Mast Cell Tryptase Promotes Airway Remodeling by Inducing Anti-Apoptotic and Cell Growth Properties in Human Alveolar and Bronchial Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mammalian Cells
2.2. In Vitro Stimulations
2.3. Cigarette Smoke Extraction
2.4. Holographic Live Cell Imaging (HoloMonitor M4)
2.5. RNA Extraction and PCR Array
2.6. Luminex and ELISA
2.7. Immunocytochemistry
2.8. Statistical Analysis
3. Results
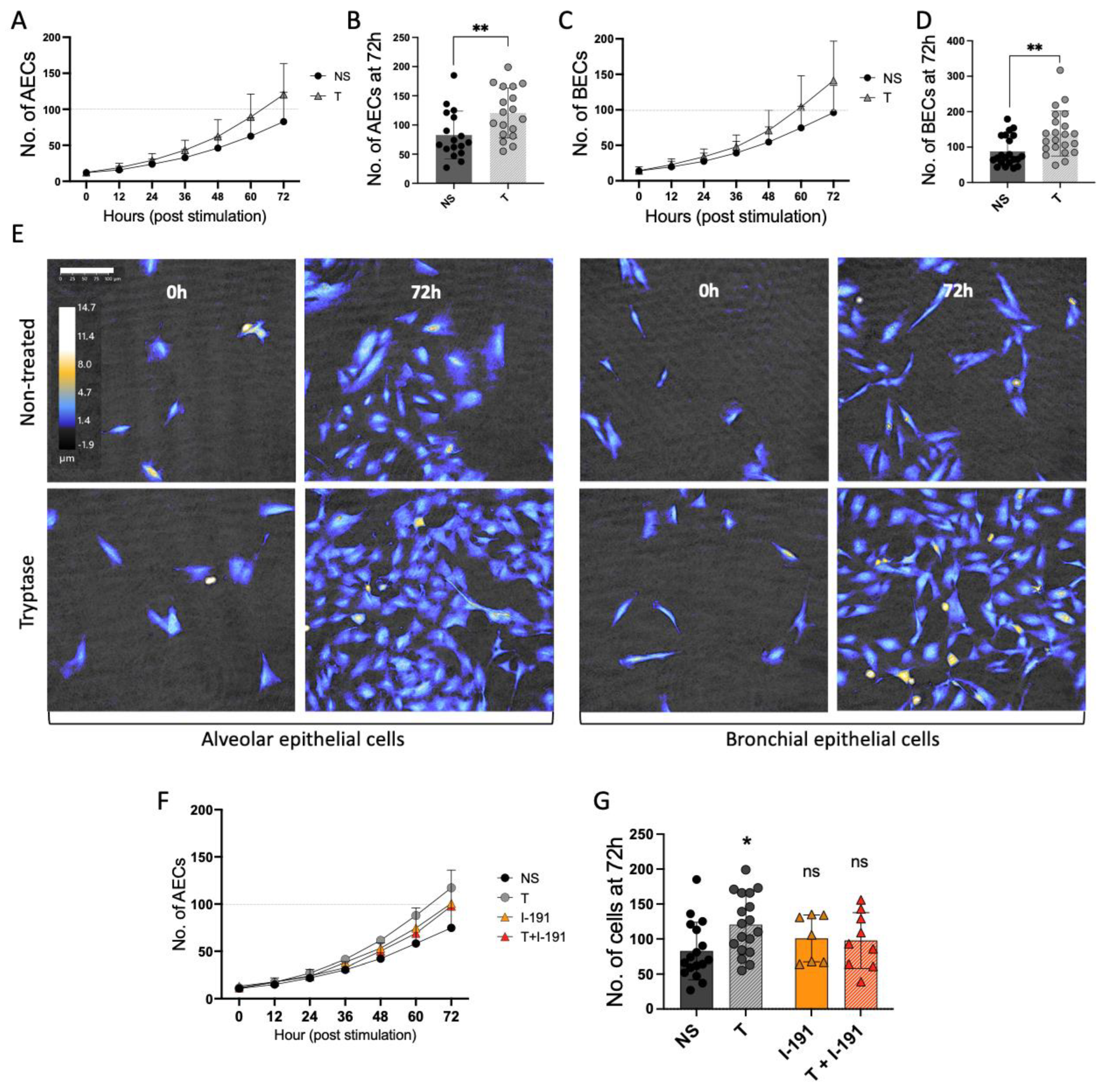
3.1. Mast Cell Tryptase Enhances Cell Growth in Alveolar and Bronchial Epithelial Cells
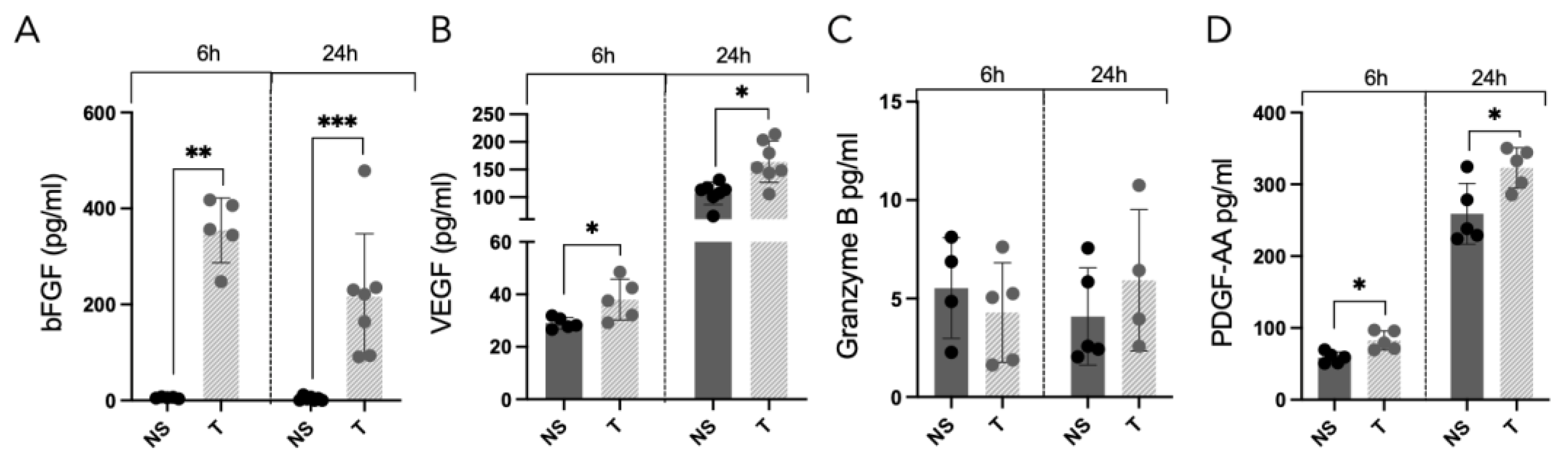
3.2. Elevated Release of Growth Factors from Alveolar Cells Is Induced by Tryptase
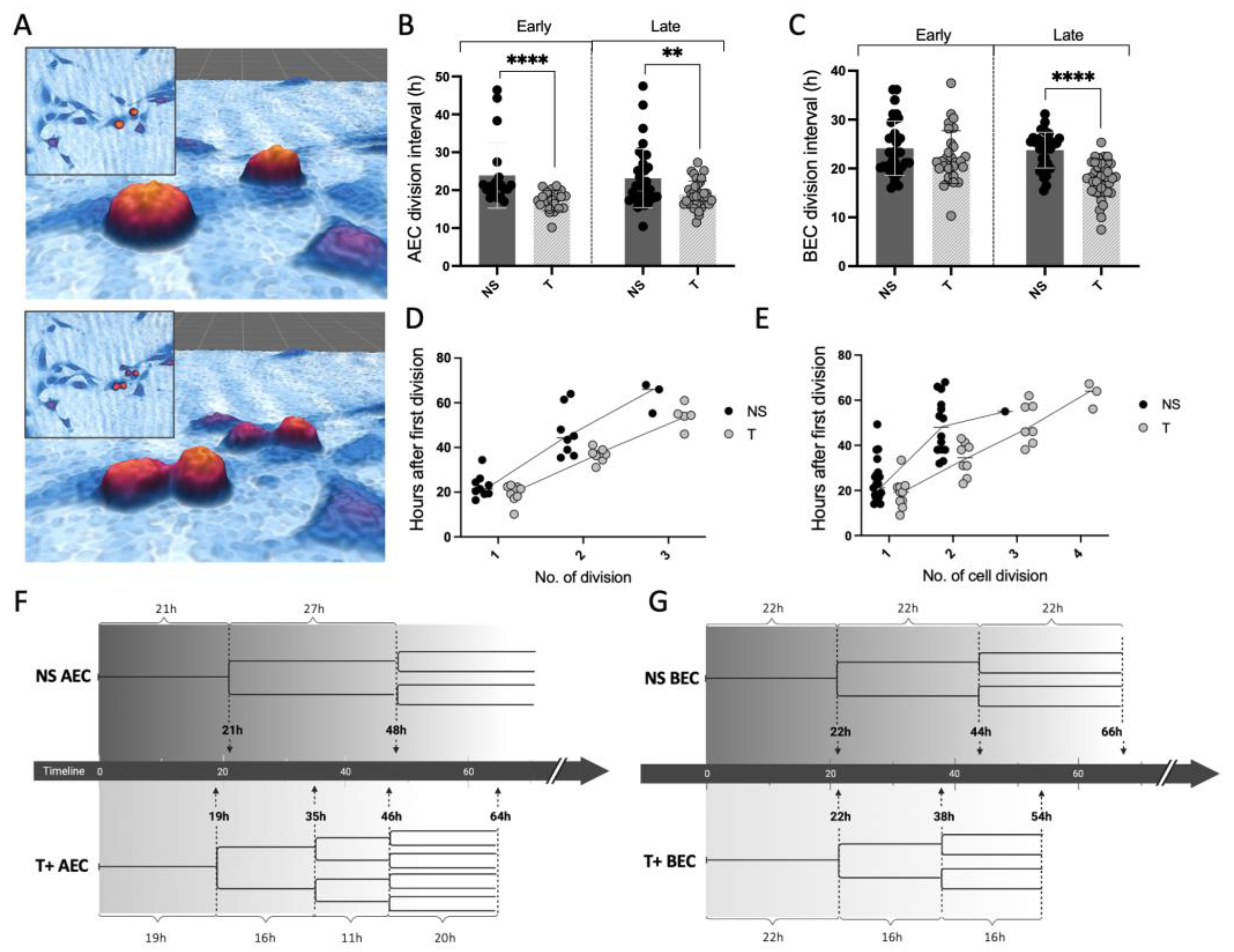
3.3. MC Tryptase Alters Cell Division Rate and Intervals in Epithelial Cells
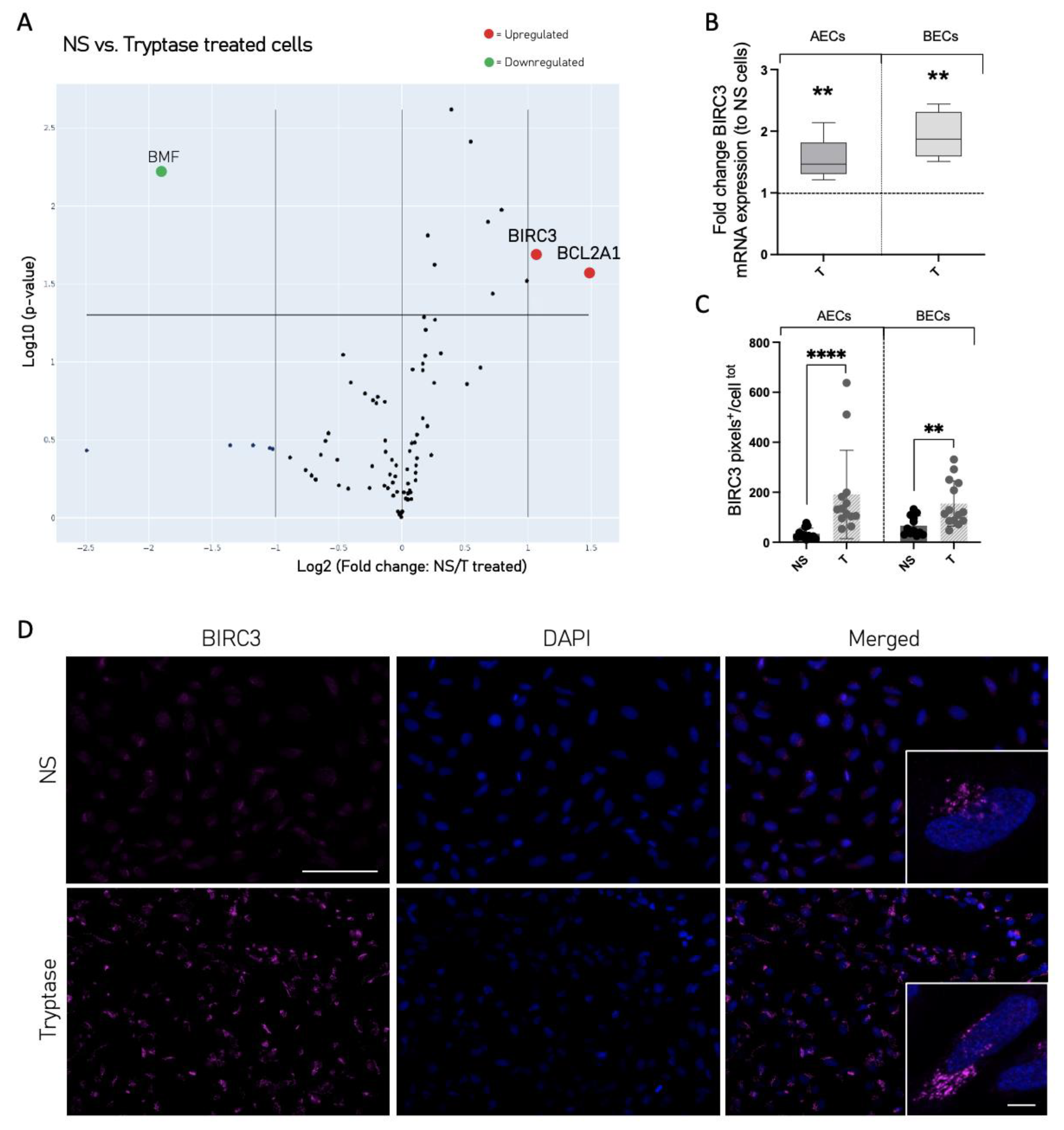
3.4. Cell Death and Survival Regulatory Protein Expression Was Altered in Tryptase-Treated Cells
3.5. The Mitogenic Effect of Tryptase Remained in Pro-Inflammatory Environments
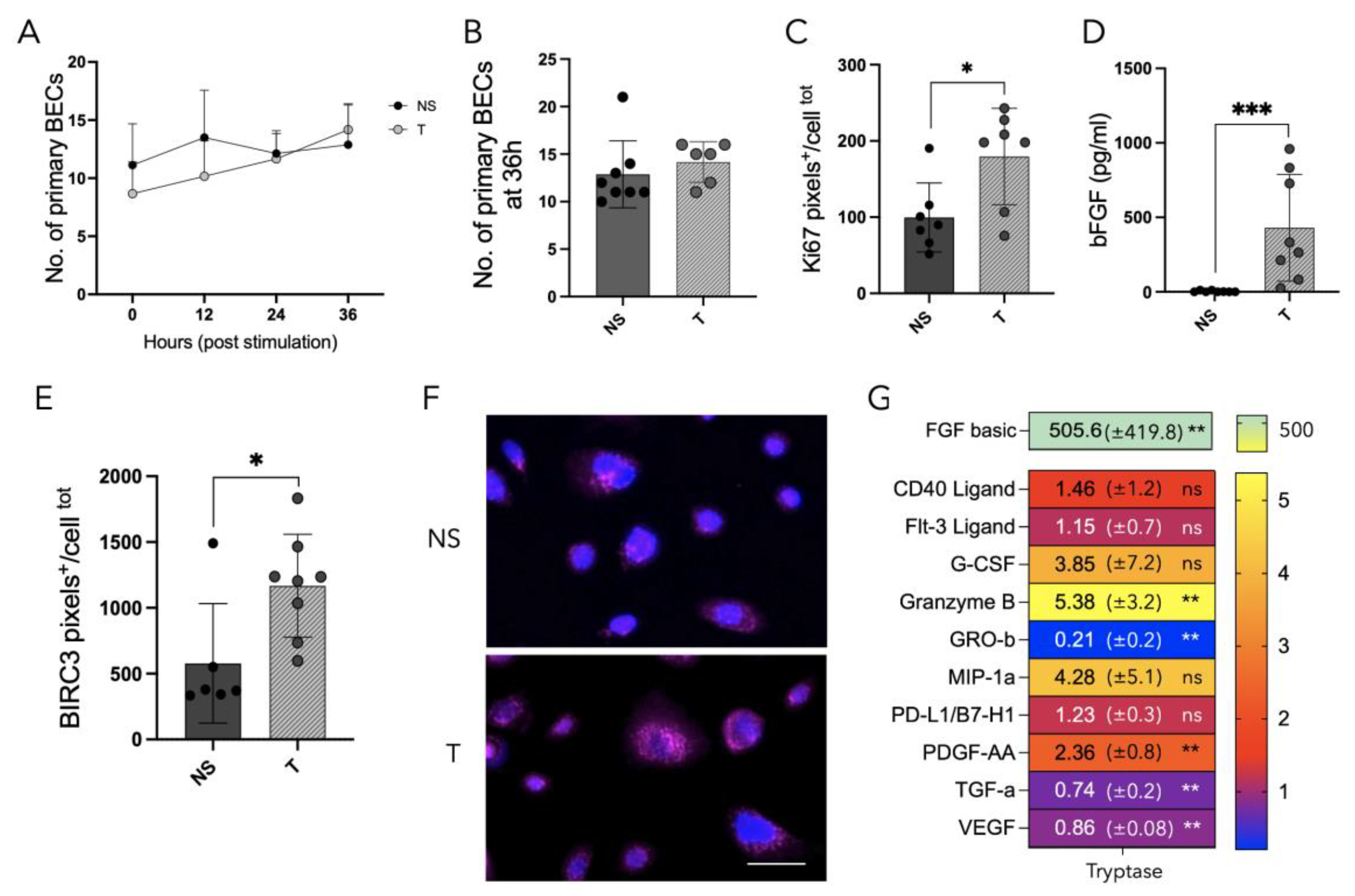
3.6. Tryptase Enhanced Protein Expression of Ki67 and BIRC3 as Well as Increased Growth Factor Release in Primary Human Bronchial Epithelial Cells from Patients with Asthma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradding, P.; Walls, A.F.; Holgate, S.T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006, 117, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M. New aspects of mast cell biology. Int. Arch. Allergy Immunol. 1997, 114, 1–9. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef]
