Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT
Abstract
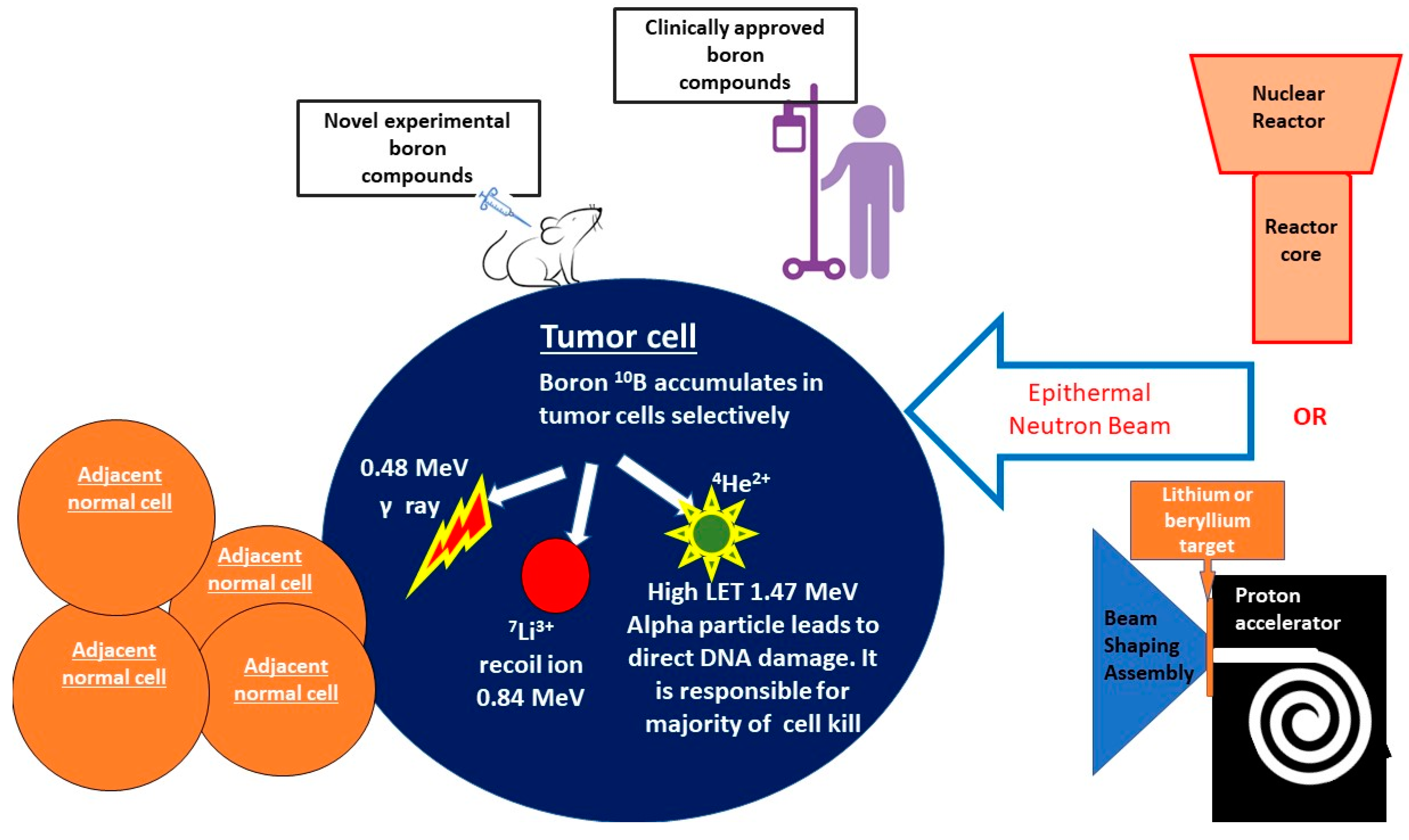
1. General Overview of BNCT
2. Unique Advantages of BNCT
3. Investigations into Novel Boron Carriers
4. Translational Work to Date Involving BNCT
5. Immunogenic Potential of BNCT
5.1. Va: Radiation-Induced Immune Response Pathways
5.2. IVb: Existing Data Regarding the Immunogenicity of BNCT and the Potential for Combining with Immunotherapies
6. The Potential for Combining CAR-T and BNCT
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron Neutron Capture Therapy: Current Status and Future Perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Barth, R.F. Boron Neutron Capture Therapy at the Crossroads: Challenges and Opportunities. Appl. Radiat. Isot. 2009, 67, S3–S6. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Olejniczak, K.; Kaniowski, D.; Araszkiewicz, M.; Tymińska, K.; Korgul, A. Molecular Mechanisms of Specific Cellular DNA Damage Response and Repair Induced by the Mixed Radiation Field During Boron Neutron Capture Therapy. Front. Oncol. 2021, 11, 676575. [Google Scholar] [CrossRef] [PubMed]
- Altieri, S.; Protti, N. A Brief Review on Reactor-Based Neutron Sources for Boron Neutron Capture Therapy. Ther. Radiol. Oncol. 2018, 2, 47. [Google Scholar] [CrossRef]
- Porra, L.; Wendland, L.; Seppälä, T.; Koivunoro, H.; Revitzer, H.; Tervonen, J.; Kankaanranta, L.; Anttonen, A.; Tenhunen, M.; Joensuu, H. From Nuclear Reactor-Based to Proton Accelerator-Based Therapy: The Finnish Boron Neutron Capture Therapy Experience. Cancer Biother. Radiopharm. 2022, 38, 184–191. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron Delivery Agents for Neutron Capture Therapy of Cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Kato, I.; Fujita, Y.; Maruhashi, A.; Kumada, H.; Ohmae, M.; Kirihata, M.; Imahori, Y.; Suzuki, M.; Sakrai, Y.; Sumi, T.; et al. Effectiveness of Boron Neutron