Microvascular Leakage as Therapeutic Target for Ischemia and Reperfusion Injury
Abstract
1. Introduction
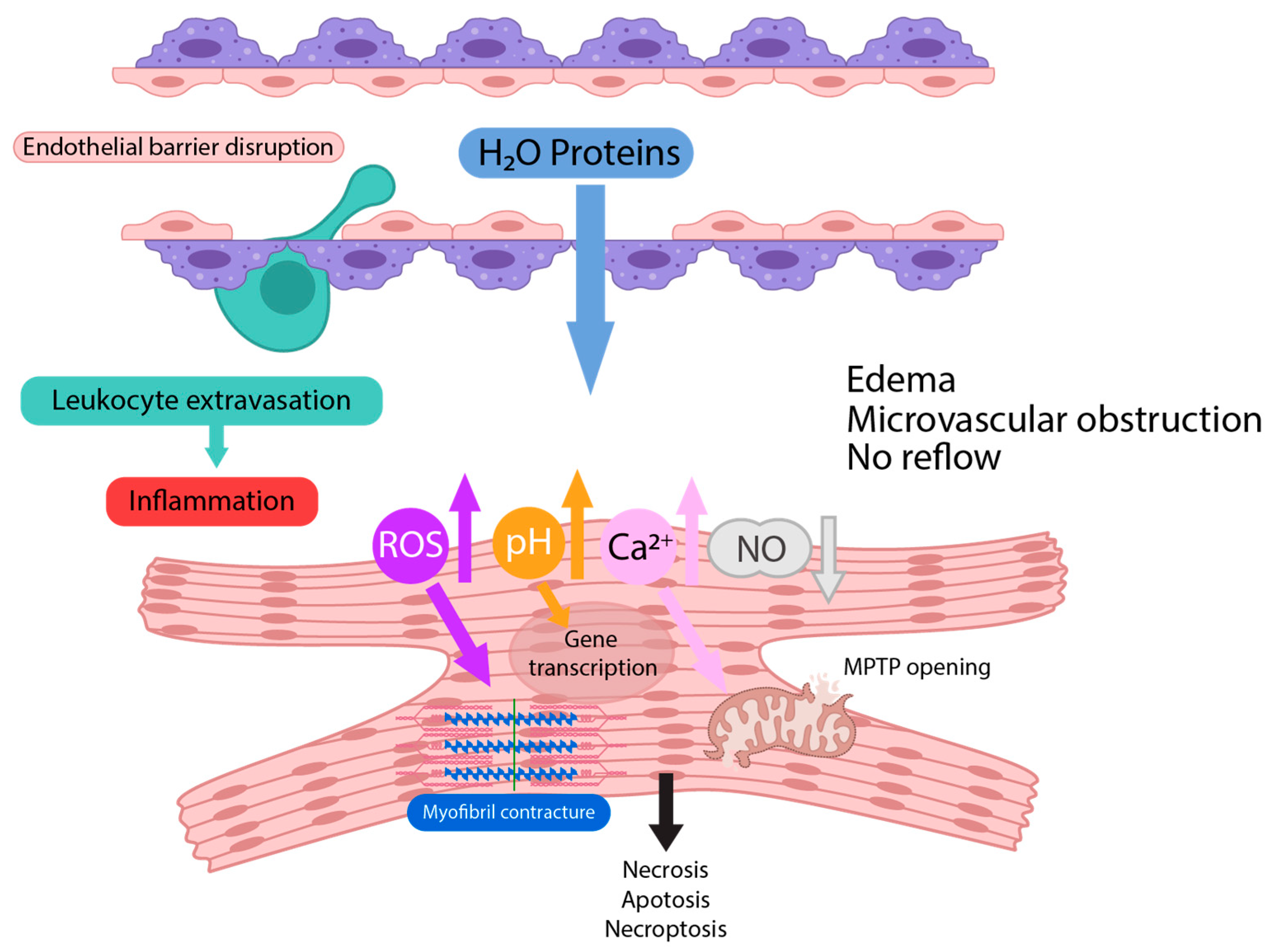
2. Pathophysiology of Ischemia/Reperfusion Injury
2.1. Myocardial IRI
2.2. IRI in the Brain
2.3. IRI in Organ Transplantation
2.4. IRI Following Global Ischemia after Cardiac Arrest
3. Microvascular Dysfunction and Capillary Leakage in IRI
4. Therapeutic Approaches
4.1. Experimental
4.2. Clinical
5. Conclusions and Outlook
- -
- Before being applied in a clinical setting, the effectiveness of the cardioprotective intervention must be verified in several models, preferably including large animals and comorbidities.
- -
- The data should be reproducible between different study centers in multi-center trials.
- -
- Endpoints in the latter stages of preclinical studies should mirror the clinical endpoints that will have an impact on medical practices, such as mortality.
