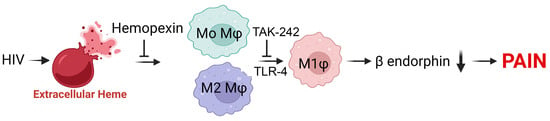
Heme-Induced Macrophage Phenotype Switching and Impaired Endogenous Opioid Homeostasis Correlate with Chronic Widespread Pain in HIV
Abstract
1. Introduction
2. Methods
2.1. Human Participants and Measurements
2.2. Reagents
2.3. Cell Culture
2.4. Macrophage Polarization
2.5. Flow Cytometry of THP-1 Cells
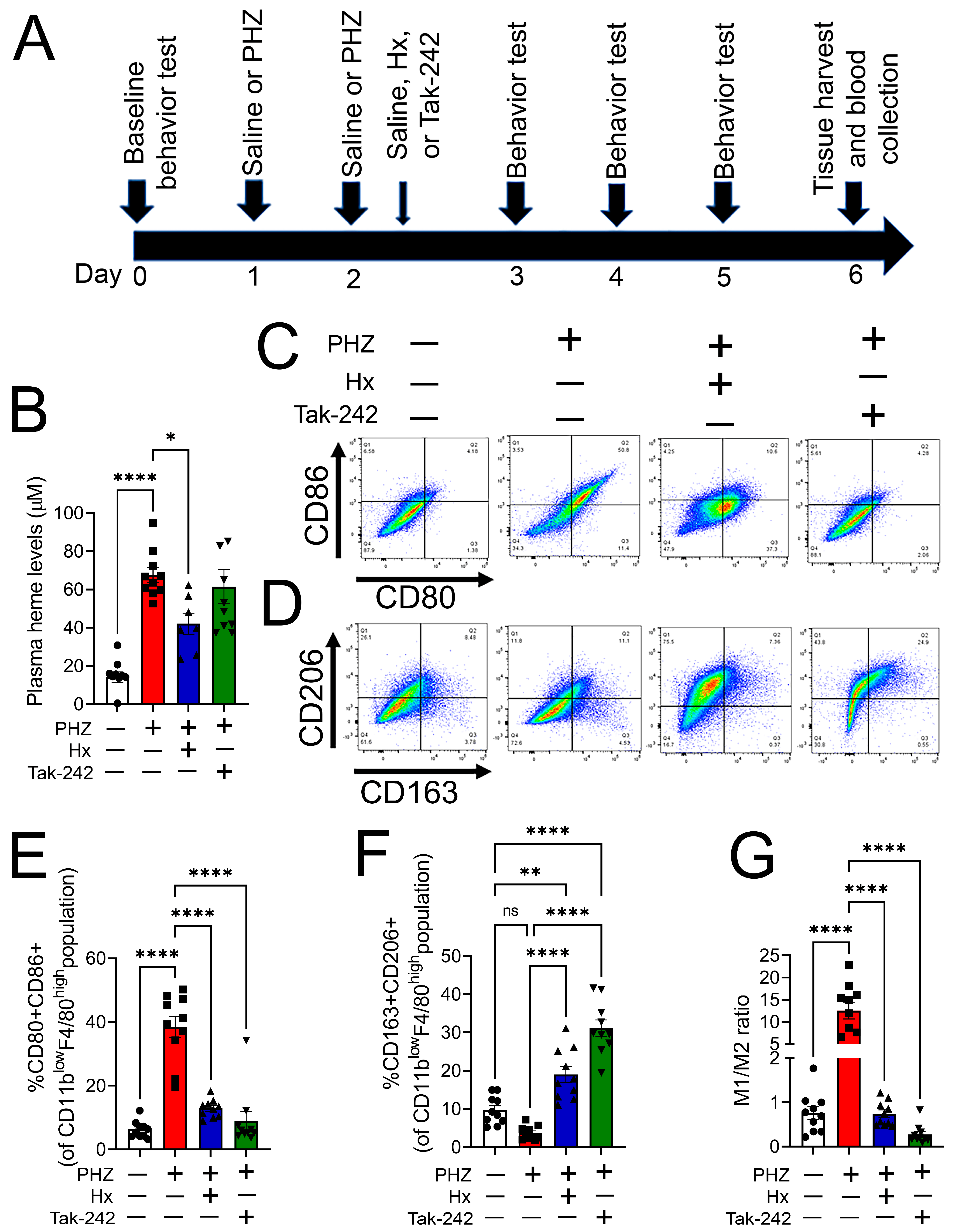
2.6. Mouse Model and Treatments
2.7. Preparation of Single Cell Suspension from Mouse Liver
2.8. Flow Cytometric Analysis of Single Cell Macrophage Suspension from Liver
2.9. cDNA Isolation and Real-Time PCR
2.10. Heme Quantification
2.11. β-Endorphin Assay
2.12. Behavioral Assay
2.13. Von Frey Microfilament Test
2.14. Acetone Evaporation Test
2.15. Hot Plate Test
2.16. Statistics
2.17. Study Approval
3. Results
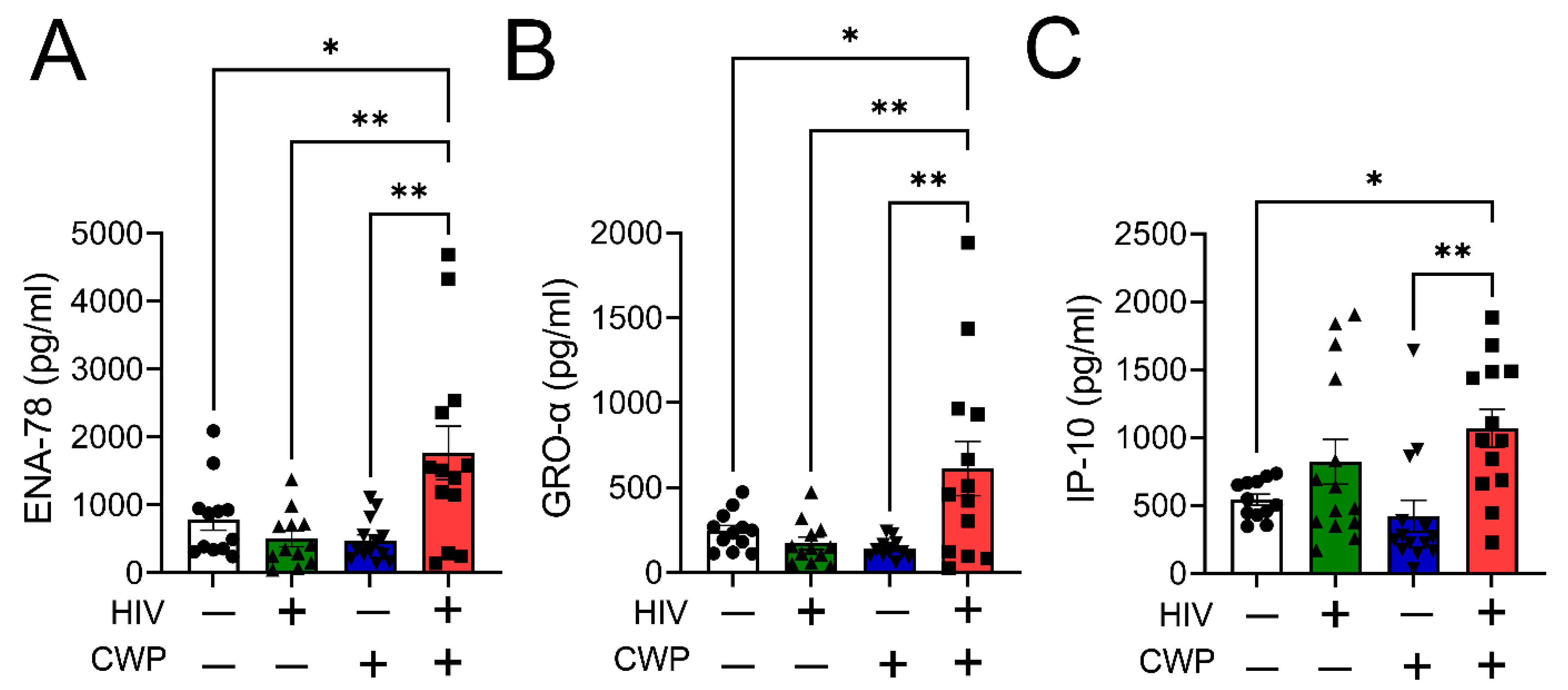
3.1. M1 Macrophage-Specific Chemokines Were Elevated in PWH with CWP
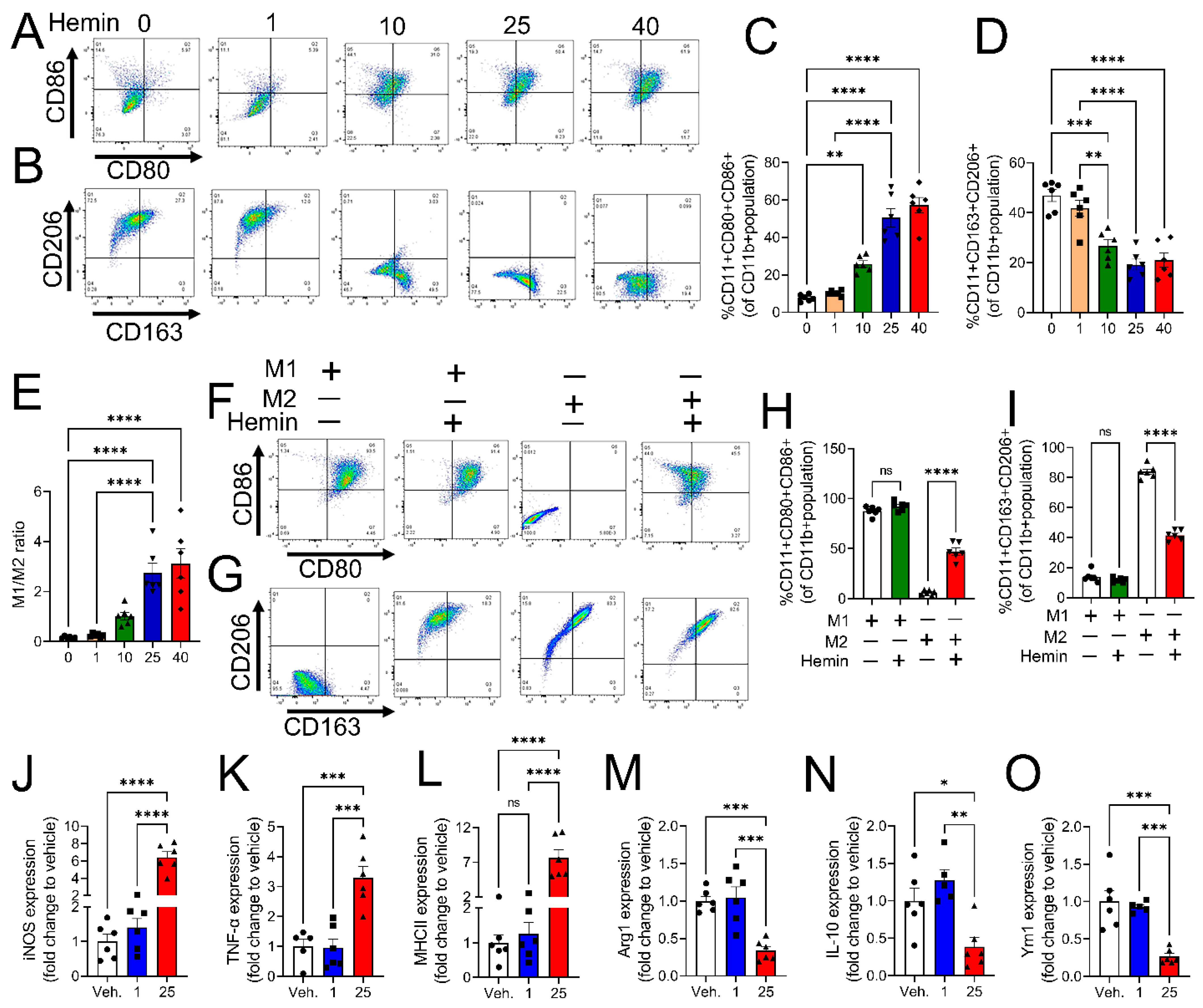
3.2. Heme-Induced Transition of M0 and M2 Macrophages to M1 Phenotype
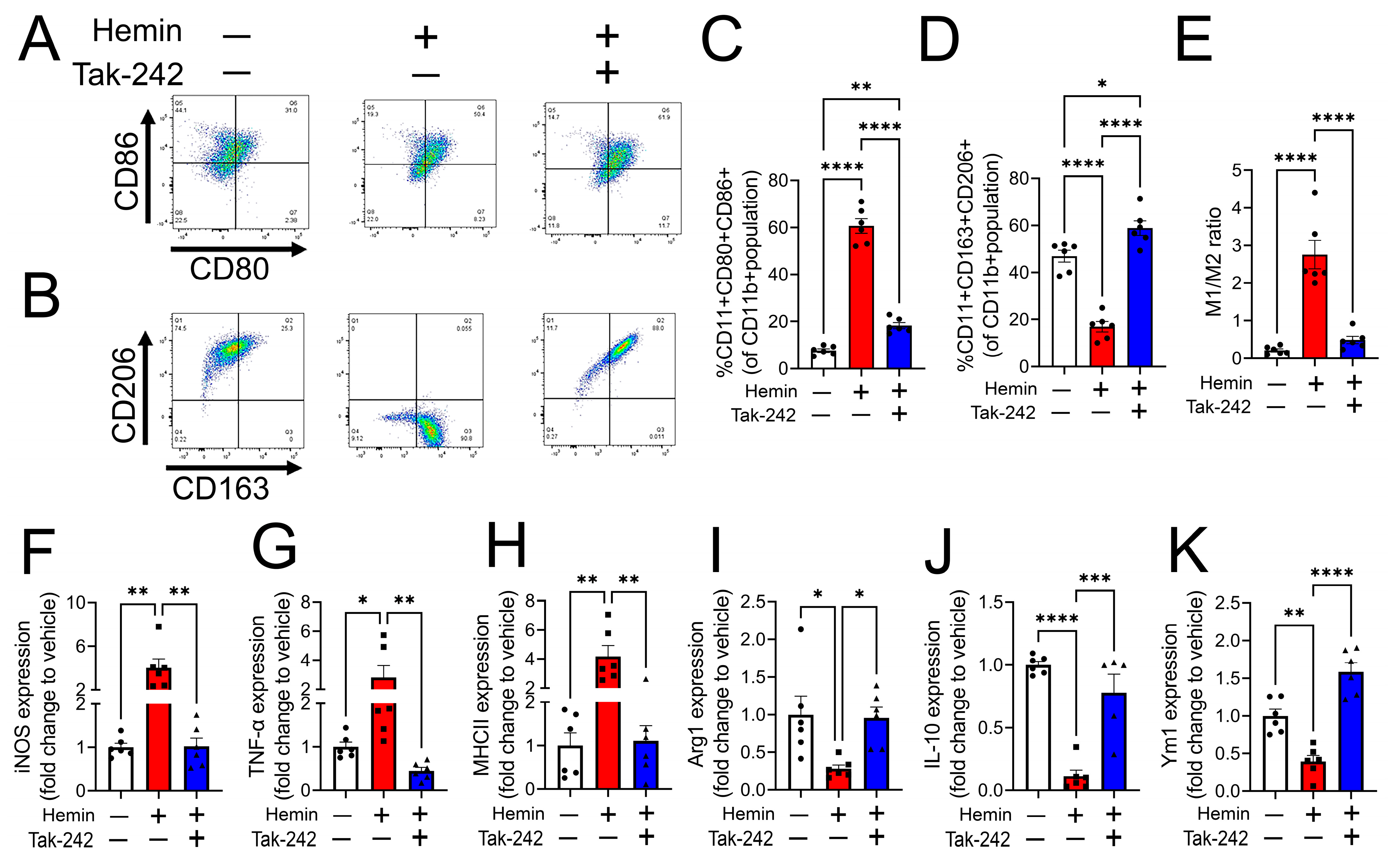
3.3. Heme-Induced M1 Phenotype Transition Is Mediated by TLR4
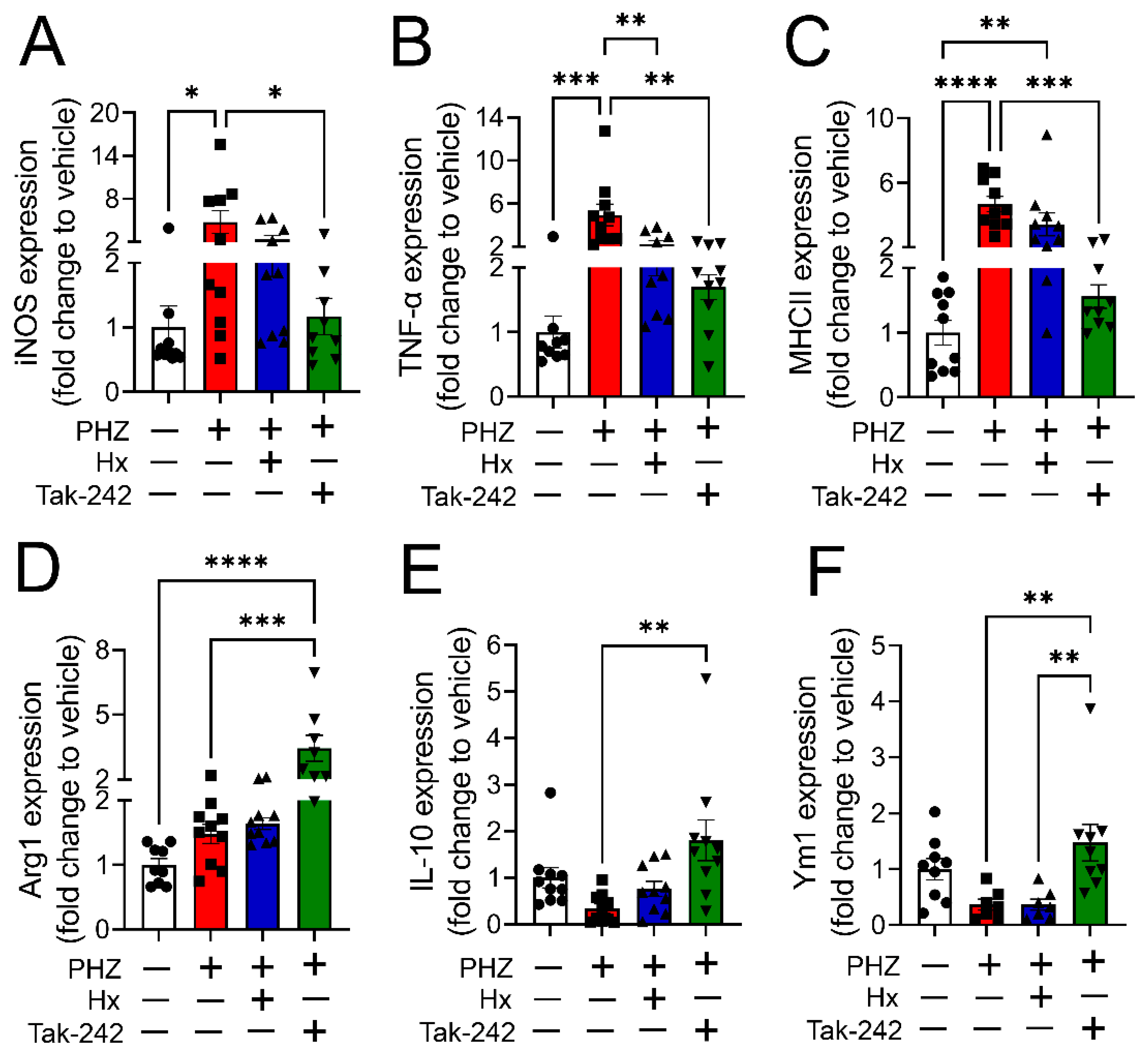
3.4. Heme Scavenging or TLR4 Inhibition Attenuated Monocyte/Macrophage Phenotype Transition in a Mouse Model of Hemolysis
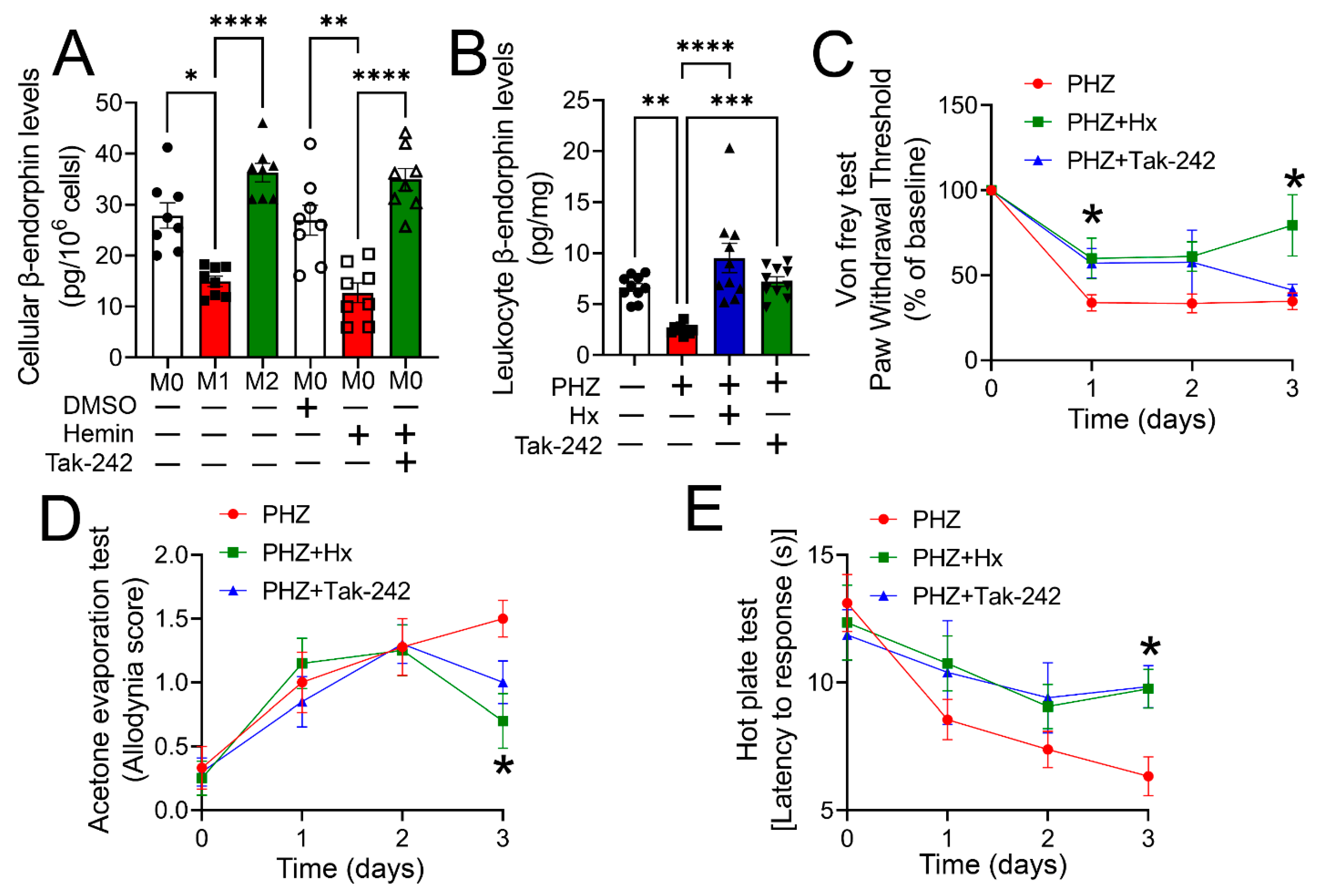
3.5. Heme Scavenging or TLR4 Inhibition Ameliorated Impaired Opioid Homeostasis and Hypersensitivity in a Mouse Model of Hemolysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miaskowski, C.; Penko, J.M.; Guzman, D.; Mattson, J.E.; Bangsberg, D.R.; Kushel, M.B. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with hiv infection. J. Pain 2011, 12, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.S.; Westfall, A.; Raper, J.L.; Zinski, A.; Norton, W.E.; Willig, J.H.; Gross, R.; Ritchie, C.S.; Saag, M.S.; Mugavero, M.J. Pain, mood, and substance abuse in hiv: Implications for clinic visit utilization, antiretroviral therapy adherence, and virologic failure. Am. J. Ther. 2012, 61, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Madden, V.J.; Parker, R.; Goodin, B.R. Chronic pain in people with hiv: A common comorbidity and threat to quality of life. Pain Manag. 2020, 10, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.S.; Cen, L.; Praestgaard, A.; Turner, M.; Obando, A.; Alpert, C.; Woolston, S.; Casarett, D.; Kostman, J.; Gross, R.; et al. Pain and physical and psychological symptoms in ambulatory hiv patients in the current treatment era. J. Pain Symptom Manag. 2011, 43, 638–645. [Google Scholar] [CrossRef]