Capture Therapy for Recurrent Head and Neck Malignancies. Appl. Radiat. Isot. 2009, 67, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Samadian, H.; Ghorbani, M.; Zakariaee, S.S.; Knaup, C. Physical, Dosimetric and Clinical Aspects and Delivery Systems in Neutron Capture Therapy. Rep. Pract. Oncol. Radiother. 2018, 23, 462–473. [Google Scholar] [CrossRef]
- Koivunoro, H.; Hippeläinen, E.; Auterinen, I.; Kankaanranta, L.; Kulvik, M.; Laakso, J.; Seppälä, T.; Savolainen, S.; Joensuu, H. Biokinetic Analysis of Tissue Boron (10B) Concentrations of Glioma Patients Treated with BNCT in Finland. Appl. Radiat. Isot. 2015, 106, 189–194. [Google Scholar] [CrossRef]
- Kageji, T.; Nagahiro, S.; Otersen, B.; Gabel, D.; Nakaichi, M.; Nakagawa, Y. Subcellular Biodistribution of Sodium Borocaptate (BSH: Na2B12H11SH) in a Rat Glioma Model in Boron Neutron Capture Therapy. J. Neurooncol. 2002, 59, 135–142. [Google Scholar] [CrossRef]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a Boron Delivery Agent for Boron Neutron Capture Therapy, Is Transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(Vinyl Alcohol) Boosting Therapeutic Potential of p-Boronophenylalanine in Neutron Capture Therapy by Modulating Metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef]
- Fukuda, H. Boron Neutron Capture Therapy (BNCT) for Cutaneous Malignant Melanoma Using 10B-p-Boronophenylalanine (BPA) with Special Reference to the Radiobiological Basis and Clinical Results. Cells 2021, 10, 2881. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Hsiao, H.W.; Tung, C.-J.; Liu, H.M.; Chou, F.I. Microdosimetry Study of THOR BNCT Beam Using Tissue Equivalent Proportional Counter. Appl. Radiat. Isot. 2009, 67, S175–S178. [Google Scholar] [CrossRef]
- Current Status of Neutron Capture Therapy. Available online: https://www.iaea.org/publications/6168/current-status-of-neutron-capture-therapy (accessed on 22 November 2021).
