis in HNC Cells by Activating Caspase-3
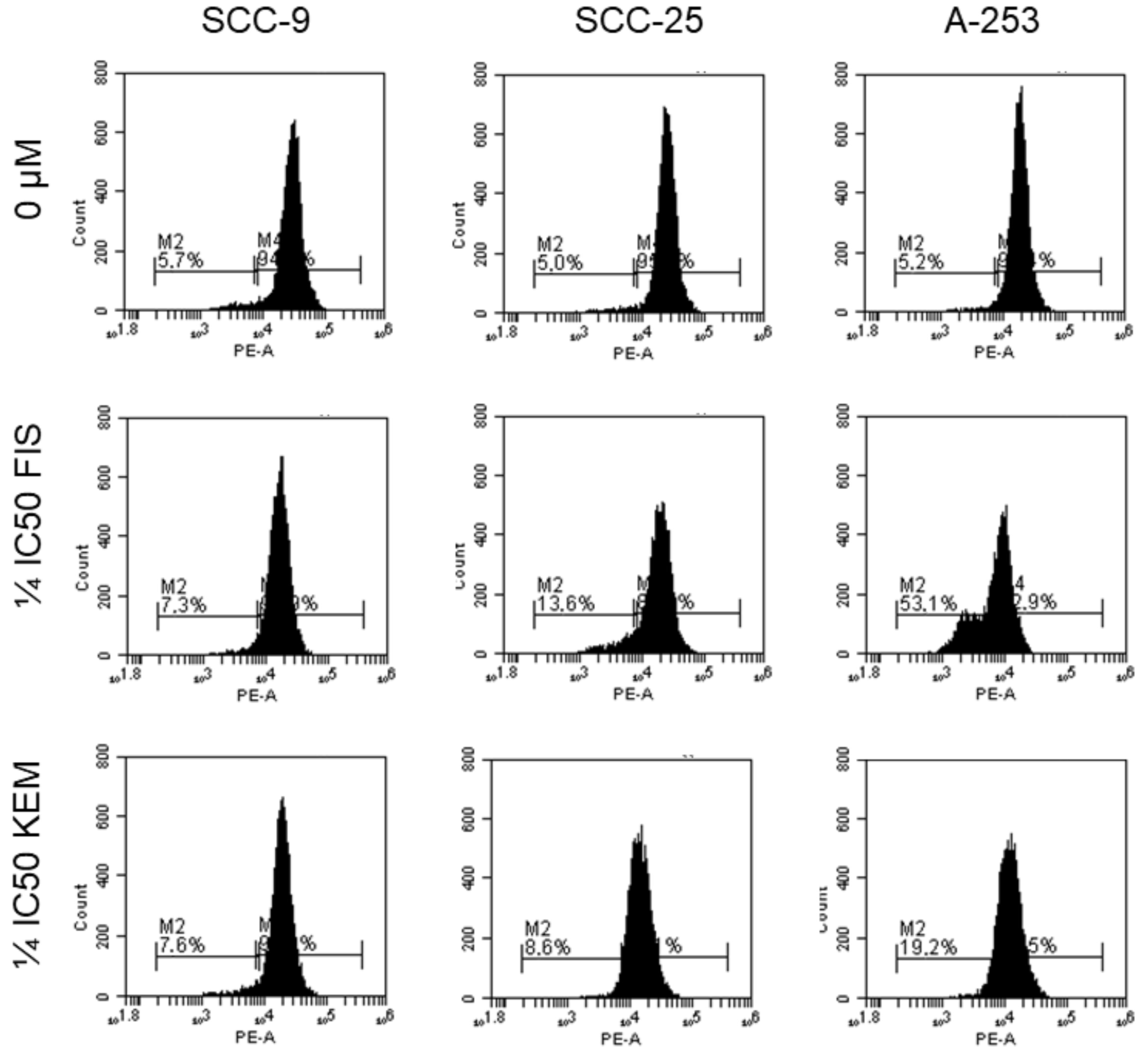
3.6. Kaempferol and Fisetin Inhibit the Anti-Apoptotic Proteins of the Bcl-2 Family
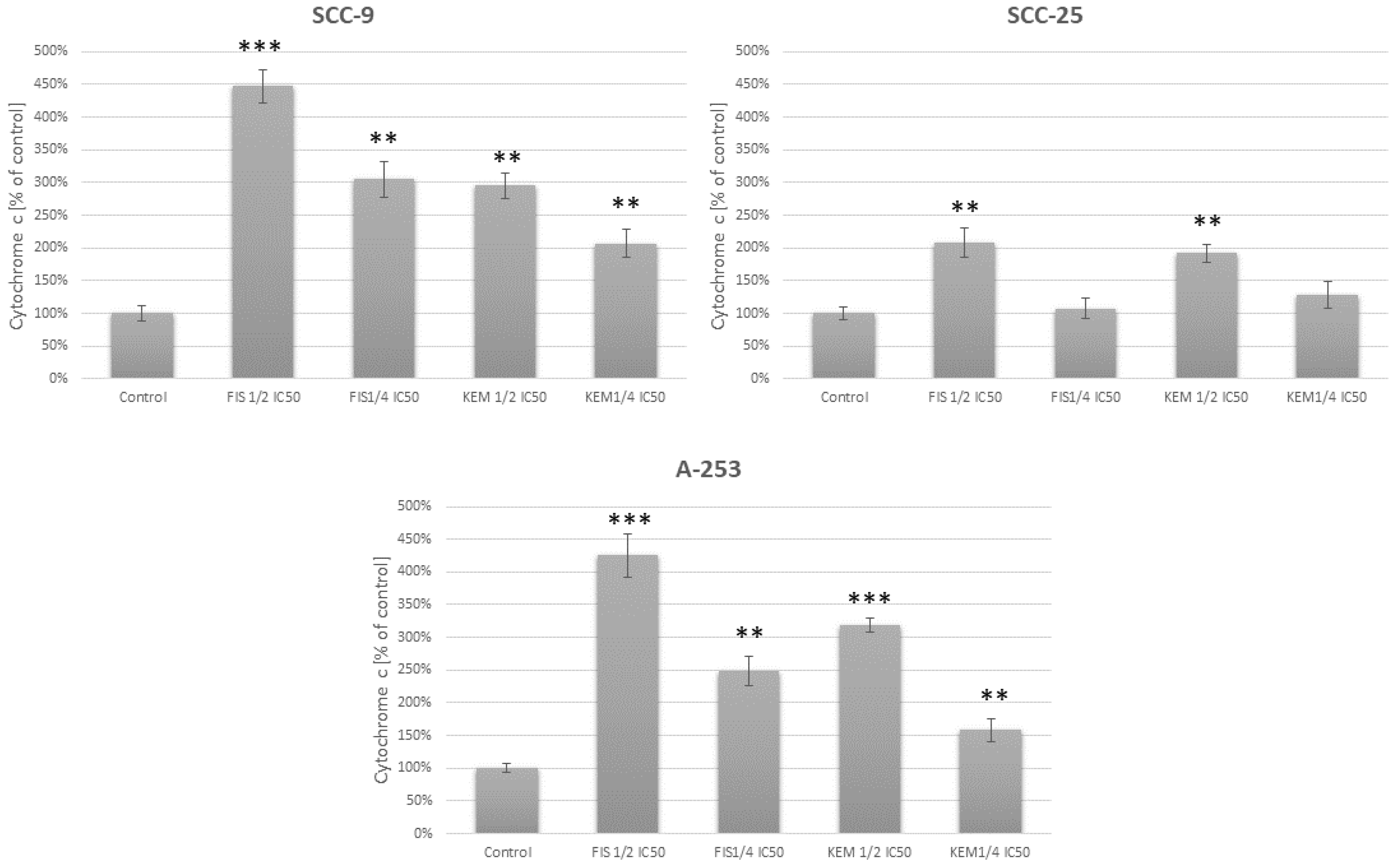
3.7. Fisetin Increases the Release of Cytochrome c in Head and Neck Cancer Cells
