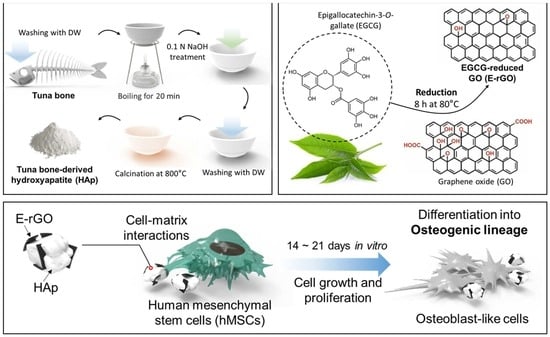
Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells by Tuna-Bone-Derived Hydroxyapatite Composites with Green Tea Polyphenol-Reduced Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of E-rGO/HAp Composites
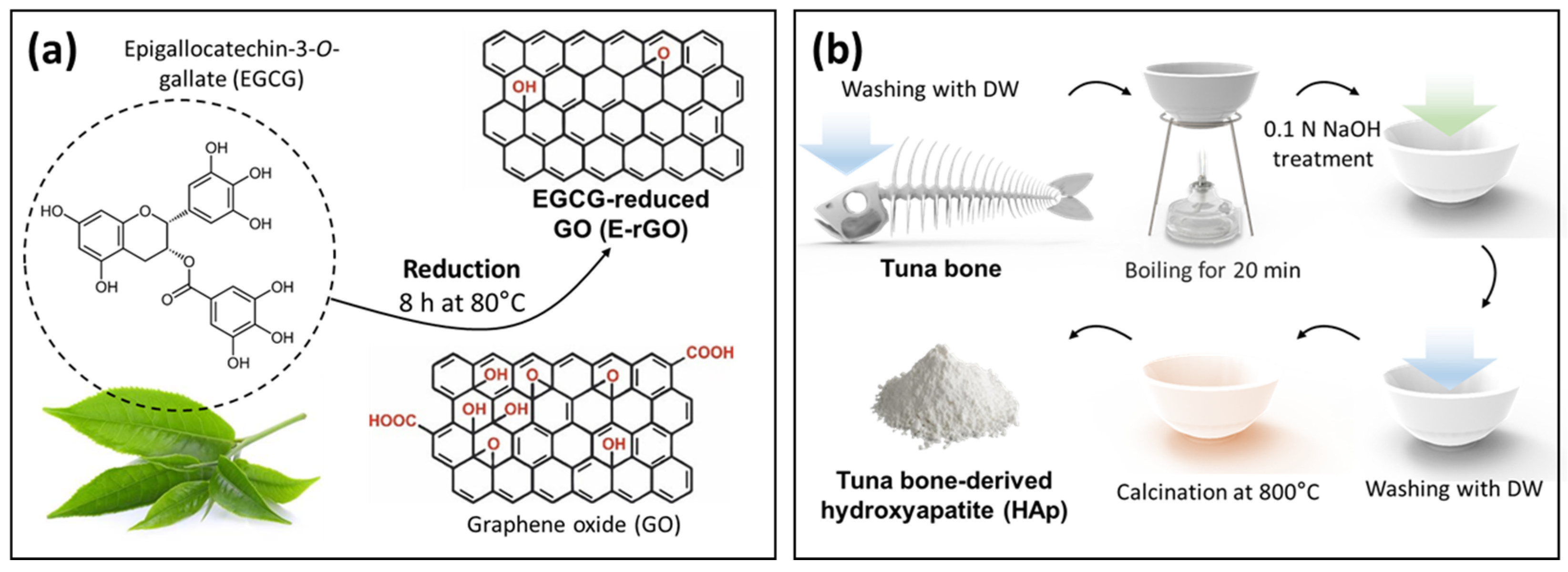
2.1.1. Preparation of E-rGO and Tuna-Bone-Derived HAp
2.1.2. Synthesis of E-rGO/HAp Composites
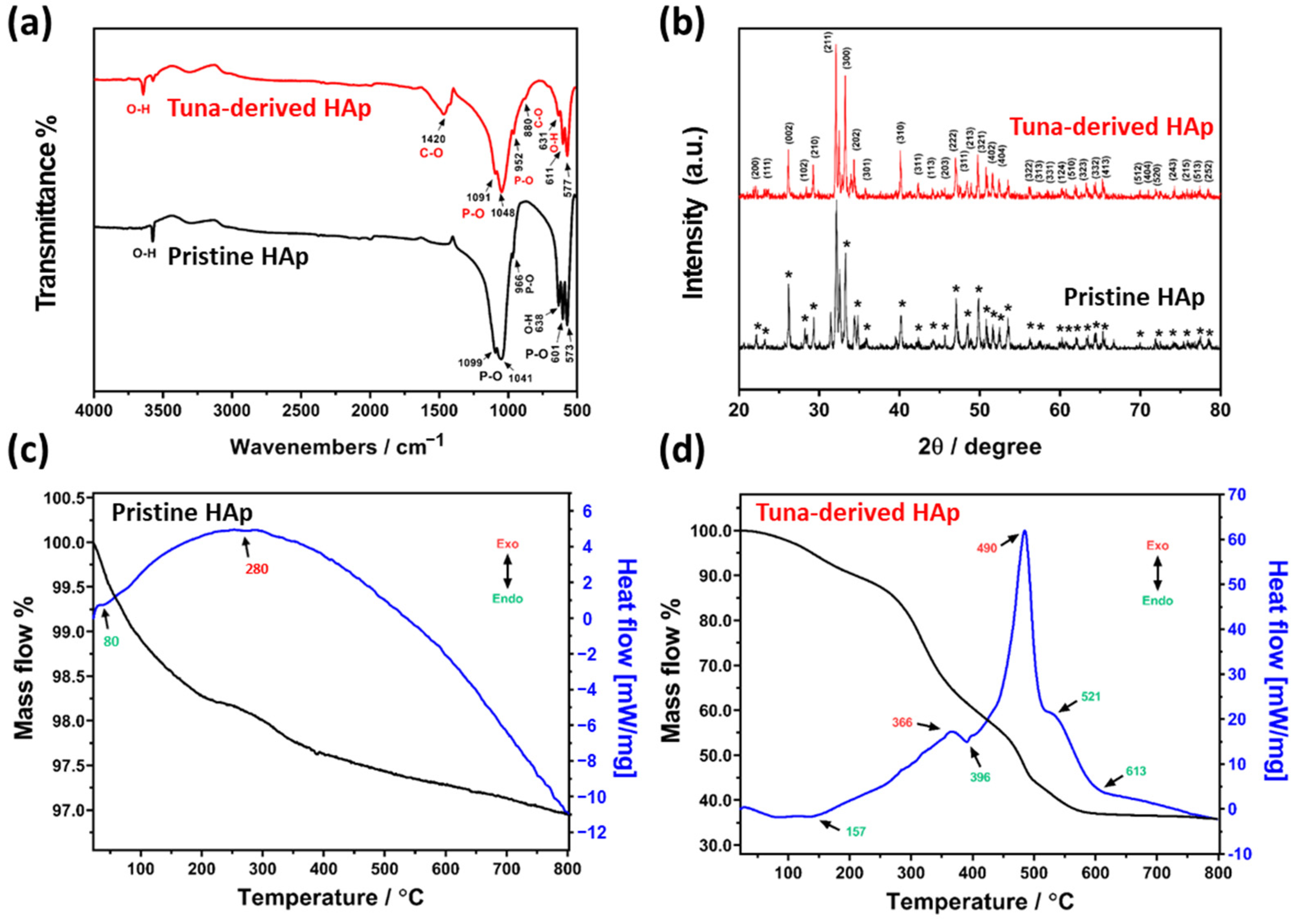
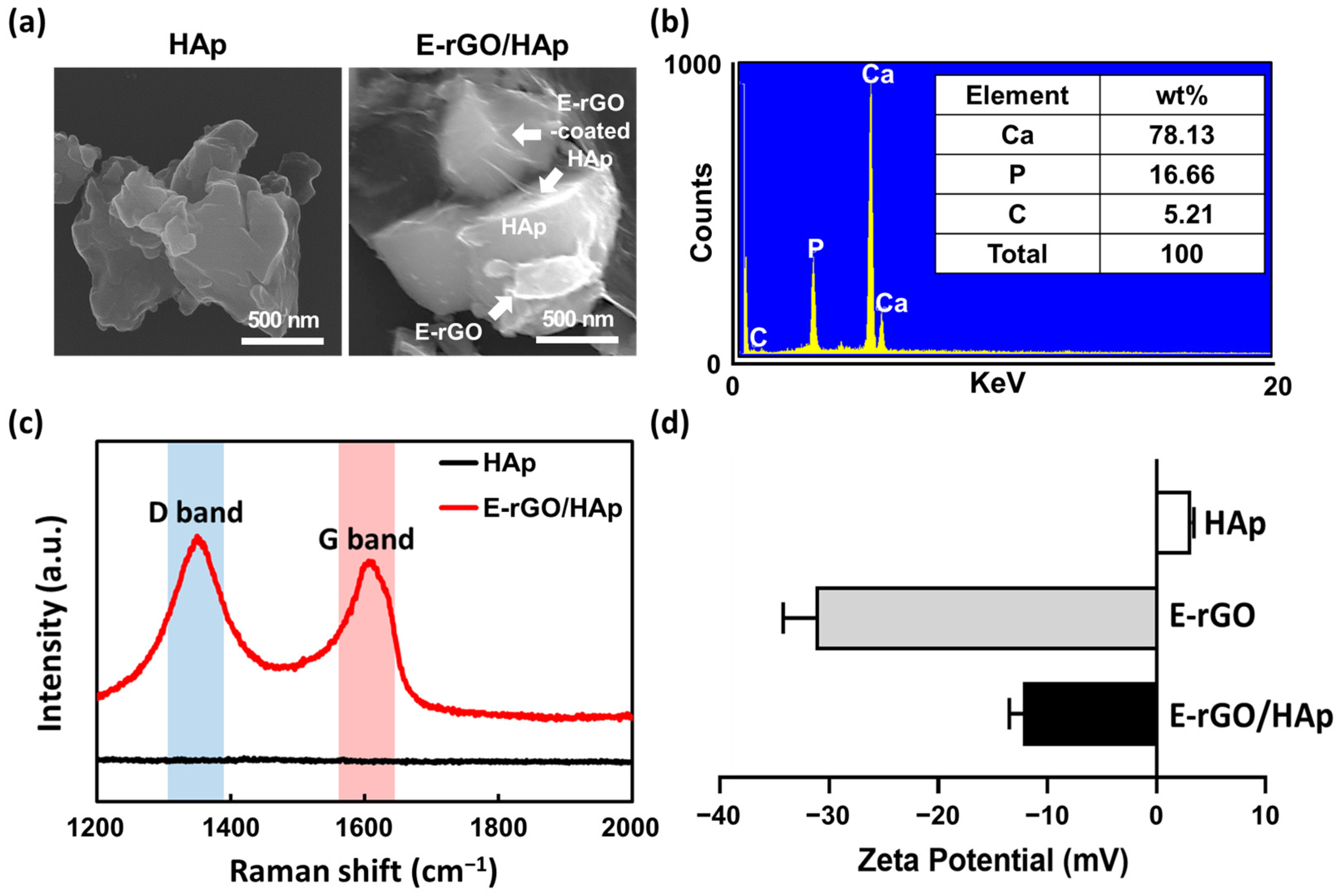
2.2. Physicochemical Characterizations
2.2.1. Physicochemical Characterizations of Tuna-Bone-Derived HAp
2.2.2. Physicochemical Characterizations of E-rGO/HAp Composites
2.3. Cell Culture Conditions
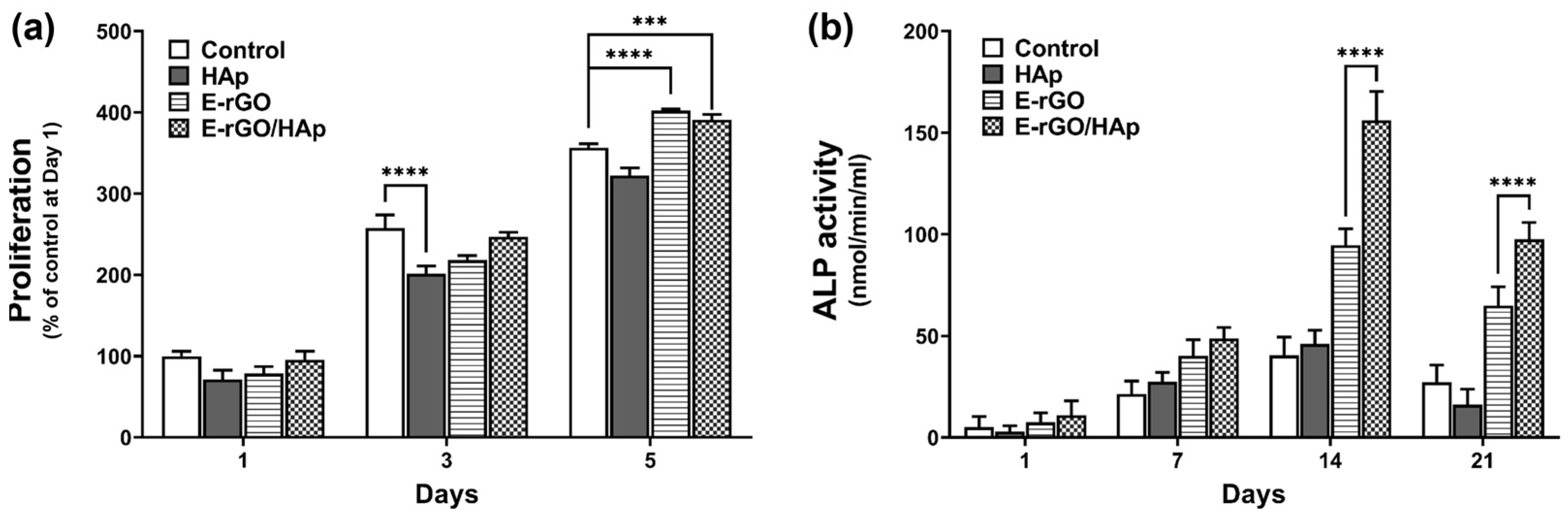
2.4. Cell Proliferation Assay
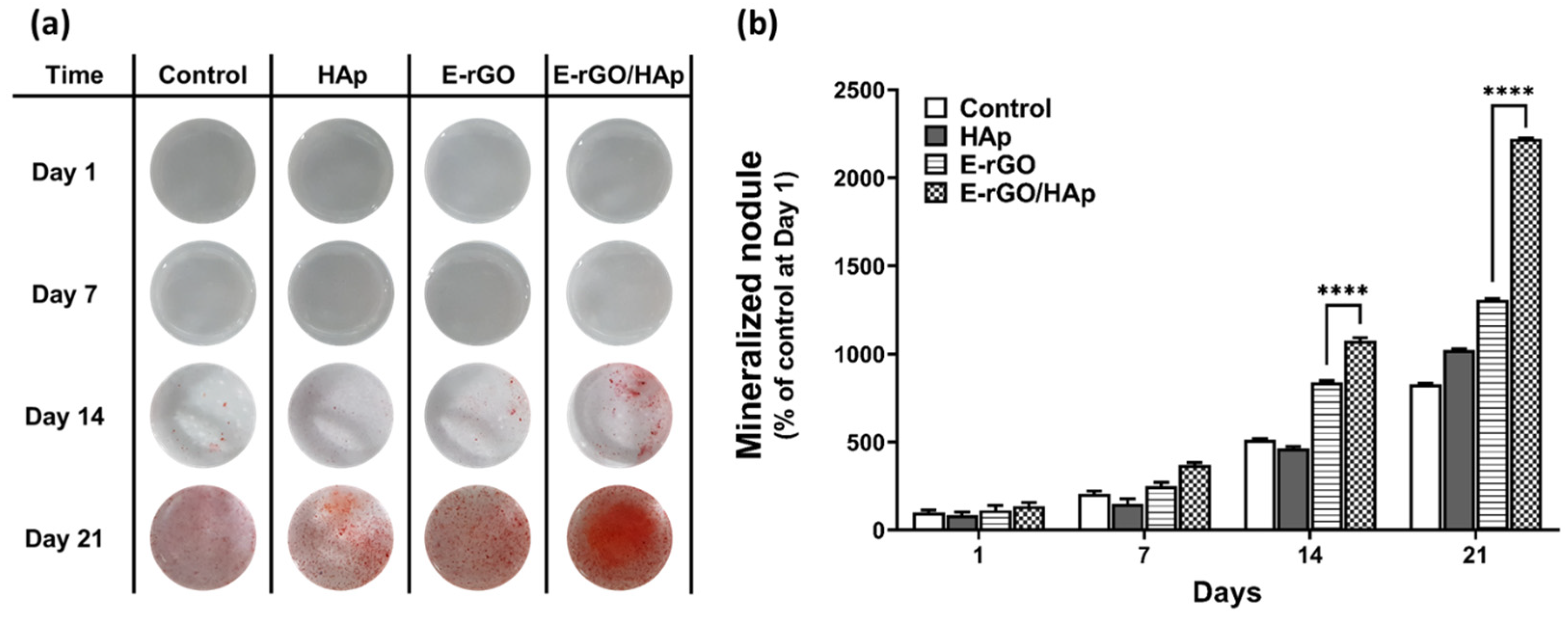
2.5. Alkaline Phosphatase (ALP) Activity Assay and Alizarin Red S (ARS) Staining
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2021, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, M.; Su, H.; Chen, H.; Zhu, Y. Up-to-date progress in bioprinting of bone tissue. Int. J. Bioprint. 2022, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental applications of systems based on hydroxyapatite nanoparticles—An evidence-based update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Biodegradable bone implants in orthopedic applications: A review. Biocybern. Biomed. Eng. 2020, 40, 596–610. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Lin, T.-L.; Lin, Y.-H.; Lee, A.K.-X.; Ng, S.Y.; Huang, T.-H.; Hsu, T.-T. Synergistic effect of static magnetic fields and 3D-printed iron-oxide-nanoparticle-containing calcium silicate/poly-ε-caprolactone scaffolds for bone tissue engineering. Cells 2022, 11, 3967. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, S.; Lu, R.; Wang, X.; Chen, S. In vitro and in vivo studies of hydrogenated titanium dioxide nanotubes with superhydrophilic surfaces during early osseointegration. Cells 2022, 11, 3417. [Google Scholar] [CrossRef]