- Sato, T.; Masunaga, S.; Kumada, H.; Hamada, N. Microdosimetric Modeling of Biological Effectiveness for Boron Neutron Capture Therapy Considering Intra- and Intercellular Heterogeneity in 10B Distribution. Sci. Rep. 2018, 8, 988. [Google Scholar] [CrossRef]
- Ono, K. An Analysis of the Structure of the Compound Biological Effectiveness Factor. J. Radiat. Res. 2016, 57, i83–i89. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, S.; Sakurai, Y.; Tanaka, H.; Tano, K.; Suzuki, M.; Kondo, N.; Narabayashi, M.; Nakagawa, Y.; Watanabe, T.; Maruhashi, A.; et al. The Dependency of Compound Biological Effectiveness Factors on the Type and the Concentration of Administered Neutron Capture Agents in Boron Neutron Capture Therapy. SpringerPlus 2014, 3, 128. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Vicente, M.H.; Harling, O.K.; Kiger, W.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current Status of Boron Neutron Capture Therapy of High Grade Gliomas and Recurrent Head and Neck Cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Suzuki, M.; Hirose, K.; Tanaka, H.; Kato, T.; Goto, H.; Narita, Y.; Miyatake, S.-I. Accelerator-Based BNCT for Patients with Recurrent Glioblastoma: A Multicenter Phase II Study. Neurooncol. Adv. 2021, 3, vdab067. [Google Scholar] [CrossRef]
- Matsumura, A.; Asano, T.; Hirose, K.; Igaki, H.; Kawabata, S.; Kumada, H. Initiatives Toward Clinical Boron Neutron Capture Therapy in Japan. Cancer Biother. Radiopharm. 2022, 38, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Zhang, Z.; Liu, T. A Realistic Appraisal of Boron Neutron Capture Therapy as a Cancer Treatment Modality. Cancer Commun. 2018, 38, 36. [Google Scholar] [CrossRef]
- Kumari, S.; Mukherjee, S.; Sinha, D.; Abdisalaam, S.; Krishnan, S.; Asaithamby, A. Immunomodulatory Effects of Radiotherapy. Int. J. Mol. Sci. 2020, 21, 8151. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Brenner, D.J.; Formenti, S.C. Does Heavy Ion Therapy Work Through the Immune System? Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 934–936. [Google Scholar] [CrossRef]
- Trivillin, V.A.; Pozzi, E.C.C.; Colombo, L.L.; Thorp, S.I.; Garabalino, M.A.; Monti Hughes, A.; González, S.J.; Farías, R.O.; Curotto, P.; Santa Cruz, G.A.; et al. Abscopal Effect of Boron Neutron Capture Therapy (BNCT): Proof of Principle in an Experimental Model of Colon Cancer. Radiat. Environ. Biophys. 2017, 56, 365–375. [Google Scholar] [CrossRef]
- Trivillin, V.A.; Langle, Y.V.; Palmieri, M.A.; Pozzi, E.C.C.; Thorp, S.I.; Benitez Frydryk, D.N.; Garabalino, M.A.; Monti Hughes, A.; Curotto, P.M.; Colombo, L.L.; et al. Evaluation of Local, Regional and Abscopal Effects of Boron Neutron Capture Therapy (BNCT) Combined with Immunotherapy in an Ectopic Colon Cancer Model. BJR Br. J. Radiol. 2021, 94, 20210593. [Google Scholar] [CrossRef]
- Kimura, S.; Masunaga, S.; Harada, T.; Kawamura, Y.; Ueda, S.; Okuda, K.; Nagasawa, H. Synthesis and Evaluation of Cyclic RGD-Boron Cluster Conjugates to Develop Tumor-Selective Boron Carriers for Boron Neutron Capture Therapy. Bioorg. Med. Chem. 2011, 19, 1721–1728. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.; Ohmori, I.; et al. The Acceleration of Boron Neutron Capture Therapy Using Multi-Linked Mercaptoundecahydrododecaborate (BSH) Fused Cell-Penetrating Peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nishimura, K.; Okada, S.; Sato, S.; Suzuki, M.; Takata, T.; Nakamura, H. Cyclic RGD-Functionalized Closo-Dodecaborate Albumin Conjugates as Integrin Targeting Boron Carriers for Neutron Capture Therapy. Mol. Pharm. 2020, 17, 3740–3747. [Google Scholar] [CrossRef]