- Robinson-Papp, J.; Simpson, D.M. Neuromuscular diseases associated with hiv-1 infection. Muscle Nerve 2009, 40, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Mawuntu, A.H.; Mahama, C.N.; Khosama, H.; Estiasari, R.; Imran, D. Early detection of peripheral neuropathy using stimulated skin wrinkling test in human immunodeficiency virus infected patients: A cross-sectional study. Medicine 2018, 97, e11526. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001, 6, 14–20. [Google Scholar] [CrossRef]
- Merlin, J.S.; Westfall, A.O.; Heath, S.L.; Goodin, B.R.; Stewart, J.C.; Sorge, R.E.; Younger, J. Brief report: Il-1β levels are associated with chronic multisite pain in people living with hiv. Am. J. Ther. 2017, 75, e99–e103. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in m1(lps+) vs. Classically and m2(lps-) vs. Alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Cabot, P.J.; Carter, L.; Gaiddon, C.; Zhang, Q.; Schäfer, M.; Loeffler, J.P.; Stein, C. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J. Clin. Investig. 1997, 100, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Cabot, P.J.; Carter, L.; Schäfer, M.; Stein, C. Methionine-enkephalin-and dynorphin a-release from immune cells and control of inflammatory pain. Pain 2001, 93, 207–212. [Google Scholar] [CrossRef]
- Mousa, S.A.; Shakibaei, M.; Sitte, N.; Schäfer, M.; Stein, C. Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology 2004, 145, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Sibinga, N.E.S.; Goldstein, A. Opioid peptides and opioid receptors in cells of the immune system. Annu. Rev. Immunol. 1988, 6, 219–249. [Google Scholar] [CrossRef] [PubMed]
- Pannell, M.; Labuz, D.; Celik, M.; Keye, J.; Batra, A.; Siegmund, B.; Machelska, H. Adoptive transfer of m2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflamm. 2016, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; DeBerry, J.J.; Ahmad, I.; Lynn, P.; Dewitte, C.; Malik, S.; Merlin, J.S.; Goodin, B.R.; Heath, S.L.; Matalon, S. Heme attenuates beta-endorphin levels in leukocytes of hiv positive individuals with chronic widespread pain. Redox Biol. 2020, 36, 101684. [Google Scholar] [CrossRef] [PubMed]
- Fortes, G.B.; Alves, L.S.; De Oliveira, R.; Dutra, F.F.; Rodrigues, D.; Fernandez, P.L.; Souto-Padron, T.; DE Rosa, M.J.; Kelliher, M.; Golenbock, D.; et al. Heme induces programmed necrosis on macrophages through autocrine tnf and ros production. Blood 2012, 119, 2368–2375. [Google Scholar] [CrossRef]
- Vinchi, F.; Costa da Silva, M.; Ingoglia, G.; Petrillo, S.; Brinkman, N.; Zuercher, A.; Cerwenka, A.; Tolosano, E.; Muckenthaler, M.U. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 2016, 127, 473–486. [Google Scholar] [CrossRef]