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Andersson, C.K.; Mori, M.; Bjermer, L.; Lofdahl, C.G.; Erjefalt, J.S. Alterations in lung mast cell populations in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Balzar, S.; Fajt, M.L.; Comhair, S.A.; Erzurum, S.C.; Bleecker, E.; Busse, W.W.; Castro, M.; Gaston, B.; Israel, E.; Schwartz, L.B.; et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2011, 183, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Balzar, S.; Chu, H.W.; Strand, M.; Wenzel, S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 431–439. [Google Scholar] [CrossRef]
- Altman, M.C.; Lai, Y.; Nolin, J.D.; Long, S.; Chen, C.C.; Piliponsky, A.M.; Altemeier, W.A.; Larmore, M.; Frevert, C.W.; Mulligan, M.S.; et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J. Clin. Investig. 2019, 129, 4979–4991. [Google Scholar] [CrossRef]
- Al-Shaikhly, T.; Murphy, R.C.; Parker, A.; Lai, Y.; Altman, M.C.; Larmore, M.; Altemeier, W.A.; Frevert, C.W.; Debley, J.S.; Piliponsky, A.M.; et al. Location of eosinophils in the airway wall is critical for specific features of airway hyperresponsiveness and T2 inflammation in asthma. Eur. Respir. J. 2022, 60, 2101865. [Google Scholar] [CrossRef]
- Bagher, M.; Larsson-Callerfelt, A.K.; Rosmark, O.; Hallgren, O.; Bjermer, L.; Westergren-Thorsson, G. Mast cells and mast cell tryptase enhance migration of human lung fibroblasts through protease-activated receptor 2. Cell Commun. Signal. 2018, 16, 59. [Google Scholar] [CrossRef]