- Wu, G.; Barth, R.F.; Yang, W.; Chatterjee, M.; Tjarks, W.; Ciesielski, M.J.; Fenstermaker, R.A. Site-Specific Conjugation of Boron-Containing Dendrimers to Anti-EGF Receptor Monoclonal Antibody Cetuximab (IMC-C225) and Its Evaluation as a Potential Delivery Agent for Neutron Capture Therapy. Bioconjug. Chem. 2004, 15, 185–194. [Google Scholar] [CrossRef]
- Nakase, I.; Aoki, A.; Sakai, Y.; Hirase, S.; Ishimura, M.; Takatani-Nakase, T.; Hattori, Y.; Kirihata, M. Antibody-Based Receptor Targeting Using an Fc-Binding Peptide-Dodecaborate Conjugate and Macropinocytosis Induction for Boron Neutron Capture Therapy. ACS Omega 2020, 5, 22731–22738. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Y.; Huang, Y.; Zhang, Z.; Yang, W.; Du, Z.; Zhou, Y. Targeting Glioma Stem Cells Enhances Anti-Tumor Effect of Boron Neutron Capture Therapy. Oncotarget 2016, 7, 43095–43108. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.W.; Miura, M.; Easson, M.W.; Vicente, M.G.H. Recent Progress in the Syntheses and Biological Evaluation of Boronated Porphyrins for Boron Neutron-Capture Therapy. Anticancer Agents Med. Chem. 2006, 6, 145–157. [Google Scholar] [CrossRef]
- Kawabata, S.; Yang, W.; Barth, R.F.; Wu, G.; Huo, T.; Binns, P.J.; Riley, K.J.; Ongayi, O.; Gottumukkala, V.; Vicente, M.G.H. Convection Enhanced Delivery of Carboranylporphyrins for Neutron Capture Therapy of Brain Tumors. J. Neurooncol. 2011, 103, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gottumukkala, V.; Luguya, R.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and Cellular Studies of an Octa-Anionic 5,10,15,20-Tetra[3,5-(Nido-Carboranylmethyl)Phenyl]Porphyrin (H(2)OCP) for Application in BNCT. Bioorg. Med. Chem. 2005, 13, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, R.; Kawabata, S.; Tanaka, H.; Sakurai, Y.; Suzuki, M.; Ono, K.; Miyatake, S.-I.; Kuroiwa, T.; Hao, E.; Vicente, M.G.H. Tetrakis(p-Carboranylthio-Tetrafluorophenyl)Chlorin (TPFC): Application for Photodynamic Therapy and Boron Neutron Capture Therapy. J. Pharm. Sci. 2015, 104, 962–970. [Google Scholar] [CrossRef]
- Luguya, R.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis of Novel Carboranylchlorins with Dual Application in Boron Neutron Capture Therapy (BNCT) and Photodynamic Therapy (PDT). Appl. Radiat. Isot. 2004, 61, 1117–1123. [Google Scholar] [CrossRef]
- Ozawa, T.; Afzal, J.; Lamborn, K.R.; Bollen, A.W.; Bauer, W.F.; Koo, M.-S.; Kahl, S.B.; Deen, D.F. Toxicity, Biodistribution, and Convection-Enhanced Delivery of the Boronated Porphyrin BOPP in the 9L Intracerebral Rat Glioma Model. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 247–252. [Google Scholar] [CrossRef]
- Wei, Q.; Kullberg, E.B.; Gedda, L. Trastuzumab-Conjugated Boron-Containing Liposomes for Tumor-Cell Targeting; Development and Cellular Studies. Int. J. Oncol. 2003, 23, 1159–1165. [Google Scholar] [CrossRef]
- Kueffer, P.J.; Maitz, C.A.; Khan, A.A.; Schuster, S.A.; Shlyakhtina, N.I.; Jalisatgi, S.S.; Brockman, J.D.; Nigg, D.W.; Hawthorne, M.F. Boron Neutron Capture Therapy Demonstrated in Mice Bearing EMT6 Tumors Following Selective Delivery of Boron by Rationally Designed Liposomes. Proc. Natl. Acad. Sci. USA 2013, 110, 6512–6517. [Google Scholar] [CrossRef]
- Development of High Boron Content Liposomes and Their Promising Antitumor Effect for Neutron Capture Therapy of Cancers|Bioconjugate Chemistry. Available online: https://pubs.acs.org/doi/10.1021/bc300527n (accessed on 6 November 2022).