- Aggarwal, S.; Lam, A.; Bolisetty, S.; Carlisle, M.A.; Traylor, A.; Agarwal, A.; Matalon, S. Heme attenuation ameliorates irritant gas inhalation-induced acute lung injury. Antioxid. Redox Signal. 2016, 24, 99–112. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme triggers tlr4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graça-Souza, A.V.; Bozza, M.T. Characterization of heme as activator of toll-like receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.F.; Alves, L.S.; Rodrigues, D.; Fernandez, P.L.; de Oliveira, R.B.; Golenbock, D.T.; Zamboni, D.S.; Bozza, M.T. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. USA 2014, 111, E4110-8. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.S.M.; Sathyapalan, T.; Diboun, I.; Atkin, S.L.; Butler, A.E. Identification of macrophage activation-related biomarkers in obese type 2 diabetes that may be indicative of enhanced respiratory risk in covid-19. Sci. Rep. 2021, 11, 6428. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesthesia 2018, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Abrial, C.; Salvator, H.; Brollo, M.; Naline, E.; Devillier, P. The role of toll-like receptors in the production of cytokines by human lung macrophages. J. Innate Immun. 2020, 12, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Bruno, K.; Woller, S.; Miller, Y.I.; Yaksh, T.L.; Wallace, M.; Beaton, G.; Chakravarthy, K. Targeting toll-like receptor-4 (tlr4)-an emerging therapeutic target for persistent pain states. Pain 2018, 159, 1908–1915. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and m2 macrophages derived from thp-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Savelkoul, H.F.J.; Wichers, H.J. Characterization of polarized thp-1 macrophages and polarizing ability of lps and food compounds. Food Funct. 2013, 4, 266–276. [Google Scholar] [CrossRef]
- Kegel, V.; Deharde, D.; Pfeiffer, E.; Zeilinger, K.; Seehofer, D.; Damm, G. Protocol for isolation of primary human hepatocytes and corresponding major populations of non-parenchymal liver cells. J. Vis. Exp. 2016, e53069. [Google Scholar] [CrossRef]
- Chatterjee, T.; Pattanayak, R.; Ukil, A.; Chowdhury, S.; Bhattacharyya, M. Autophagy protects peripheral blood mononuclear cells against inflammation, oxidative and nitrosative stress in diabetic dyslipidemia. Free. Radic. Biol. Med. 2019, 143, 309–323. [Google Scholar] [CrossRef]
- Chatterjee, T.; De, D.; Chowdhury, S.; Bhattacharyya, M. Nuclear factor nf-κb1 functional promoter polymorphism and its expression conferring the risk of type 2 diabetes-associated dyslipidemia. Mamm. Genome 2020, 31, 252–262. [Google Scholar] [CrossRef]