- Berger, P.; Perng, D.W.; Thabrew, H.; Compton, S.J.; Cairns, J.A.; McEuen, A.R.; Marthan, R.; Tunon De Lara, J.M.; Walls, A.F. Tryptase and agonists of PAR-2 induce the proliferation of human airway smooth muscle cells. J. Appl. Physiol. 2001, 91, 1372–1379. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Saglani, S. Asthma and allergy: The emerging epithelium. Nat. Med. 2010, 16, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Kardas, G.; Kuna, P.; Panek, M. Biological Therapies of Severe Asthma and Their Possible Effects on Airway Remodeling. Front. Immunol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Frazzi, R. BIRC3 and BIRC5, multi-faceted inhibitors in cancer. Cell Biosci. 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Kardas, G.; Daszyńska-Kardas, A.; Marynowski, M.; Brząkalska, O.; Kuna, P.; Panek, M. Role of Platelet-Derived Growth Factor (PDGF) in Asthma as an Immunoregulatory Factor Mediating Airway Remodeling and Possible Pharmacological Target. Front. Pharmacol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Doherty, T.; Broide, D. Cytokines and growth factors in airway remodeling in asthma. Curr. Opin. Immunol. 2007, 19, 676–680. [Google Scholar] [CrossRef]
- Berlin, F.; Mogren, S.; Tutzauer, J.; Andersson, C.K. Mast Cell Proteases Tryptase and Chymase Induce Migratory and Morphological Alterations in Bronchial Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5250. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Weidinger, S.; Meineke, V.; Köhn, F.M.; Mayerhofer, A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma: Possible relevance to human fibrotic disorders. Proc. Natl. Acad. Sci. USA 2002, 99, 15072–15077. [Google Scholar] [CrossRef]
- Hvidtfeldt, M.; Sverrild, A.; Pulga, A.; Frøssing, L.; Silberbrandt, A.; Hostrup, M.; Thomassen, M.; Sanden, C.; Clausson, C.M.; Siddhuraj, P.; et al. Airway Hyperresponsiveness Reflects Corticosteroid-Sensitive Mast Cell Involvement Across Asthma Phenotypes. J. Allergy Clin. Immunol. 2023, in press. [Google Scholar] [CrossRef]
- Uller, L.; Leino, M.; Bedke, N.; Sammut, D.; Green, B.; Lau, L.; Howarth, P.H.; Holgate, S.T.; Davies, D.E. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax 2010, 65, 626–632. [Google Scholar] [CrossRef]
- Gellner, C.A.; Reynaga, D.D.; Leslie, F.M. Cigarette Smoke Extract: A Preclinical Model of Tobacco Dependence. Curr. Protoc. Neurosci. 2016, 77, 9–54. [Google Scholar] [CrossRef] [PubMed]
- Mogren, S.; Berlin, F.; Eskilsson, L.; Van Der Burg, N.; Tufvesson, E.; Andersson, C.K. Mast Cell Proteases Promote Diverse Effects on the Plasminogen Activation System and Wound Healing in A549 Alveolar Epithelial Cells. Cells 2022, 11, 2916. [Google Scholar] [CrossRef]
