scViewer: An Interactive Single-Cell Gene Expression Visualization Tool
Abstract
1. Introduction
2. Materials and Methods
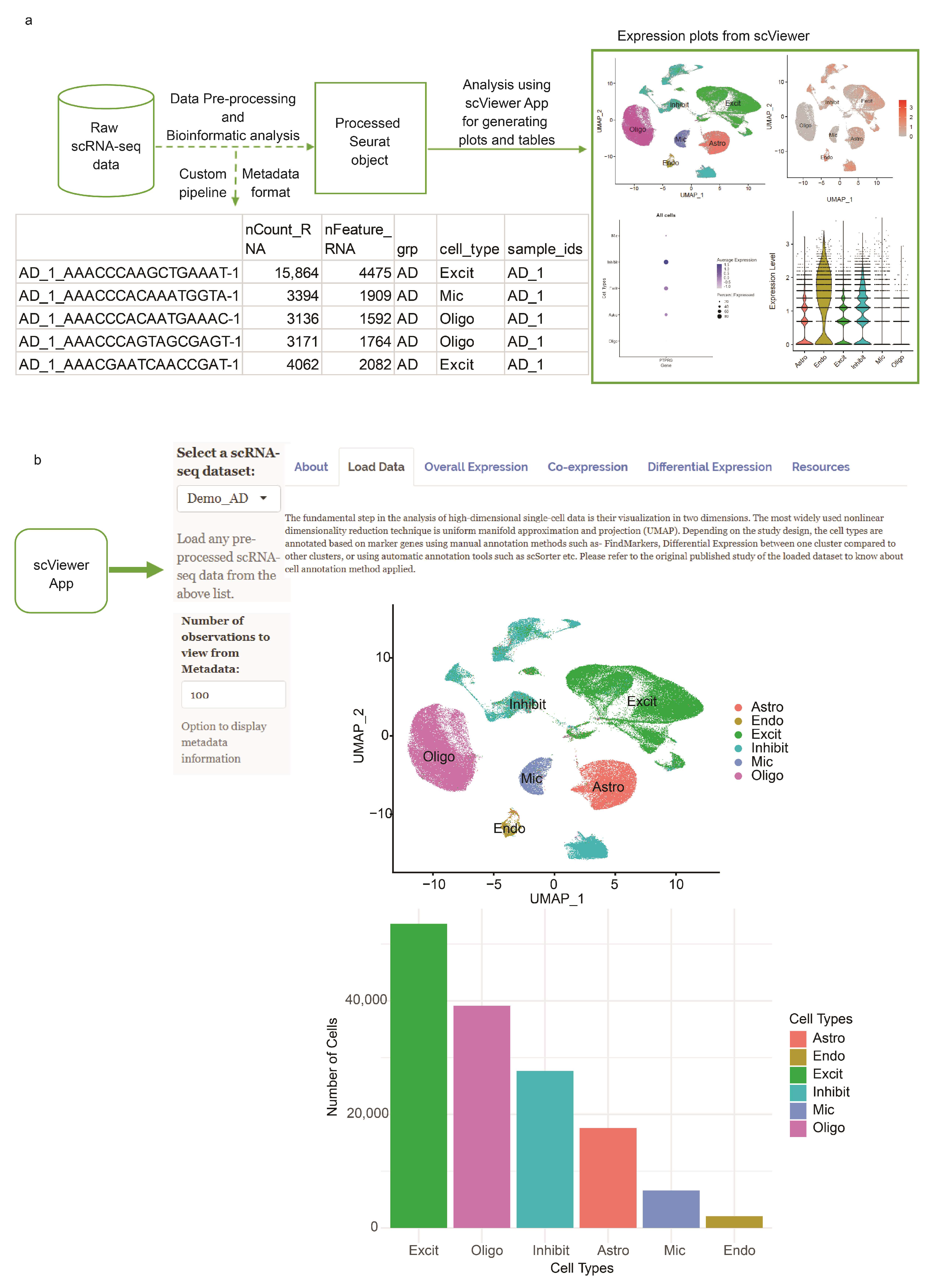
2.1. Infrastructure
2.2. Data Input
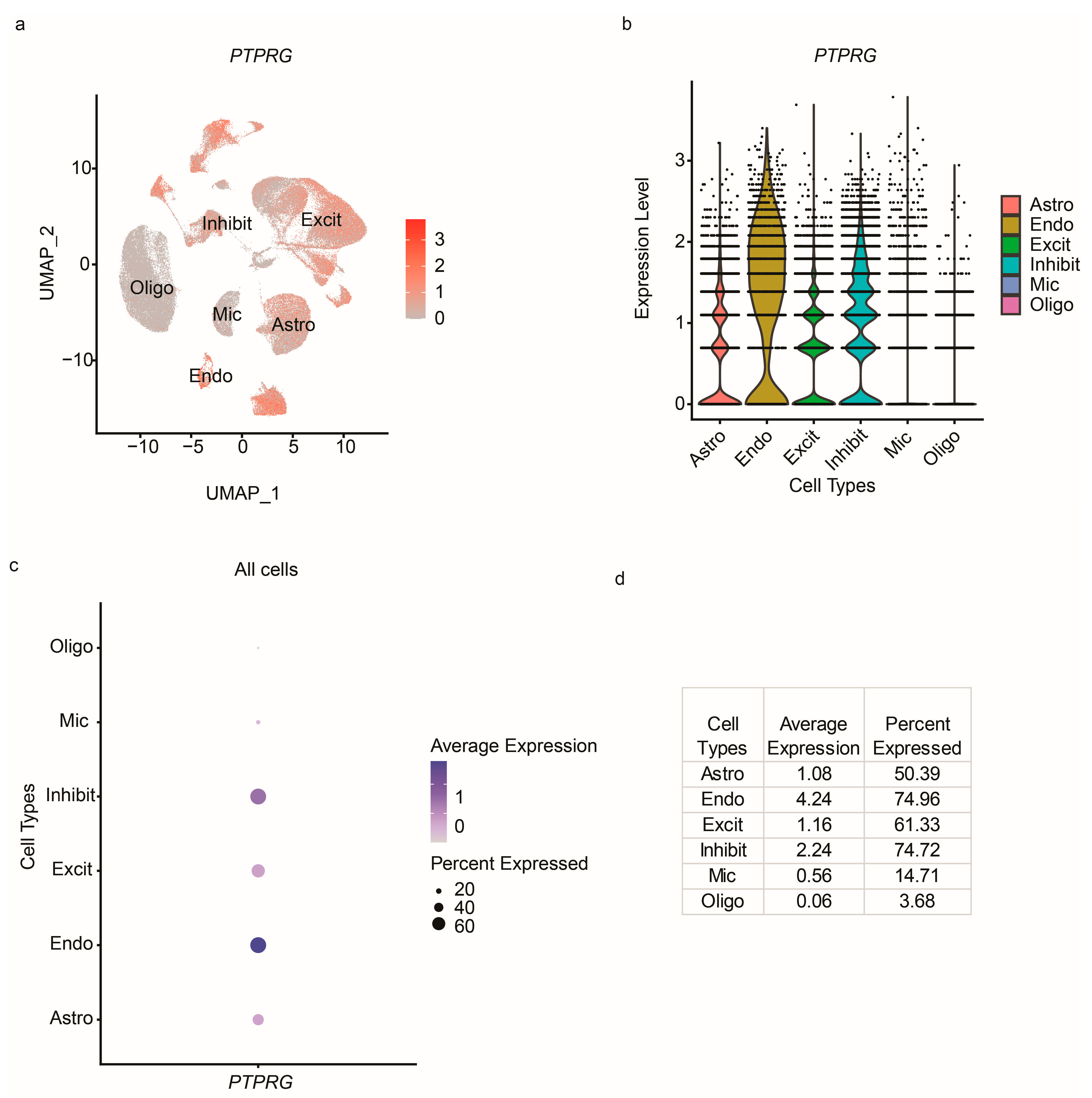
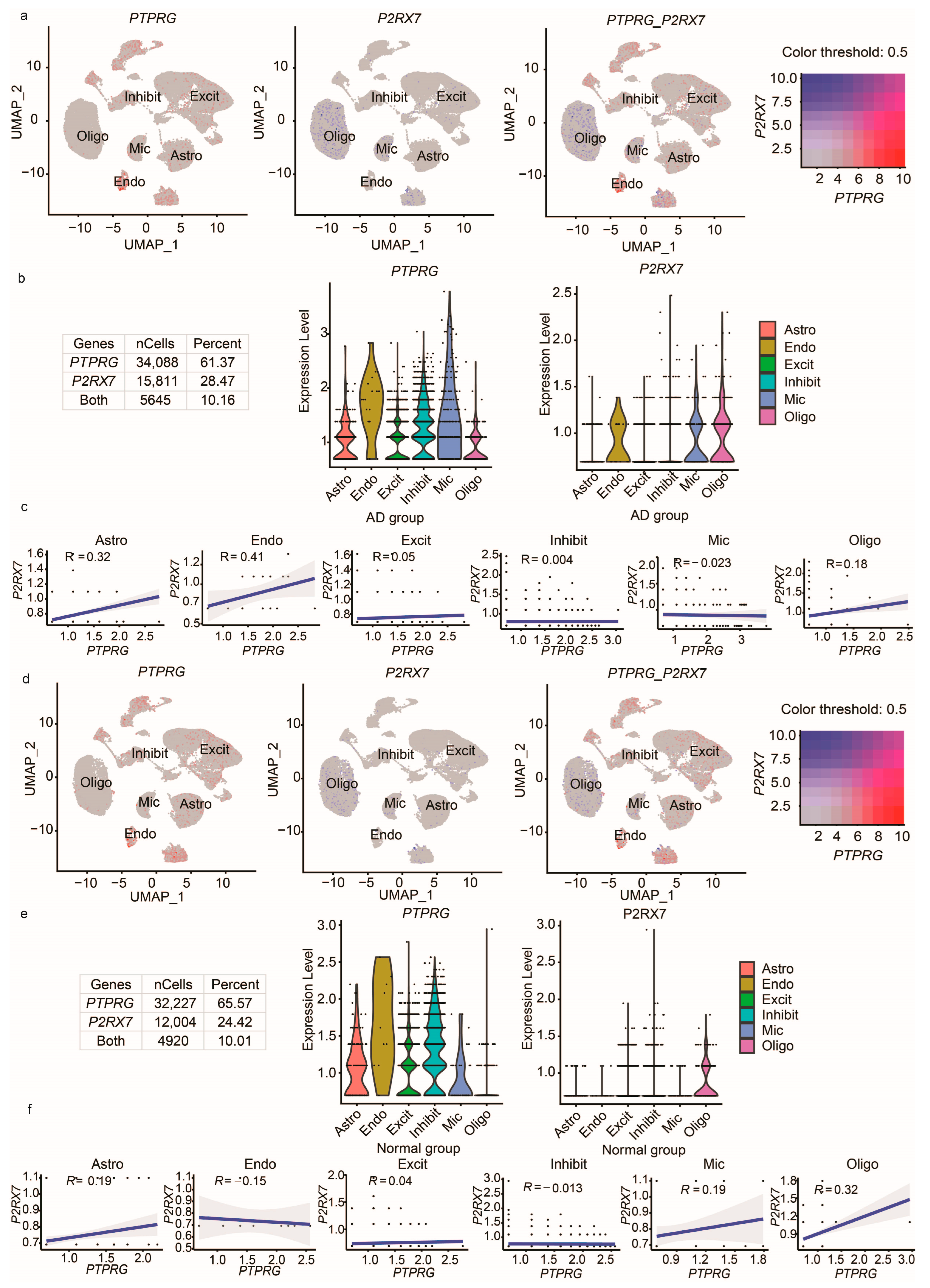
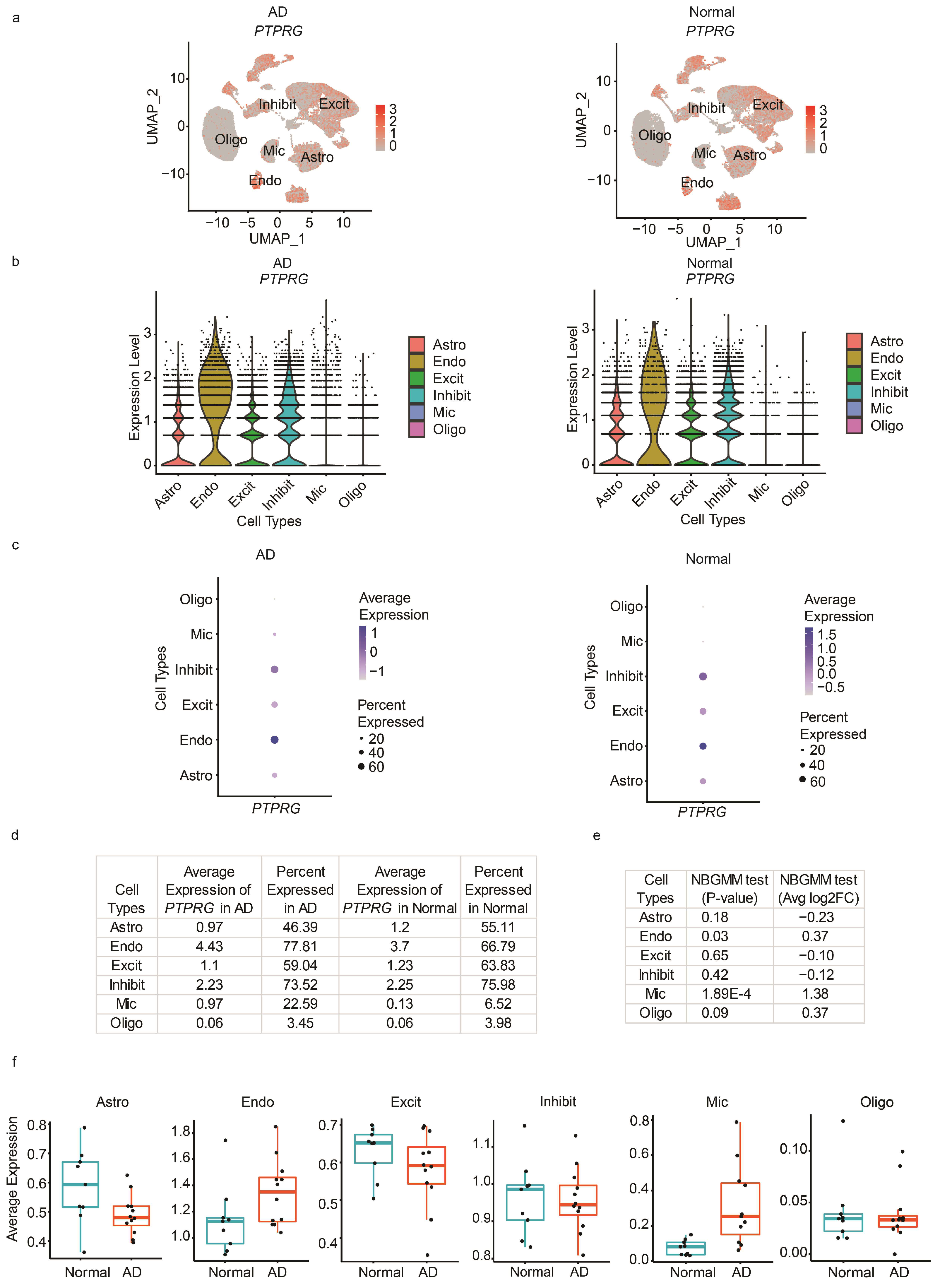
2.3. Data Analysis
2.4. User Interface
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewsey, M.G.; Yi, C.; Berkowitz, O.; Ayora, F.; Bernado, M.; Whelan, J. scCloudMine: A cloud-based app for visualization, comparison, and exploration of single-cell transcriptomic data. Plant Commun. 2022, 3, 100302. [Google Scholar] [CrossRef]
- Jagla, B.; Libri, V.; Chica, C.; Rouilly, V.; Mella, S.; Puceat, M.; Hasan, M. SCHNAPPs-Single Cell sHiNy APPlication(s). J. Immunol. Methods 2021, 499, 113176. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e3529. [Google Scholar] [CrossRef]
- Lun, A.T.L.; Bach, K.; Marioni, J.C. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 2016, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.F.; Kamaraj, U.S.; Cao, E.Y.; Rackham, O.J.L. ShinyCell: Simple and sharable visualization of single-cell gene expression data. Bioinformatics 2021, 37, 3374–3376. [Google Scholar] [CrossRef] [PubMed]
- David, F.P.A.; Litovchenko, M.; Deplancke, B.; Gardeux, V. ASAP 2020 update: An open, scalable and interactive web-based portal for (single-cell) omics analyses. Nucleic Acids Res. 2020, 48, W403–W414. [Google Scholar] [CrossRef]
- Hillje, R.; Pelicci, P.G.; Luzi, L. Cerebro: Interactive visualization of scRNA-seq data. Bioinformatics 2019, 36, 2311–2313. [Google Scholar] [CrossRef]
- Stein, D.F.; Chen, H.; Vinyard, M.E.; Qin, Q.; Combs, R.D.; Zhang, Q.; Pinello, L. singlecellVR: Interactive Visualization of Single-Cell Data in Virtual Reality. Front. Genet. 2021, 12, 764170. [Google Scholar] [CrossRef]
- Legetth, O.; Rodhe, J.; Lang, S.; Dhapola, P.; Wallergård, M.; Soneji, S. CellexalVR: A virtual reality platform to visualize and analyze single-cell omics data. iScience 2021, 24, 103251. [Google Scholar] [CrossRef]