3.8. Kaempferol and Fisetin Cause Morphological Changes in HNC Cells
3.9. Kaempferol and Fisetin Induce the Formation of Apoptotic Bodies in HNC Cells
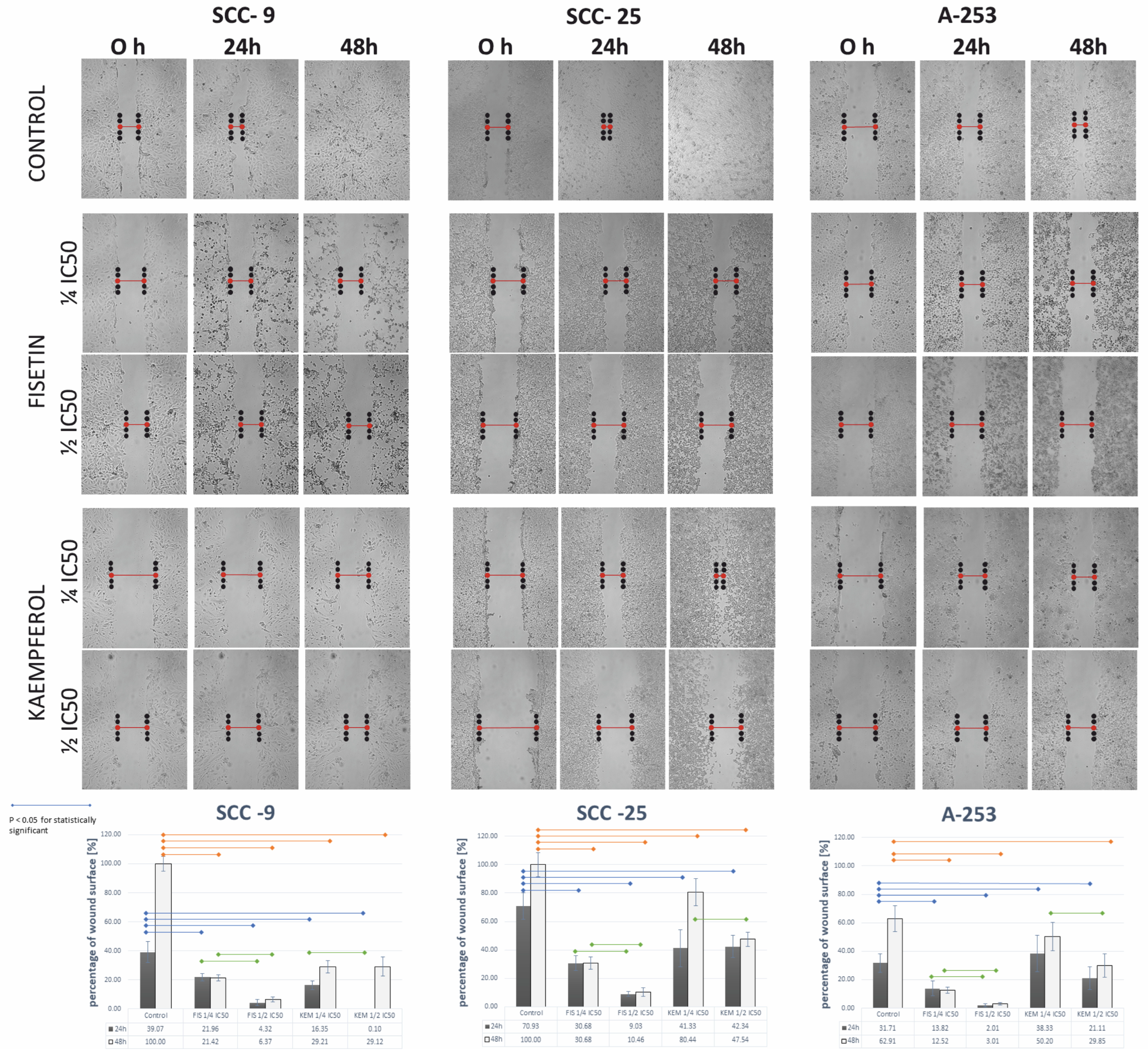
3.10. Kaempferol and Fisetin Inhibit the Migration of HNCs Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, K.B.; Lang, J.J.; Compton, K.; Xu, R.; Acheson, A.R.; Henrikson, H.J.; Kocarnik, J.M.; Penberthy, L.; Aali, A.; Abbas, Q.; et al. The Global Burden of Cancer Attributable to Risk Factors, 2010–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Cancer (INAC). International Agency for Research on Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 17 April 2022).