- Bonin, R.P.; Bories, C.; De Koninck, Y. A simplified up-down method (sudo) for measuring mechanical nociception in rodents using von frey filaments. Mol. Pain 2014, 10, 26. [Google Scholar] [CrossRef]
- CChoi, J.; Liu, R.-M.; Kundu, R.K.; Sangiorgi, F.; Wu, W.; Maxson, R.; Forman, H.J. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 tat-transgenic mice. J. Biol. Chem. 2000, 275, 3693–3698. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.T.; Roederer, M.; Israelski, D.M.; Bubp, J.; Mole, L.A.; McSHANE, D.; Deresinski, S.C.; Ross, W.; Sussman, H.; Raju, P.A.; et al. Intracellular glutathione levels in t cell subsets decrease in hiv-infected individuals. AIDS Res. Hum. Retroviruses 1992, 8, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kline, E.R.; Kleinhenz, D.J.; Liang, B.; Dikalov, S.; Guidot, D.M.; Hart, C.M.; Jones, D.P.; Sutliff, R.L. “Vascular oxidative stress and nitric oxide depletion in hiv-1 transgenic rats are reversed by glutathione restoration”. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2792–H2804. [Google Scholar] [CrossRef] [PubMed]
- East, J.; Blanton, L.S. Symptomatic hyperbilirubinemia secondary to dapsone-induced hemolysis and atazanavir therapy. Antimicrob. Agents Chemother. 2011, 56, 1081–1083. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Flores-Cantu, H.; Calderon-Hernandez, H.J.; A Diaz-Torres, M.; Villareal-Velazquez, H.J. Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with hiv and cryptococcal meningitis: A diagnostic challenge. Int. J. STD AIDS 2015, 26, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nóvoa, S.; Morello, J.; González, M.; Vispo, E.; Barreiro, P.; González-Pardo, G.; Jiménez-Nácher, I.; Gonzalez-Lahoz, J.; Soriano, V. Increase in serum bilirubin in hiv/hepatitis-c virus-coinfected patients on atazanavir therapy following initiation of pegylated-interferon and ribavirin. Aids 2008, 22, 2535–2537. [Google Scholar] [CrossRef]
- Becker, S.; Fusco, G.; Fusco, J.; Balu, R.; Gangjee, S.; Brennan, C.; Feinberg, J. Hiv-associated thrombotic microangiopathy in the era of highly active antiretroviral therapy: An observational study. Clin. Infect. Dis. 2004, 39 (Suppl. S5), S267–S275. [Google Scholar] [CrossRef]
- Louw, S.; Gounden, R.; Mayne, E.S. Gounden and E. S. Mayne. Thrombotic thrombocytopenic purpura (ttp)-like syndrome in the hiv era. Thromb. J. 2018, 16, 35. [Google Scholar] [CrossRef]
- Burke, P.A., Jr.; Aljadir, D.; Raman, T. Diagnosis, management, and pathogenesis of ttp/hus in an hiv positive patient. Del. Med J. 2010, 82, 309–312. [Google Scholar]