- Sykes, A.; Macintyre, J.; Edwards, M.R.; Del Rosario, A.; Haas, J.; Gielen, V.; Kon, O.M.; McHale, M.; Johnston, S.L. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax 2014, 69, 240–246. [Google Scholar] [CrossRef]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and airway remodeling in severe asthma. Allergy 2022, 77, 3538–3552. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis Primers 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Martin, J.G. Mechanisms of airway remodeling. Chest 2013, 144, 1026–1032. [Google Scholar] [CrossRef]
- Bjermer, L. The role of small airway disease in asthma. Curr. Opin. Pulm. Med. 2014, 20, 23–30. [Google Scholar] [CrossRef]
- Erjefält, J.S. Anatomical and histopathological approaches to asthma phenotyping. Respir. Med. 2023, 210, 107168. [Google Scholar] [CrossRef]
- Andersson, C.K.; Andersson-Sjoland, A.; Mori, M.; Hallgren, O.; Pardo, A.; Eriksson, L.; Bjermer, L.; Lofdahl, C.G.; Selman, M.; Westergren-Thorsson, G.; et al. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respir. Res. 2011, 12, 139. [Google Scholar] [CrossRef]
- Andersson, C.; Tufvesson, E.; Diamant, Z.; Bjermer, L. Revisiting the role of the mast cell in asthma. Curr. Opin. Pulm. Med. 2016, 22, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.K.; Weitoft, M.; Rydell-Törmänen, K.; Bjermer, L.; Westergren-Thorsson, G.; Erjefält, J.S. Uncontrolled asthmatics have increased FceRI(+) and TGF-β-positive MC(TC) mast cells and collagen VI in the alveolar parenchyma. Clin. Exp. Allergy 2018, 48, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J. Immunol. 