- Cakir, B.; Prete, M.; Huang, N.; van Dongen, S.; Pir, P.; Kiselev, V.Y. Comparison of visualization tools for single-cell RNAseq data. NAR Genom. Bioinform. 2020, 2, lqaa052. [Google Scholar] [CrossRef]
- Megill, C.; Martin, B.; Weaver, C.; Bell, S.; Prins, L.; Badajoz, S.; McCandless, B.; Pisco, A.O.; Kinsella, M.; Griffin, F.; et al. cellxgene: A performant, scalable exploration platform for high dimensional sparse matrices. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rue-Albrecht, K.; Marini, F.; Soneson, C.; Lun, A.T.L. iSEE: Interactive SummarizedExperiment Explorer. F1000Res 2018, 7, 741. [Google Scholar] [CrossRef]
- Davie, K.; Janssens, J.; Koldere, D.; De Waegeneer, M.; Pech, U.; Kreft, Ł.; Aibar, S.; Makhzami, S.; Christiaens, V.; Bravo González-Blas, C.; et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell 2018, 174, 982–998.e920. [Google Scholar] [CrossRef]
- Feng, D.; Whitehurst, C.E.; Shan, D.; Hill, J.D.; Yue, Y.G. Single Cell Explorer, collaboration-driven tools to leverage large-scale single cell RNA-seq data. BMC Genom. 2019, 20, 676. [Google Scholar] [CrossRef]