- Aghali, A. Craniofacial bone tissue engineering: Current approaches and potential therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Nasri, C.S.S.M.; Arshad, S.E.B. Hydrothermal synthesis of hydroxyapatite powders using Response Surface Methodology (RSM). PLoS ONE 2021, 16, e0251009. [Google Scholar] [CrossRef]
- Fathi, M.; Zahrani, E.M. Mechanical alloying synthesis and bioactivity evaluation of nanocrystalline fluoridated hydroxyapatite. J. Cryst. Growth 2009, 311, 1392–1403. [Google Scholar] [CrossRef]
- Yeong, K.; Wang, J.; Ng, S. Mechanochemical synthesis of nanocrystalline hydroxyapatite from CaO and CaHPO4. Biomaterials 2001, 22, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Kuo, C.-S.; Li, Y.-Y.; Huang, C.-P. Polymer-assisted synthesis of hydroxyapatite nanoparticle. Mater. Sci. Eng. C 2009, 29, 819–822. [Google Scholar] [CrossRef]
- Simon, V.; Lazăr, D.; Turcu, R.; Mocuta, H.; Magyari, K.; Prinz, M.; Neumann, M.; Simon, S. Atomic environment in sol–gel derived nanocrystalline hydroxyapatite. Mater. Sci. Eng. B 2009, 165, 247–251. [Google Scholar] [CrossRef]
- Park, S.J.; Gupta, K.C.; Kim, H.; Kim, S.; Kang, I.-K. Osteoblast behaviours on nanorod hydroxyapatite-grafted glass surfaces. Biomater. Res. 2019, 23, 28. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.W.; Heo, J.H.; Park, C.; Kim, D.-H.; Yi, G.S.; Kang, H.C.; Jung, H.S.; Shin, H.; Lee, J.H. Natural bone-mimicking nanopore-incorporated hydroxyapatite scaffolds for enhanced bone tissue regeneration. Biomater. Res. 2022, 26, 7. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Ronan, K.; Kannan, M.B. Novel sustainable route for synthesis of hydroxyapatite biomaterial from biowastes. ACS Sustain. Chem. Eng. 2017, 5, 2237–2245. [Google Scholar] [CrossRef]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Sivakumar, M.; Kumar, T.S.; Shantha, K.; Rao, K.P. Development of hydroxyapatite derived from Indian coral. Biomaterials 1996, 17, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mondal, S.; Mondal, A.; Mandal, N. Fish scale derived hydroxyapatite scaffold for bone tissue engineering. Mater. Charact. 2016, 121, 112–124. [Google Scholar] [CrossRef]
- Barakat, N.A.; Khil, M.S.; Omran, A.; Sheikh, F.A.; Kim, H.Y. Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J. Mater. Process. Technol. 2009, 209, 3408–3415. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, Q.; Li, Z.; Zhang, H.; Chen, Z. Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci. Technol. Adv. Mater. 2017, 18, 110–121. [Google Scholar] [PubMed]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Paul, S.; Pal, A.; Choudhury, A.R.; Bodhak, S.; Balla, V.K.; Sinha, A.; Das, M. Effect of trace elements on the sintering effect of fish scale derived hydroxyapatite and its bioactivity. Ceram. Int. 2017, 43, 15678–15684. [Google Scholar] [CrossRef]
- Culurgioni, J.; Mele, S.; Merella, P.; Addis, P.; Figus, V.; Cau, A.; Karakulak, F.S.; Garippa, G. Metazoan gill parasites of the Atlantic bluefin tuna Thunnus thynnus (Linnaeus) (Osteichthyes: Scombridae) from the Mediterranean and their possible use as biological tags. Folia Parasitol. 2014, 61, 148. [Google Scholar]
- Venkatesan, J.; Kim, S.K. Effect of temperature on isolation and characterization of hydroxyapatite from tuna (Thunnus obesus) bone. Materials 2010, 3, 4761–4772. [Google Scholar] [CrossRef]
- Li, Q.; Feng, C.; Cao, Q.; Wang, W.; Ma, Z.; Wu, Y.; He, T.; Jing, Y.; Tan, W.; Liao, T. Strategies of strengthening mechanical properties in the osteoinductive calcium phosphate bioceramics. Regen. Biomater. 2023, 10, rbad013. [Google Scholar] [CrossRef]