- Serpa, J.A.; Villarreal-Williams, E.; Giordano, T.P. Prevalence of g6pd deficiency in a large cohort of hiv-infected patients. J. Infect. 2010, 61, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Spinazzola, F.; Barletta, C.; DeMartino, G.; Martini, F.; Natili, S.; Noto, P.; Ferri, F.; Tossini, G.; Visco, G. Beta-endorphins acth and cortisol in csf and plasma of hiv infected patients. Riv. Eur. Sci. Med. Farmacol 1995, 17, 161–165. [Google Scholar]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble cd14 independently predict mortality in hiv infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Labuz, D.; Keye, J.; Glauben, R.; Machelska, H. Il-4 induces m2 macrophages to produce sustained analgesia via opioids. J. Clin. Investig. 2020, 5, e133093. [Google Scholar] [CrossRef] [PubMed]
- Adisa, O.A.; Hu, Y.; Ghosh, S.; Aryee, D.; Osunkwo, I.; Ofori-Acquah, S.F. Association between plasma free haem and incidence of vaso-occlusive episodes and acute chest syndrome in children with sickle cell disease. Br. J. Haematol. 2013, 162, 702–705. [Google Scholar] [CrossRef]
- Ghosh, S.; Adisa, O.A.; Chappa, P.; Tan, F.; Jackson, K.A.; Archer, D.R.; Ofori-Acquah, S.F. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J. Clin. Investig. 2013, 123, 4809–4820. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Kapitzke, D.; Vetter, I.; Cabot, P.J. Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control. Ther. Clin. Risk Manag. 2005, 1, 279–297. [Google Scholar]
- Wen, S.; Jiang, Y.; Liang, S.; Cheng, Z.; Zhu, X.; Guo, Q. Opioids regulate the immune system: Focusing on macrophages and their organelles. Front. Pharmacol. 2022, 12, 814241. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-F.; Chen, Y.-J.; Zhang, J.-X.; Shen, L.; Wang, R.; Zhou, J.-S.; Hu, J.-G.; Lü, H.-Z. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav. Immun. 2015, 45, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Willemen, H.L.; Eijkelkamp, N.; Carbajal, A.G.; Wang, H.; Mack, M.; Zijlstra, J.; Heijnen, C.J.; Kavelaars, A. Monocytes/macrophages control resolution of transient inflammatory pain. J. Pain 2014, 15, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The m1 and m2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.D.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-De-Arellano, M. Novel markers to delineate murine m1 and m2 macrophages. PLOS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Meng, J.; Lu, C.; Li, X.; Wang, E.; Shan, F. Macrophage polarization induced by neuropeptide methionine enkephalin (menk) promotes tumoricidal responses. Cancer Immunol. Immunother. 2012, 61, 1755–1768. [Google Scholar] [CrossRef]