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Bai, X.; Cui, C.; Yin, J.; Li, H.; Gong, Q.; Wei, B.; Lu, Y. The Association between Oral Hygiene and Head and Neck Cancer: A Meta-Analysis. Acta Odontol. Scand. 2023, 81, 174–395. [Google Scholar] [CrossRef]
- Starzyńska, A.; Sobocki, B.K.; Alterio, D. Current Challenges in Head and Neck Cancer Management. Cancers 2022, 14, 358. [Google Scholar] [CrossRef]
- Brook, I. Late Side Effects of Radiation Treatment for Head and Neck Cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [Green Version]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Kiliçkan, E.; Ayar, A.; Çolak, R. Effects of Two Antioxidants; α-Lipoic Acid and Fisetin against Diabetic Cataract in Mice. Int. Ophthalmol. 2015, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Elsallabi, O.; Patruno, A.; Pesce, M.; Cataldi, A.; Carradori, S.; Gallorini, M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules 2022, 27, 738. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Seguin, J.; Ly, N.K.; Henríquez, L.C.; Plansart, E.; Hammad, K.; Gahoual, R.; Dhôtel, H.; Izabelle, C.; Saubamea, B.; et al. Designing Fisetin Nanocrystals for Enhanced in Cellulo Anti-Angiogenic and Anticancer Efficacy. Int. J. Pharm. X 2022, 4, 100138. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Krzykawski, K.; Kabała-Dzik, A.; Wojtyczka, R.D.; Chodurek, E.; Dziedzic, A. Fisetin, a Potent Anticancer Flavonol Exhibiting Cytotoxic Activity against Neoplastic Malignant Cells and Cancerous Conditions: A Scoping, Comprehensive Review. Nutrients 2022, 14, 2604. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutrients 2021, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Kimura, R.; Matsunaga, H.; Matsunaga, T.; Yoshino, Y.; Endo, S.; Ikari, A. Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression. Int. J. Mol. Sci. 2022, 23, 7536. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Baran, Y. Fisetin and Hesperetin Induced Apoptosis and Cell Cycle Arrest in Chronic Myeloid Leukemia Cells Accompanied by Modulation of Cellular Signaling. Tumor Biol. 2016, 37, 5781–5795. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.-X.; Chen, J. Fisetin Inhibits Inflammation and Induces Autophagy by Mediating PI3K/AKT/MTOR Signaling in LPS-Induced RAW264.7 Cells. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Kim, T.W. Fisetin, an Anti-Inflammatory Agent, Overcomes Radioresistance by Activating the PERK-ATF4-CHOP Axis in Liver Cancer. Int. J. Mol. Sci. 2023, 24, 9076. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef]
- Su, C.-H.; Kuo, C.-L.; Lu, K.-W.; Yu, F.-S.; Ma, Y.-S.; Yang, J.-L.; Chu, Y.-L.; Chueh, F.-S.; Liu, K.-C.; Chung, J.-G. Fisetin-Induced Apoptosis of Human Oral Cancer SCC-4 Cells through Reactive Oxygen Species Production, Endoplasmic Reticulum Stress, Caspase-, and Mitochondria-Dependent Signaling Pathways. Env. Toxicol. 2017, 32, 1725–1741. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Suh, Y.; Afaq, F.; Mukhtar, H. Dietary Agents for Chemoprevention of Prostate Cancer. Cancer Lett. 2008, 265, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, H.C.; Pearlman, R.L.; Afaq, F. Fisetin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 213–244. [Google Scholar] [CrossRef]