- Han, D.-W.; Hong, S.W. Multifaceted Biomedical Applications of Graphene, 1st ed.; Springer: Singapore, 2022; pp. 1–264. [Google Scholar]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.-S.; Park, J.-C.; Hong, S.W.; Han, D.-W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef]
- Shin, Y.C.; Bae, J.-H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.-B.; Han, D.-W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Lee, J.H.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Kim, B.; Park, J.-C.; Han, D.-W. Synergistic effects of reduced graphene oxide and hydroxyapatite on osteogenic differentiation of MC3T3-E1 preosteoblasts. Carbon 2015, 95, 1051–1060. [Google Scholar] [CrossRef]
- Raja, I.S.; Preeth, D.R.; Vedhanayagam, M.; Hyon, S.-H.; Lim, D.; Kim, B.; Rajalakshmi, S.; Han, D.-W. Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration. Biomater. Res. 2021, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Song, H.; Zhan, Z.; Lv, Y. Multifunctional reduced graphene oxide-based nanoplatform for synergistic targeted chemo-photothermal therapy. ACS Appl. Bio. Mater. 2020, 3, 5213–5222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Yin, J. Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Mathirat, A.; Dalavi, P.A.; Prabhu, A.; GV, Y.D.; Anil, S.; Senthilkumar, K.; Seong, G.H.; Sargod, S.S.; Bhat, S.S.; Venkatesan, J. Remineralizing potential of natural nano-hydroxyapatite obtained from epinephelus chlorostigma in artificially induced early enamel lesion: An in vitro study. Nanomaterials 2022, 12, 3993. [Google Scholar] [CrossRef]
- Bouyer, E.; Gitzhofer, F.; Boulos, M. Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 2000, 11, 523–531. [Google Scholar] [CrossRef]