1996, 156, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mogren, S.; Berlin, F.; Ramu, S.; Sverrild, A.; Porsbjerg, C.; Uller, L.; Andersson, C.K. Mast cell tryptase enhances wound healing by promoting migration in human bronchial epithelial cells. Cell Adh. Migr. 2021, 15, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.O.; Sommerhoff, C.P.; Paivandy, A.; Pejler, G. Mast cell chymase regulates extracellular matrix remodeling-related events in primary human small airway epithelial cells. J. Allergy Clin. Immunol. 2022, 150, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.K.; Tufvesson, E.; Aronsson, D.; Bergqvist, A.; Mori, M.; Bjermer, L.; Erjefalt, J.S. Alveolar mast cells shift to an FcepsilonRI-expressing phenotype in mild atopic asthma: A novel feature in allergic asthma pathology. Allergy 2011, 66, 1590–1597. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Ross, R.; Raines, E.W.; Bowen-Pope, D.F. The biology of platelet-derived growth factor. Cell 1986, 46, 155–169. [Google Scholar] [CrossRef]
- Antoniades, H.N.; Bravo, M.A.; Avila, R.E.; Galanopoulos, T.; Neville-Golden, J.; Maxwell, M.; Selman, M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J. Clin. Investig. 1990, 86, 1055–1064. [Google Scholar] [CrossRef]
- Kranenburg, A.R.; Willems-Widyastuti, A.; Mooi, W.J.; Saxena, P.R.; Sterk, P.J.; de Boer, W.I.; Sharma, H.S. Chronic obstructive pulmonary disease is associated with enhanced bronchial expression of FGF-1, FGF-2, and FGFR-1. J. Pathol. 2005, 206, 28–38. [Google Scholar] [CrossRef]