- Tedesco, S.; Bolego, C.; Toniolo, A.; Nassi, A.; Fadini, G.P.; Locati, M.; Cignarella, A. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology 2015, 220, 545–554. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Sui, L.; Xu, H.; Piao, Q.; Liu, Y.; Qu, X.; Sun, Y.; Song, L.; Li, D.; et al. Antibiotics induce polarization of pleural macrophages to m2-like phenotype in patients with tuberculous pleuritis. Sci. Rep. 2017, 7, 14982. [Google Scholar] [CrossRef]
- Dong, H.; Yang, Y.; Gao, C.; Sun, H.; Wang, H.; Hong, C.; Wang, J.; Gong, F.; Gao, X. Lactoferrin-containing immunocomplex mediates antitumor effects by resetting tumor-associated macrophages to m1 phenotype. J. Immunother. Cancer 2020, 8, e000339. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Soldano, S.; Contini, P.; Tomatis, V.; Ruaro, B.; Paolino, S.; Brizzolara, R.; Montagna, P.; Sulli, A.; Pizzorni, C.; et al. A circulating cell population showing both m1 and m2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir. Res. 2018, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Ambarus, C.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.; Reedquist, K.; Tak, P.; Baeten, D. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Kalsum, S.; Loreti, M.G.; Rekha, R.S.; Muvva, J.R.; Lourda, M.; Brighenti, S. Polarization of m1 and m2 human monocyte-derived cells and analysis with flow cytometry upon mycobacterium tuberculosis infection. J. Vis. Exp. 2020, 163, e61807. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoshida, S.; Kubo, Y.; Yoshimura, T.; Kobayashi, Y.; Nakama, T.; Yamaguchi, M.; Ishikawa, K.; Oshima, Y.; Ishibashi, T. Different distributions of m1 and m2 macrophages in a mouse model of laser-induced choroidal neovascularization. Mol. Med. Rep. 2017, 15, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Merle, N.S.; Grunenwald, A.; Figueres, M.-L.; Chauvet, S.; Daugan, M.; Knockaert, S.; Robe-Rybkine, T.; Noé, R.; May, O.; Frimat, M.; et al. Characterization of renal injury and inflammation in an experimental model of intravascular hemolysis. Front. Immunol. 2018, 9, 179. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory m1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed]
- HHaldar, M.; Kohyama, M.; So, A.Y.; Kc, W.; Wu, X.; Briseno, C.G.; Satpathy, A.T.; Kretzer, N.M.; Arase, H.; Rajasekaran, N.S.; et al. Heme-mediated spi-c induction promotes monocyte differentiation into iron-recycling macrophages. Cell 2014, 156, 1223–1234. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Nieuwenhuizen, L.; Schutgens, R.E.; Coeleveld, K.; Mastbergen, S.C.; Roosendaal, G.; Biesma, D.H.; Lafeber, F.P. Hemarthrosis in hemophilic mice results in alterations in m1-m2 monocyte/macrophage polarization. Thromb. Res. 2014, 133, 390–395. [Google Scholar] [CrossRef]