- Dorrigiv, M.; Zareiyan, A.; Hosseinzadeh, H. Onion (Allium Cepa) and Its Main Constituents as Antidotes or Protective Agents against Natural or Chemical Toxicities: A Comprehensive Review. Iran. J. Pharm. Res. 2021, 20, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Roshangar, L. Kaempferol: A Potential Agent in the Prevention of Colorectal Cancer. Physiol. Rep. 2022, 10, e15488. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol Induces ROS-Dependent Apoptosis in Pancreatic Cancer Cells via TGM2-Mediated Akt/MTOR Signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef]
- Swanson, H.I.; Choi, E.-Y.; Helton, W.B.; Gairola, C.G.; Valentino, J. Impact of Apigenin and Kaempferol on Human Head and Neck Squamous Cell Carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2014, 117, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of Apoptosis by Cancer Chemotherapy. Exp. Cell. Res. 2000, 256, 42–49. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, H.T.-L.; Truong, K.D. Comparative Cytotoxic Effects of Methanol, Ethanol and DMSO on Human Cancer Cell Lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Tanasiewicz, M.; Varoni, E.M.; Iriti, M. Flavonoids Induce Migration Arrest and Apoptosis in Detroit 562 Oropharynx Squamous Cell Carcinoma Cells. Processes 2021, 9, 426. [Google Scholar] [CrossRef]
- Kleczka, A.; Kubina, R.; Dzik, R.; Jasik, K.; Stojko, J.; Cholewa, K.; Kabała-Dzik, A. Caffeic Acid Phenethyl Ester (CAPE) Induced Apoptosis in Serous Ovarian Cancer OV7 Cells by Deregulation of BCL2/BAX Genes. Molecules 2020, 25, 3514. [Google Scholar] [CrossRef]
- Rahman, N.; Khan, H.; Zia, A.; Khan, A.; Fakhri, S.; Aschner, M.; Gul, K.; Saso, L. Bcl-2 Modulation in P53 Signaling Pathway by Flavonoids: A Potential Strategy towards the Treatment of Cancer. Int. J. Mol. Sci. 2021, 22, 11315. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome c Release from Mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afroze, N.; Pramodh, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Sundaram, M.K.; Haque, S.; Hussain, A. Fisetin Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress and Inflammation in Human Cancer Cells, HeLa. Int. J. Mol. Sci. 2022, 23, 1707. [Google Scholar] [CrossRef]
- Hassani, S.; Maghsoudi, H.; Fattahi, F.; Malekinejad, F.; Hajmalek, N.; Sheikhnia, F.; Kheradmand, F.; Fahimirad, S.; Ghorbanpour, M. Flavonoids Nanostructures Promising Therapeutic Efficiencies in Colorectal Cancer. Int. J. Biol. Macromol. 2023, 241, 124508. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The Mechanism of Anticancer Action and Potential Clinical Use of Kaempferol in the Treatment of Breast Cancer. Biomed. Pharm. 2019, 117, 109086. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Alarifi, A.; Karami, A.M.; Ayub, R.; Abduh, N.A.Y.; Saeed, W.S.; Muddassir, M. Antiproliferative Mechanisms of a Polyphenolic Combination of Kaempferol and Fisetin in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 6393. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.-G. Kaempferol Induces Autophagic Cell Death via IRE1-JNK-CHOP Pathway and Inhibition of G9a in Gastric Cancer Cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. P-Coumaric Acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 8602. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Fang, W.; Liang, K.; Li, X.; Zhang, F.; Pang, Y.; Fang, G.; Wang, X. Kaempferol Suppresses Androgen-Dependent and Androgen-Independent Prostate Cancer by Regulating Ki67 Expression. Mol. Biol. Rep. 2022, 49, 4607–4617. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.H. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway In Vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.-H.; Chen, Y.C. Kaempferol Nanoparticles Achieve Strong and Selective Inhibition of Ovarian Cancer Cell Viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xing, J.; Aikemu, B.; Sun, J.; Zheng, M. Kaempferol Exhibits a Synergistic Effect with Doxorubicin to Inhibit Proliferation, Migration, and Invasion of Liver Cancer. Oncol. Rep. 2021, 45, 32. [Google Scholar] [CrossRef] [PubMed]
- Imtiyaz, K.; Husain Rahmani, A.; Alsahli, M.A.; Almatroodi, S.A.; Rizvi, M.M.A. Fisetin Induces Apoptosis in Human Skin Cancer Cells through Downregulating MTH1. J. Biomol. Struct. Dyn. 2022, 1–15. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, J.W. Fisetin Induces Apoptosis in Human Nonsmall Lung Cancer Cells via a Mitochondria-Mediated Pathway. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 300–309. [Google Scholar] [CrossRef]
- Chen, C.-M.; Hsieh, Y.-H.; Hwang, J.-M.; Jan, H.-J.; Hsieh, S.-C.; Lin, S.-H.; Lai, C.-Y. Fisetin Suppresses ADAM9 Expression and Inhibits Invasion of Glioma Cancer Cells through Increased Phosphorylation of ERK1/2. Tumor Biol. 2015, 36, 3407–3415. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Chen, M.-C.; Tseng, Y.-S.; Chen, M.-C.; Zhou, Z.; Yang, J.-J.; Lin, Y.-M.; Viswanadha, V.P.; Wang, G.; Huang, C.-Y. Fisetin Activates Hippo Pathway and JNK/ERK/AP-1 Signaling to Inhibit Proliferation and Induce Apoptosis of Human Osteosarcoma Cells via ZAK Overexpression. Environ. Toxicol. 2019, 34, 902–911. [Google Scholar] [CrossRef]
- Sechi, M.; Lall, R.K.; Afolabi, S.O.; Singh, A.; Joshi, D.C.; Chiu, S.-Y.; Mukhtar, H.; Syed, D.N. Fisetin Targets YB-1/RSK Axis Independent of Its Effect on ERK Signaling: Insights from in Vitro and in Vivo Melanoma Models. Sci. Rep. 2018, 8, 15726. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.Y.; Jeong, S.-J.; Kim, S.-H.; Jung, J.H.; Kim, J.-H.; Koh, W.; Chen, C.-Y.; Kim, S.-H. Activation of Reactive Oxygen Species/AMP Activated Protein Kinase Signaling Mediates Fisetin-Induced Apoptosis in Multiple Myeloma U266 Cells. Cancer Lett. 2012, 319, 197–202. [Google Scholar] [CrossRef]
- Sarvarian, P.; Samadi, P.; Gholipour, E.; Akhodadadi, M.; Pourakbari, R.; Akbarzadelale, P.; Shamsasenjan, K. Fisetin-Loaded Grape-Derived Nanoparticles Improve Anticancer Efficacy in MOLT-4 Cells. Biochem. Biophys. Res. Commun. 2023, 658, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.-Y.; Ye, L.-X.; Lin, J.-S.; Lin, H.-Y.; Yu, L.-R.; Wang, C.-Y.; Mao, X.-D.; Zhang, S.-H.; Sun, P.-M. An Integrated Approach of Network Pharmacology, Molecular Docking, and Experimental Verification Uncovers Kaempferol as the Effective Modulator of HSD17B1 for Treatment of Endometrial Cancer. J. Transl. Med. 2023, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-L.; Hung, F.-M.; Lee, C.-H.; Yeh, M.-Y.; Lee, M.-H.; Lu, H.-F.; Chen, Y.