- Leal-Egaña, A.; Díaz-Cuenca, A.; Boccaccini, A.R. Tuning of cell–biomaterial anchorage for tissue regeneration. Adv. Mater. 2013, 25, 4049–4057. [Google Scholar] [CrossRef]
- Gross, K.A.; Gross, V.; Berndt, C.C. Thermal analysis of amorphous phases in hydroxyapatite coatings. J. Am. Ceram. Soc. 1998, 81, 106–112. [Google Scholar] [CrossRef]
- Liao, C.-J.; Lin, F.-H.; Chen, K.-S.; Sun, J.-S. Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomaterials 1999, 20, 1807–1813. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, S.M.; Silva, A.R.; Ciminelli, V.S.; Mansur, H.S. Niobium-doped hydroxyapatite bioceramics: Synthesis, characterization and in vitro cytocompatibility. Materials 2015, 8, 4191–4209. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Antoniac, I.; Ciocan, L.T.; Miculescu, M.; Brânzei, M.; Ernuteanu, A.; Batalu, D.; Berbecaru, A. Complex analysis on heat treated human compact bones. UPB Sci. Bull. B 2011, 73, 203–211. [Google Scholar]
- Amirthalingam, N.; Deivarajan, T.; Paramasivam, M. Mechano chemical synthesis of hydroxyapatite using dolomite. Mater. Lett. 2019, 254, 379–382. [Google Scholar] [CrossRef]
- Wang, H.; Lee, J.K.; Moursi, A.; Lannutti, J.J. Ca/P ratio effects on the degradation of hydroxyapatite in vitro. J. Biomed. Mater. Res. A 2003, 67, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yazici, H.; Ergun, C.; Webster, T.J.; Bermek, H. An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta. Biomater. 2008, 4, 1472–1479. [Google Scholar] [CrossRef]
- Shin, M.C.; Kang, M.S.; Park, R.; Chae, S.Y.; Han, D.-W.; Hong, S.W. Differential cellular interactions and responses to ultrathin micropatterned graphene oxide arrays with or without ordered in turn RGD peptide films. Appl. Surf. Sci. 2021, 561, 150115. [Google Scholar] [CrossRef]