- Lin, T.; Kwak, Y.H.; Sammy, F.; He, P.; Thundivalappil, S.; Sun, G.; Chao, W.; Warren, H.S. Synergistic inflammation is induced by blood degradation products with microbial toll-like receptor agonists and is blocked by hemopexin. J. Infect. Dis. 2010, 202, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Schluesener, H.J. The innate immunity of the central nervous system in chronic pain: The role of toll-like receptors. Cell. Mol. Life Sci. 2007, 64, 1128–1136. [Google Scholar] [CrossRef]
- Christianson, C.A.; Dumlao, D.S.; Stokes, J.A.; Dennis, E.A.; Svensson, C.I.; Corr, M.; Yaksh, T.L. Spinal tlr4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011, 152, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.-S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, M.A.; Cho, I.-H.; Kim, M.S.; Lee, S.; Jo, E.-K.; Choi, S.-Y.; Park, K.; Kim, J.S.; Akira, S.; et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007, 282, 14975–14983. [Google Scholar] [CrossRef]
- Qian, N.-S.; Liao, Y.-H.; Feng, Q.-X.; Tang, Y.; Dou, K.-F.; Tao, K.-S. Spinal toll like receptor 3 is involved in chronic pancreatitis-induced mechanical allodynia of rat. Mol. Pain 2011, 7, 15. [Google Scholar] [CrossRef]
- Das, N.; Dewan, V.; Grace, P.M.; Gunn, R.J.; Tamura, R.; Tzarum, N.; Watkins, L.R.; Wilson, I.A.; Yin, H. Hmgb1 activates proinflammatory signaling via tlr5 leading to allodynia. Cell Rep. 2016, 17, 1128–1140. [Google Scholar] [CrossRef]
- Park, C.-K.; Xu, Z.-Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.-J.; Ji, R.-R. Extracellular micrornas activate nociceptor neurons to elicit pain via tlr7 and trpa1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef]
- David, B.T.; Ratnayake, A.; Amarante, M.A.; Reddy, N.P.; Dong, W.; Sampath, S.; Heary, R.F.; Elkabes, S. A toll-like receptor 9 antagonist reduces pain hypersensitivity and the inflammatory response in spinal cord injury. Neurobiol. Dis. 2013, 54, 194–205. [Google Scholar] [CrossRef]
- Stokes, J.A.; Corr, M.; Yaksh, T.L. Spinal toll-like receptor signaling and nociceptive processing: Regulatory balance between tirap and trif cascades mediated by tnf and ifnβ. Pain 2013, 154, 733–742. [Google Scholar] [CrossRef]
- Chang, J.J.; Lacas, A.; Lindsay, R.J.; Doyle, E.; Axten, K.L.; Pereyra, F.; Rosenberg, E.S.; Walker, B.D.; Allen, T.; Altfeld, M. Differential regulation of toll-like receptor pathways in acute and chronic hiv-1 infection. Aids 2012, 26, 533–541. [Google Scholar] [CrossRef]