-L.; Liu, J.-Y.; Chung, J.-G. Fisetin Induces Apoptosis of HSC3 Human Oral Cancer Cells Through Endoplasmic Reticulum Stress and Dysfunction of Mitochondria-Mediated Signaling Pathways. In Vivo 2017, 31, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Won, D.-H.; Chung, S.H.; Shin, J.-A.; Hong, K.-O.; Yang, I.-H.; Yun, J.-W.; Cho, S.-D. Induction of Sestrin 2 Is Associated with Fisetin-Mediated Apoptosis in Human Head and Neck Cancer Cell Lines. J. Clin. Biochem. Nutr. 2019, 64, 97. [Google Scholar] [CrossRef] [Green Version]
- Park, B.-S.; Choi, N.-E.; Lee, J.H.; Kang, H.-M.; Yu, S.-B.; Kim, H.-J.; Kang, H.-K.; Kim, I.-R. Crosstalk between Fisetin-Induced Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma. J. Cancer 2019, 10, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-J.; Jia, S.-S. Fisetin Inhibits Laryngeal Carcinoma through Regulation of AKT/NF-ΚB/MTOR and ERK1/2 Signaling Pathways. Biomed. Pharmacother. 2016, 83, 1164–1174. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.H.; Song, K.; Kim, S.H.; Yoon, J.-H.; Kim, K.-S. Kaempferol and Quercetin, Components of Ginkgo Biloba Extract (EGb 761), Induce Caspase-3-Dependent Apoptosis in Oral Cavity Cancer Cells. Phytother. Res. 2010, 24, S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, P.-N.; Chen, M.-K.; Yang, W.-E.; Tang, C.-H.; Yang, S.-F.; Hsieh, Y.-S. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by Down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Park, J.H.Y. Kaempferol Induces Cell Cycle Arrest in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2013, 18, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Dang, Q.; Xu, D.; Chen, Y.; Zhu, G.; Wu, K.; Zeng, J.; Long, Q.; Wang, X.; He, D.; et al. Kaempferol Induces Cell Cycle Arrest and Apoptosis in Renal Cell Carcinoma through EGFR/P38 Signaling. Oncol. Rep. 2014, 31, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.J.; Ahn, W.S. Kaempferol Induced the Apoptosis via Cell Cycle Arrest in Human Breast Cancer MDA-MB-453 Cells. Nutr. Res. Pr. 2008, 2, 322–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-S.; Qin, X.-J.; Dai, W. Fisetin Suppresses Malignant Proliferation in Human Oral Squamous Cell Carcinoma through Inhibition of Met/Src Signaling Pathways. Am. J. Transl. Res. 2017, 9, 5678–5683. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubina, R.; Krzykawski, K.; Dziedzic, A.; Kabała-Dzik, A. Kaempferol and Fisetin-Related Signaling Pathways Induce Apoptosis in Head and Neck Cancer Cells. Cells 2023, 12, 1568. https://doi.org/10.3390/cells12121568
Kubina R, Krzykawski K, Dziedzic A, Kabała-Dzik A. Kaempferol and Fisetin-Related Signaling Pathways Induce Apoptosis in Head and Neck Cancer Cells. Cells. 2023; 12(12):1568. https://doi.org/10.3390/cells12121568
Chicago/Turabian StyleKubina, Robert, Kamil Krzykawski, Arkadiusz Dziedzic, and Agata Kabała-Dzik. 2023. "Kaempferol and Fisetin-Related Signaling Pathways Induce Apoptosis in Head and Neck Cancer Cells" Cells 12, no. 12: 1568. https://doi.org/10.3390/cells12121568
APA StyleKubina, R., Krzykawski, K., Dziedzic, A., & Kabała-Dzik, A. (2023). Kaempferol and Fisetin-Related Signaling Pathways Induce Apoptosis in Head and Neck Cancer Cells. Cells, 12(12), 1568. https://doi.org/10.3390/cells12121568