- Tan, Y.; Qiao, Y.; Chen, Z.; Liu, J.; Guo, Y.; Tran, T.; Tan, K.S.; Wang, D.Y.; Yan, Y. FGF2, an Immunomodulatory Factor in Asthma and Chronic Obstructive Pulmonary Disease (COPD). Front. Cell Dev. Biol. 2020, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Ngan, D.A.; Vickerman, S.V.; Granville, D.J.; Man, S.F.; Sin, D.D. The possible role of granzyme B in the pathogenesis of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2009, 3, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M. BCL2A1: The underdog in the BCL2 family. Cell Death Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Karasawa, H.; Sasaki, I. cIAP2 as a therapeutic target in colorectal cancer and other malignancies. Expert. Opin. Ther. Targets 2009, 13, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Xu, C.; Zeng, Z.; Chen, F.; Tang, K.; Liang, Y.; Guo, Y. Exploration of induced sputum BIRC3 levels and clinical implications in asthma. BMC Pulm. Med. 2022, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.K.; Iwasaki, J.; Cook, J.; Robinson, P.; Nagakumar, P.; Mogren, S.; Fleming, L.; Bush, A.; Saglani, S.; Lloyd, C.M. Impaired airway epithelial cell wound-healing capacity is associated with airway remodelling following RSV infection in severe preschool wheeze. Allergy 2020, 75, 3195–3207. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Arshad, H.S.; Roberts, G.C.; Howarth, P.H.; Thurner, P.; Davies, D.E. A new look at the pathogenesis of asthma. Clin. Sci. 2009, 118, 439–450. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlin, F.; Mogren, S.; Ly, C.; Ramu, S.; Hvidtfeldt, M.; Uller, L.; Porsbjerg, C.; Andersson, C.K. Mast Cell Tryptase Promotes Airway Remodeling by Inducing Anti-Apoptotic and Cell Growth Properties in Human Alveolar and Bronchial Epithelial Cells. Cells 2023, 12, 1439. https://doi.org/10.3390/cells12101439
Berlin F, Mogren S, Ly C, Ramu S, Hvidtfeldt M, Uller L, Porsbjerg C, Andersson CK. Mast Cell Tryptase Promotes Airway Remodeling by Inducing Anti-Apoptotic and Cell Growth Properties in Human Alveolar and Bronchial Epithelial Cells. Cells. 2023; 12(10):1439. https://doi.org/10.3390/cells12101439
Chicago/Turabian StyleBerlin, Frida, Sofia Mogren, Camilla Ly, Sangeetha Ramu, Morten Hvidtfeldt, Lena Uller, Celeste Porsbjerg, and Cecilia K. Andersson. 2023. "Mast Cell Tryptase Promotes Airway Remodeling by Inducing Anti-Apoptotic and Cell Growth Properties in Human Alveolar and Bronchial Epithelial Cells" Cells 12, no. 10: 1439. https://doi.org/10.3390/cells12101439
APA StyleBerlin, F., Mogren, S., Ly, C., Ramu, S., Hvidtfeldt, M., Uller, L., Porsbjerg, C., & Andersson, C. K. (2023). Mast Cell Tryptase Promotes Airway Remodeling by Inducing Anti-Apoptotic and Cell Growth Properties in Human Alveolar and Bronchial Epithelial Cells. Cells, 12(10), 1439. https://doi.org/10.3390/cells12101439