- Sumitani, S.; Oishi, M.; Nagasaki, Y. Carborane Confined Nanoparticles for Boron Neutron Capture Therapy: Improved Stability, Blood Circulation Time and Tumor Accumulation. React. Funct. Polym. 2011, 71, 684–693. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-J.; Chang, W.-Y.; Hsieh, C.-Y.; Wu, C.-C.; Chen, H.-S.; Hsu, H.-J.; Yang, A.-S.; Hsu, M.-H.; Kuo, W.-Y. Development of Theranostic Active-Targeting Boron-Containing Gold Nanoparticles for Boron Neutron Capture Therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kim, B.K.; Mackeyev, Y.; Rohani, P.; Mahajan, S.D.; Swihart, M.T.; Krishnan, S.; Prasad, P.N. Boron-Nanoparticle-Loaded Folic-Acid-Functionalized Liposomes to Achieve Optimum Boron Concentration for Boron Neutron Capture Therapy of Cancer. J. Biomed. Nanotechnol. 2019, 15, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.R.; Flieger, S.; Colorina, A.; Wozny, J.; Hosmane, N.S.; Becker, D.P. Carborane-Containing Matrix Metalloprotease (MMP) Ligands as Candidates for Boron Neutron-Capture Therapy (BNCT). ChemMedChem 2020, 15, 1897–1908. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured Boron Nitride With High Water Dispersibility For Boron Neutron Capture Therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Koganei, H.; Miyoshi, T.; Sakurai, Y.; Ono, K.; Suzuki, M. Antitumor Effect of Boron Nitride Nanotubes in Combination with Thermal Neutron Irradiation on BNCT. Bioorg. Med. Chem. Lett. 2015, 25, 172–174. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shi, Y.; Du, P.; Zhang, Z.; Liu, T.; Zhang, R.; Liu, Z. On-Demand Biodegradable Boron Nitride Nanoparticles for Treating Triple Negative Breast Cancer with Boron Neutron Capture Therapy. ACS Nano 2019, 13, 13843–13852. [Google Scholar] [CrossRef]
- Ou, M.; Wang, X.; Yu, L.; Liu, C.; Tao, W.; Ji, X.; Mei, L. The Emergence and Evolution of Borophene. Adv. Sci. 2021, 8, 2001801. [Google Scholar] [CrossRef]
- Kreimann, E.L.; Itoiz, M.E.; Dagrosa, A.; Garavaglia, R.; Farías, S.; Batistoni, D.; Schwint, A.E. The Hamster Cheek Pouch as a Model of Oral Cancer for Boron Neutron Capture Therapy Studies: Selective Delivery of Boron by Boronophenylalanine. Cancer Res. 2001, 61, 8775–8781. [Google Scholar]
- Kreimann, E.L.; Itoiz, M.E.; Longhino, J.; Blaumann, H.; Calzetta, O.; Schwint, A.E. Boron Neutron Capture Therapy for the Treatment of Oral Cancer in the Hamster Cheek Pouch Model. Cancer Res. 2001, 61, 8638–8642. [Google Scholar]
- Ono, K.; Masunaga, S.; Suzuki, M.; Kinashi, Y.; Takagaki, M.; Akaboshi, M. The Combined Effect of Boronophenylalanine and Borocaptate in Boron Neutron Capture Therapy for SCCVII Tumors in Mice. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.-I.; Kawabata, S.; Kajimoto, Y.; Aoki, A.; Yokoyama, K.; Yamada, M.; Kuroiwa, T.; Tsuji, M.; Imahori, Y.; Kirihata, M.; et al. Modified Boron Neutron Capture Therapy for Malignant Gliomas Performed Using Epithermal Neutron and Two Boron Compounds with Different Accumulation Mechanisms: An Efficacy Study Based on Findings on Neuroimages. J. Neurosurg. 2005, 103, 1000–1009. [Google Scholar] [CrossRef]
- Heber, E.M.; Trivillin, V.A.; Nigg, D.W.; Itoiz, M.E.; Gonzalez, B.N.; Rebagliati, R.J.; Batistoni, D.; Kreimann, E.L.; Schwint, A.E. Homogeneous Boron Targeting of Heterogeneous Tumors for Boron Neutron Capture Therapy (BNCT): Chemical Analyses in the Hamster Cheek Pouch Oral Cancer Model. Arch. Oral. Biol. 2006, 51, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Heber, E.; Trivillin, V.A.; Nigg, D.; Kreimann, E.L.; Itoiz, M.E.; Rebagliati, R.J.; Batistoni, D.; Schwint, A.E. Biodistribution of GB-10 (Na(2)(10)B10H10 Compound for Boron Neutron Capture Therapy (BNCT) in an Experimental Model of Oral Cancer in the Hamster Cheek Pouch. Arch. Oral. Biol. 2004, 49, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Trivillin, V.A.; Heber, E.M.; Nigg, D.W.; Itoiz, M.E.; Calzetta, O.; Blaumann, H.; Longhino, J.; Schwint, A.E. Therapeutic Success of Boron Neutron Capture Therapy (BNCT) Mediated by a Chemically Non-Selective Boron Agent in an Experimental Model of Oral Cancer: A New Paradigm in BNCT Radiobiology. Radiat. Res. 2006, 166, 387–396. [Google Scholar] [CrossRef]