- Park, K.O.; Lee, J.H.; Park, J.H.; Shin, Y.C.; Huh, J.B.; Bae, J.-H.; Kang, S.H.; Hong, S.W.; Kim, B.; Yang, D.J. Graphene oxide-coated guided bone regeneration membranes with enhanced osteogenesis: Spectroscopic analysis and animal study. Appl. Spectrosc. Rev. 2016, 51, 540–551. [Google Scholar] [CrossRef]
- Kang, M.S.; Kang, J.I.; Le Thi, P.; Park, K.M.; Hong, S.W.; Choi, Y.S.; Han, D.-W.; Park, K.D. Three-dimensional printable gelatin hydrogels incorporating graphene oxide to enable spontaneous myogenic differentiation. ACS Macro Lett. 2021, 10, 426–432. [Google Scholar] [CrossRef]
- Tang, B.; Guoxin, H.; Gao, H. Raman spectroscopic characterization of graphene. Appl. Spectrosc. Rev. 2010, 45, 369–407. [Google Scholar] [CrossRef]
- Li, M.-j.; Liu, C.-m.; Xie, Y.-b.; Cao, H.-b.; Zhao, H.; Zhang, Y. The evolution of surface charge on graphene oxide during the reduction and its application in electroanalysis. Carbon 2014, 66, 302–311. [Google Scholar] [CrossRef]
- Everett, D.H. Basic Principles of Colloid Science, 1st ed.; Royal Society of Chemistry: London, UK, 2007; pp. 1–243. [Google Scholar]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. B 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, E.; Leszczynska, J.; Brzoska, E. The characteristics of human bone-derived cells (HBDCS) during osteogenesis in vitro. Cell Mol. Biol. Lett. 2016, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In vitro uptake of hydroxyapatite nanoparticles and their effect on osteogenic differentiation of human mesenchymal stem cells. Stem. Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Ran, K.; Zhang, Y.; Pan, H.; Shangguan, J.; Tong, M.; Yang, J.; Yao, Q.; Xu, H. Porous hydroxyapatite scaffold orchestrated with bioactive coatings for rapid bone repair. Biomater. Adv. 2023, 144, 213202. [Google Scholar] [CrossRef]
- Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2016, 60, 45–53. [Google Scholar] [CrossRef]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 89, 326–335. [Google Scholar] [CrossRef]
- Liu, L.; Gao, X.; Li, X.; Zhu, G.; Li, N.; Shi, X.; Wang, Y. Calcium alendronate-coated composite scaffolds promote osteogenesis of ADSCs via integrin and FAK/ERK signalling pathways. J. Mater. Chem. B 2020, 8, 6912–6924. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; da Silva, B.C.; Hamouda, A.E.; de Toledo, M.A.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Wiesmann, A.; Bühring, H.-J.; Mentrup, C.; Wiesmann, H.-P. Decreased CD90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006, 2, 8. [Google Scholar] [CrossRef]
- Kopczyńska, E.; Makarewicz, R. Endoglin—A marker of vascular endothelial cell proliferation in cancer. Contemp. Oncol. 2012, 16, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Leyva, M.; Barrera, L.; López-Camarillo, C.; Arriaga-Pizano, L.; Orozco-Hoyuela, G.; Carrillo-Casas, E.M.; Calderón-Pérez, J.; López-Díaz, A.; Hernandez-Aguilar, F.; González-Ramírez, R. Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential. Stem Cells Dev. 2013, 22, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fierens, K.; Zhang, Z.; Vanparijs, N.; Schuijs, M.J.; Van Steendam, K.; Feiner Gracia, N.; De Rycke, R.; De Beer, T.; De Beuckelaer, A. Spontaneous protein adsorption on graphene oxide nanosheets allowing efficient intracellular vaccine protein delivery. ACS Appl. Mater. Interfaces 2016, 8, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.-M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.-M.; Huh, J.B.; Han, D.-W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, K.; Wang, Z. Enhanced growth and osteogenic differentiation of MC3T3-E1 cells on Ti6Al4V alloys modified with reduced graphene oxide. RSC Adv. 2017, 7, 14430–14437. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Han, P.; Xu, M.; Fang, B.; Wang, J.; Chang, J.; Xiao, Y. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon 2015, 93, 116–129. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, Q.; Xu, L.; Li, G.; Yang, G.; Ding, X.; Wang, X.; Cui, D.; Jiang, X. Graphene oxide-copper nanocomposite-coated porous CaP scaffold for vascularized bone regeneration via activation of Hif-1α. Adv. Healthc. Mater. 2019, 8, 1900067. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.S.; Park, R.; Jo, H.J.; Shin, Y.C.; Kim, C.-S.; Hyon, S.-H.; Hong, S.W.; Oh, J.; Han, D.-W. Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells by Tuna-Bone-Derived Hydroxyapatite Composites with Green Tea Polyphenol-Reduced Graphene Oxide. Cells 2023, 12, 1448. https://doi.org/10.3390/cells12111448
Kang MS, Park R, Jo HJ, Shin YC, Kim C-S, Hyon S-H, Hong SW, Oh J, Han D-W. Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells by Tuna-Bone-Derived Hydroxyapatite Composites with Green Tea Polyphenol-Reduced Graphene Oxide. Cells. 2023; 12(11):1448. https://doi.org/10.3390/cells12111448
Chicago/Turabian StyleKang, Moon Sung, Rowoon Park, Hyo Jung Jo, Yong Cheol Shin, Chang-Seok Kim, Suong-Hyu Hyon, Suck Won Hong, Junghwan Oh, and Dong-Wook Han. 2023. "Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells by Tuna-Bone-Derived Hydroxyapatite Composites with Green Tea Polyphenol-Reduced Graphene Oxide" Cells 12, no. 11: 1448. https://doi.org/10.3390/cells12111448
APA StyleKang, M. S., Park, R., Jo, H. J., Shin, Y. C., Kim, C.-S., Hyon, S.-H., Hong, S. W., Oh, J., & Han, D.-W. (2023). Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells by Tuna-Bone-Derived Hydroxyapatite Composites with Green Tea Polyphenol-Reduced Graphene Oxide. Cells, 12(11), 1448. https://doi.org/10.3390/cells12111448