- Speir, M.L.; Bhaduri, A.; Markov, N.S.; Moreno, P.; Nowakowski, T.J.; Papatheodorou, I.; Pollen, A.A.; Raney, B.J.; Seninge, L.; Kent, W.J.; et al. UCSC Cell Browser: Visualize your single-cell data. Bioinformatics 2021, 37, 4578–4580. [Google Scholar] [CrossRef] [PubMed]
- Tabaka, M.; Gould, J.; Regev, A. scSVA: An interactive tool for big data visualization and exploration in single-cell omics. bioRxiv 2019, 512582. [Google Scholar] [CrossRef]
- Weinreb, C.; Wolock, S.; Klein, A.M. SPRING: A kinetic interface for visualizing high dimensional single-cell expression data. Bioinformatics 2017, 34, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 28 December 2022).
- Chang, W.; Cheng, J.; Allaire, J.; Sievert, C.; Schloerke, B.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; Borges, B. shiny: Web Application Framework for R. Available online: https://shiny.rstudio.com/ (accessed on 28 December 2022).
- Chang, W. shinythemes: Themes for shiny. Available online: https://CRAN.R-project.org/package=shinythemes (accessed on 28 December 2022).
- Attali, D. shinyjs: Easily Improve the User Experience of Your Shiny Apps in Seconds. Available online: https://CRAN.R-project.org/package=shinyjs (accessed on 28 December 2022).
- Amezquita, R.A.; Lun, A.T.L.; Becht, E.; Carey, V.J.; Carpp, L.N.; Geistlinger, L.; Marini, F.; Rue-Albrecht, K.; Risso, D.; Soneson, C.; et al. Orchestrating single-cell analysis with Bioconductor. Nat. Methods 2020, 17, 137–145. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Campbell, K.R.; Lun, A.T.L.; Wills, Q.F. Scater: Pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 2017, 33, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Davila-Velderrain, J.; Sumida, T.S.; Hafler, D.A.; Kellis, M.; Kulminski, A.M. NEBULA is a fast negative binomial mixed model for differential or co-expression analysis of large-scale multi-subject single-cell data. Commun. Biol. 2021, 4, 629. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R.; Henry, L.; Muller, K. dplyr: A Grammar of Data Manipulation. Available online: https://github.com/tidyverse/dplyr (accessed on 28 December 2022).