- Hernández, J.C.; Stevenson, M.; Latz, E.; Urcuqui-Inchima, S. Hiv type 1 infection up-regulates tlr2 and tlr4 expression and function in vivo and in vitro. Hum. Retroviruses 2012, 28, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Choi, B.; Jang, Y.H.; Cho, I.-H.; Lee, S.J. Heme molecule functions as an endogenous agonist of astrocyte tlr2 to contribute to secondary brain damage after intracerebral hemorrhage. Mol. Brain 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Del Cornò, M.; Cappon, A.; Donninelli, G.; Varano, B.; Marra, F.; Gessani, S. Hiv-1 gp120 signaling through tlr4 modulates innate immune activation in human macrophages and the biology of hepatic stellate cells. J. Leukoc. Biol. 2016, 100, 599–606. [Google Scholar] [CrossRef]
- Ben Haij, N.; Leghmari, K.; Planès, R.; Thieblemont, N.; Bahraoui, E. Hiv-1 tat protein binds to tlr4-md2 and signals to induce tnf-alpha and il-10. Retrovirology 2013, 10, 123. [Google Scholar] [CrossRef] [PubMed]
| Group (Number of Participants) | HIVneg, Painneg (14) | HIVneg, Painpos (14) | HIVpos, Painneg (14) | HIVpos, Painpos (13) |
|---|---|---|---|---|
| Avg. age (SD) | 50.1 (12.6) | 45.3 (15.0) | 48.6 (8.4) | 54.1 (5.8) |
| Percent Females | 64.3 | 64.3 | 50 | 38.5 |
| Percent Afr. Amer. | 64.3 | 64.3 | 71.4 | 76.9 |
| Avg. CD4+ (SD) | 715.4 (421.2) | 637.5 (385.0) | ||
| Avg. Nadir CD4+ (SD) | 165.0 (231.5) | 204.5 (202.4) | ||
| Avg. VL (SD) | 19.5 (1.9) | 59.2 (130.7) | ||
| Avg. Highest VL (SD) | 199115 (249762) | 428995 (1220712) | ||
| Plasma heme (µM) (SD) | 14.4 (4.0) | 26.3 (13.0) | 27.4 (12.4) | 80.9 (62.4) abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, T.; Arora, I.; Underwood, L.B.; Lewis, T.L.; Masjoan Juncos, J.X.; Heath, S.L.; Goodin, B.R.; Aggarwal, S. Heme-Induced Macrophage Phenotype Switching and Impaired Endogenous Opioid Homeostasis Correlate with Chronic Widespread Pain in HIV. Cells 2023, 12, 1565. https://doi.org/10.3390/cells12121565
Chatterjee T, Arora I, Underwood LB, Lewis TL, Masjoan Juncos JX, Heath SL, Goodin BR, Aggarwal S. Heme-Induced Macrophage Phenotype Switching and Impaired Endogenous Opioid Homeostasis Correlate with Chronic Widespread Pain in HIV. Cells. 2023; 12(12):1565. https://doi.org/10.3390/cells12121565
Chicago/Turabian StyleChatterjee, Tanima, Itika Arora, Lilly B. Underwood, Terry L. Lewis, Juan Xavier Masjoan Juncos, Sonya L. Heath, Burel R. Goodin, and Saurabh Aggarwal. 2023. "Heme-Induced Macrophage Phenotype Switching and Impaired Endogenous Opioid Homeostasis Correlate with Chronic Widespread Pain in HIV" Cells 12, no. 12: 1565. https://doi.org/10.3390/cells12121565
APA StyleChatterjee, T., Arora, I., Underwood, L. B., Lewis, T. L., Masjoan Juncos, J. X., Heath, S. L., Goodin, B. R., & Aggarwal, S. (2023). Heme-Induced Macrophage Phenotype Switching and Impaired Endogenous Opioid Homeostasis Correlate with Chronic Widespread Pain in HIV. Cells, 12(12), 1565. https://doi.org/10.3390/cells12121565