- Molinari, A.J.; Pozzi, E.C.C.; Monti Hughes, A.; Heber, E.M.; Garabalino, M.A.; Thorp, S.I.; Miller, M.; Itoiz, M.E.; Aromando, R.F.; Nigg, D.W.; et al. “Sequential” Boron Neutron Capture Therapy (BNCT): A Novel Approach to BNCT for the Treatment of Oral Cancer in the Hamster Cheek Pouch Model. Radiat. Res. 2011, 175, 463–472. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carpano, M.; Curotto, P.; Thorp, S.; Casal, M.; Juvenal, G.; Pisarev, M.; Dagrosa, M.A. In Vitro Studies of DNA Damage and Repair Mechanisms Induced by BNCT in a Poorly Differentiated Thyroid Carcinoma Cell Line. Radiat. Environ. Biophys. 2018, 57, 143–152. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Lu, C.; Xiao, H.; Guo, Z.; Duan, D.; Zhang, Z.; Liu, T.; Liu, Z. Boron Encapsulated in a Liposome Can Be Used for Combinational Neutron Capture Therapy. Nat. Commun. 2022, 13, 2143. [Google Scholar] [CrossRef]
- Durante, M.; Formenti, S. Harnessing Radiation to Improve Immunotherapy: Better with Particles? BJR Br. J. Radiol. 2020, 93, 20190224. [Google Scholar] [CrossRef]
- Ebner, D.K.; Tinganelli, W.; Helm, A.; Bisio, A.; Yamada, S.; Kamada, T.; Shimokawa, T.; Durante, M. The Immunoregulatory Potential of Particle Radiation in Cancer Therapy. Front. Immunol. 2017, 8, 99. [Google Scholar] [CrossRef]
- Sevenich, L. Turning “Cold” Into “Hot” Tumors—Opportunities and Challenges for Radio-Immunotherapy Against Primary and Metastatic Brain Cancers. Front. Oncol. 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; He, Y.; Tang, H.; Chen, X.; Liu, S.; Tao, Y. CGAS/STING: Novel Perspectives of the Classic Pathway. Mol. Biomed. 2020, 1, 7. [Google Scholar] [CrossRef]
- Constanzo, J.; Faget, J.; Ursino, C.; Badie, C.; Pouget, J.-P. Radiation-Induced Immunity and Toxicities: The Versatility of the CGAS-STING Pathway. Front. Immunol. 2021, 12, 680503. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Dey, S.K.; Sarma, A.; Khuda-Bukhsh, A.R. Cell Killing, Nuclear Damage and Apoptosis in Chinese Hamster V79 Cells after Irradiation with Heavy-Ion Beams of (16)O, (12)C and (7)Li. Mutat. Res. 2007, 632, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA Exonuclease Trex1 Regulates Radiotherapy-Induced Tumour Immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; et al. CGAS-STING, an Important Pathway in Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef]
- Gorin, J.-B.; Ménager, J.; Gouard, S.; Maurel, C.; Guilloux, Y.; Faivre-Chauvet, A.; Morgenstern, A.; Bruchertseifer, F.; Chérel, M.; Davodeau, F.; et al. Antitumor Immunity Induced after α Irradiation. Neoplasia 2014, 16, 319–328. [Google Scholar] [CrossRef]
- Smilowitz, H.M.; Micca, P.L.; Nawrocky, M.M.; Slatkin, D.N.; Tu, W.; Coderre, J.A. The Combination of Boron Neutron-Capture Therapy and Immunoprophylaxis for Advanced Intracerebral Gliosarcomas in Rats. J. Neurooncol. 2000, 46, 231–240. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Martins, I.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.-L. Macrophage Biology Plays a Central Role during Ionizing Radiation-Elicited Tumor Response. Biomed. J. 2017, 40, 200–211. [Google Scholar] [CrossRef]