- Wickham, H. stringr: Simple, Consistent Wrappers for Common String Operations, version 1.5. 0; R Studio package RStudio[J]. Inc.: Vienna, Austria, 2022.
- Gagolewski, M. stringi: Fast and Portable Character String Processing in R. J. Stat. Softw. 2022, 103, 1–59. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, J.; Tan, X. DT: A Wrapper of the JavaScript Library ‘DataTables’. Available online: https://CRAN.R-project.org/package=DT (accessed on 28 December 2022).
- Valero-Mora, P.M. ggplot2: Elegant Graphics for Data Analysis. J. Stat. Softw. Book Rev. 2010, 35, 1–3. [Google Scholar] [CrossRef]
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 28 December 2022).
- Auguie, B. gridExtra: Miscellaneous Functions for “Grid” Graphics. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 28 December 2022).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 28 December 2022).
- Lau, S.F.; Cao, H.; Fu, A.K.Y.; Ip, N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 25800–25809. [Google Scholar] [CrossRef]
- Squair, J.W.; Gautier, M.; Kathe, C.; Anderson, M.A.; James, N.D.; Hutson, T.H.; Hudelle, R.; Qaiser, T.; Matson, K.J.E.; Barraud, Q.; et al. Confronting false discoveries in single-cell differential expression. Nat. Commun. 2021, 12, 5692. [Google Scholar] [CrossRef]
- Zimmerman, K.D.; Espeland, M.A.; Langefeld, C.D. A practical solution to pseudoreplication bias in single-cell studies. Nat. Commun. 2021, 12, 738. [Google Scholar] [CrossRef]
- Crowell, H.L.; Soneson, C.; Germain, P.L.; Calini, D.; Collin, L.; Raposo, C.; Malhotra, D.; Robinson, M.D. muscat detects subpopulation-specific state transitions from multi-sample multi-condition single-cell transcriptomics data. Nat. Commun. 2020, 11, 6077. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lee, J.-H.; Kang, H.; Jiang, H. A Two-Part Mixed Model for Differential Expression Analysis in Single-Cell High-Throughput Gene Expression Data. Genes 2022, 13, 377. [Google Scholar] [CrossRef]
- Lahnemann, D.; Koster, J.; Szczurek, E.; McCarthy, D.J.; Hicks, S.C.; Robinson, M.D.; Vallejos, C.A.; Campbell, K.R.; Beerenwinkel, N.; Mahfouz, A.; et al. Eleven grand challenges in single-cell data science. Genome Biol. 2020, 21, 31. [Google Scholar] [CrossRef]
- Lawlor, N.; Marquez, E.J.; Lee, D.; Ucar, D. V-SVA: An R Shiny application for detecting and annotating hidden sources of variation in single-cell RNA-seq data. Bioinformatics 2020, 36, 3582–3584. [Google Scholar] [CrossRef]
- Interlandi, M.; Kerl, K.; Dugas, M. InterCellar enables interactive analysis and exploration of cell−cell communication in single-cell transcriptomic data. Commun. Biol. 2022, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, H.A.; Conley, C.J.; Stephens, W.Z.; O’Connell, R.M. CIPR: A web-based R/shiny app and R package to annotate cell clusters in single cell RNA sequencing experiments. BMC Bioinform. 2020, 21, 191. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, A.R.; Kumar, G.; Zhou, H.; Warren, L. scViewer: An Interactive Single-Cell Gene Expression Visualization Tool. Cells 2023, 12, 1489. https://doi.org/10.3390/cells12111489
Patil AR, Kumar G, Zhou H, Warren L. scViewer: An Interactive Single-Cell Gene Expression Visualization Tool. Cells. 2023; 12(11):1489. https://doi.org/10.3390/cells12111489
Chicago/Turabian StylePatil, Abhijeet R., Gaurav Kumar, Huanyu Zhou, and Liling Warren. 2023. "scViewer: An Interactive Single-Cell Gene Expression Visualization Tool" Cells 12, no. 11: 1489. https://doi.org/10.3390/cells12111489
APA StylePatil, A. R., Kumar, G., Zhou, H., & Warren, L. (2023). scViewer: An Interactive Single-Cell Gene Expression Visualization Tool. Cells, 12(11), 1489. https://doi.org/10.3390/cells12111489