- Khan, A.A.; Maitz, C.; Quanyu, C.; Hawthorne, F. BNCT Induced Immunomodulatory Effects Contribute to Mammary Tumor Inhibition. PLoS ONE 2019, 14, e0222022. [Google Scholar] [CrossRef]
- Chen, J.; Dai, Q.; Yang, Q.; Bao, X.; Zhou, Y.; Zhong, H.; Wu, L.; Wang, T.; Zhang, Z.; Lu, Y.; et al. Therapeutic Nucleus-Access BNCT Drug Combined CD47-Targeting Gene Editing in Glioblastoma. J. Nanobiotechnol. 2022, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Hirase, S.; Aoki, A.; Hattori, Y.; Morimoto, K.; Noguchi, K.; Fujii, I.; Takatani-Nakase, T.; Futaki, S.; Kirihata, M.; Nakase, I. Dodecaborate-Encapsulated Extracellular Vesicles with Modification of Cell-Penetrating Peptides for Enhancing Macropinocytotic Cellular Uptake and Biological Activity in Boron Neutron Capture Therapy. Mol. Pharm. 2022, 19, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Ding, Y.; Xue, Z.; Li, P.; Li, J.; Li, F. Roles of Exosomes as Drug Delivery Systems in Cancer Immunotherapy: A Mini-Review. Discov. Oncol. 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.W.A.; Jahangir, S.; Ghosh, B.; Yesmin, F.; Anis, A.; Satil, S.N.; Anwar, F.; Rashid, M.H. Exosomes for Regulation of Immune Responses and Immunotherapy. J. Nanotheranostics 2022, 3, 55–85. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T Cells in Solid Tumors: Challenges and Opportunities. Stem. Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- DeSelm, C.; Palomba, M.L.; Yahalom, J.; Hamieh, M.; Eyquem, J.; Rajasekhar, V.K.; Sadelain, M. Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape. Mol. Ther. 2018, 26, 2542–2552. [Google Scholar] [CrossRef]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in Radiobiological Responses: A Review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an INOS+/M1 Phenotype That Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like Receptor 4-Dependent Contribution of the Immune System to Anticancer Chemotherapy and Radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Anderson, R. Realizing the Clinical Potential of Immunogenic Cell Death in Cancer Chemotherapy and Radiotherapy. Int. J. Mol. Sci. 2019, 20, 959. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Formenti, S.C. Role of T Lymphocytes in Tumor Response to Radiotherapy. Front. Oncol. 2012, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Teitz-Tennenbaum, S.; Li, Q.; Rynkiewicz, S.; Ito, F.; Davis, M.A.; McGinn, C.J.; Chang, A.E. Radiotherapy Potentiates the Therapeutic Efficacy of Intratumoral Dendritic Cell Administration. Cancer Res. 2003, 63, 8466–8475. [Google Scholar]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Chen, Y.-W. HGG-05. Regression of Recurrent Glioblastoma after Boron Neutron Capture Therapy and Chimeric Antigen Receptor T-Cell Therapy in a Child. Neuro Oncol. 2020, 22, iii345. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seneviratne, D.S.; Saifi, O.; Mackeyev, Y.; Malouff, T.; Krishnan, S. Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT. Cells 2023, 12, 1398. https://doi.org/10.3390/cells12101398
Seneviratne DS, Saifi O, Mackeyev Y, Malouff T, Krishnan S. Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT. Cells. 2023; 12(10):1398. https://doi.org/10.3390/cells12101398
Chicago/Turabian StyleSeneviratne, Danushka S., Omran Saifi, Yuri Mackeyev, Timothy Malouff, and Sunil Krishnan. 2023. "Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT" Cells 12, no. 10: 1398. https://doi.org/10.3390/cells12101398
APA StyleSeneviratne, D. S., Saifi, O., Mackeyev, Y., Malouff, T., & Krishnan, S. (2023). Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT. Cells, 12(10), 1398. https://doi.org/10.3390/cells12101398