- -
- Pre-clinical translational studies should start to reflect the background of current medical therapy for STEMI patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef]
- Camacho, X.; Nedkoff, L.; Wright, F.L.; Nghiem, N.; Buajitti, E.; Goldacre, R.; Rosella, L.C.; Seminog, O.; Tan, E.J.; Hayes, A.; et al. Relative Contribution of Trends in Myocardial Infarction Event Rates and Case Fatality to Declines in Mortality: An International Comparative Study of 1·95 Million Events in 80·4 Million People in Four Countries. Lancet Public Health 2022, 7, e229–e239. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart Failure after Myocardial Infarction: Incidence and Predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left Ventricular Remodelling Post-Myocardial Infarction: Pathophysiology, Imaging, and Novel Therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial Ischemia Reperfusion Injury: From Basic Science to Clinical Bedside. Sem. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef]
- Beyersdorf, F.; Trummer, G.; Benk, C.; Pooth, J.S. Application of Cardiac Surgery Techniques to Improve the Results of Cardiopulmonary Resuscitation after Cardiac Arrest: Controlled Automated Reperfusion of the Whole Body. JTCVS Open 2021, 8, 47–52. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Wu, Z.J.; Zheng, J.H.; Qin, T.; Yang, Y.G.; Chen, M.H. The Incidence of Acute Kidney Injury Following Cardiac Arrest and Cardiopulmonary Resuscitation in a Rat Model. Ren. Fail. 2019, 41, 278–283. [Google Scholar] [CrossRef]
- Sandroni, C.; Cronberg, T.; Sekhon, M. Brain Injury after Cardiac Arrest: Pathophysiology, Treatment, and Prognosis. Intensive Care Med. 2021, 47, 1393–1414. [Google Scholar] [CrossRef]
- Cannon, J.W. Hemorrhagic Shock. N. Engl. J. Med. 2018, 378, 370–379. [Google Scholar] [CrossRef]
- Yeung, K.K.; Groeneveld, M.; Lu, J.J.N.; van Diemen, P.; Jongkind, V.; Wisselink, W. Organ Protection during Aortic Cross-Clamping. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 305–315. [Google Scholar] [CrossRef]
- Nielsen, E.W.; Miller, Y.; Brekke, O.L.; Grond, J.; Duong, A.H.; Fure, H.; Ludviksen, J.K.; Pettersen, K.; Reubsaet, L.; Solberg, R.; et al. A Novel Porcine Model of Ischemia-Reperfusion Injury After Cross-Clamping the Thoracic Aorta Revealed Substantial Cardiopulmonary, Thromboinflammatory and Biochemical Changes Without Effect of C1-Inhibitor Treatment. Front. Immunol. 2022, 13, 852119. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial Ischaemia–Reperfusion Injury and Cardioprotection in Perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial Ischemia/Reperfusion Injury: Mechanisms of Injury and Implications for Management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.Y.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Jennings, R.B. Historical Perspective on the Pathology of Myocardial Ischemia/Reperfusion Injury. Circ. Res. 2013, 113, 428–438. [Google Scholar] [CrossRef]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial Necrosis Induced by Temporary Occlusion of a Coronary Artery in Dogs. Arch. Pathol. 1960, 70, 68–78. [Google Scholar]
- Enzmann, G.; Kargaran, S.; Engelhardt, B. Ischemia–Reperfusion Injury in Stroke: Impact of the Brain Barriers and Brain Immune Privilege on Neutrophil Function. Ther. Adv. Neurol Disord. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Yu, Z. Ischemia-Reperfusion Injury in the Brain: Mechanisms and Potential Therapeutic Strategies. Biochem. Pharmacol. 2016, 5, 4. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-Reperfusion Injury in Liver Transplantation-from Bench to Bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. Ischemia–Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The Pathophysiology of Acute Myocardial Infarction and Strategies of Protection beyond Reperfusion: A Continual Challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef]
- Leineweber, K.; Böse, D.; Vogelsang, M.; Haude, M.; Erbel, R.; Heusch, G. Intense Vasoconstriction in Response to Aspirate from Stented Saphenous Vein Aortocoronary Bypass Grafts. J. Am. Coll. Cardiol. 2006, 47, 981–986. [Google Scholar] [CrossRef][Green Version]
- Heusch, G.; Kleinbongard, P.; Böse, D.; Levkau, B.; Haude, M.; Schulz, R.; Erbel, R. Coronary Microembolization: From Bedside to Bench and Back to Bedside. Circulation 2009, 120, 1822–1836. [Google Scholar] [CrossRef]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial No-Reflow in Humans. J. Am. Coll. Cardiol. 2010, 55, 281–292. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef]
- Hamirani, Y.S.; Wong, A.; Kramer, C.M.; Salerno, M. Effect of Microvascular Obstruction and Intramyocardial Hemorrhage by CMR on LV Remodeling and Outcomes after Myocardial Infarction: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2014, 7, 940–952. [Google Scholar] [CrossRef]
- Yang, Q.; He, G.-W.; Underwood, M.J.; Yu, C.-M. Review Article Cellular and Molecular Mechanisms of Endothelial Ischemia/Reperfusion Injury: Perspectives and Implications for Postischemic Myocardial Protection. Am. J. Transl. Res. 2016, 8, 765–777. [Google Scholar]
- Francischetti, I.; Moreno, J.B.J.; Scholz, M.; Yoshida, W.B. Leukocytes and the Inflammatory Response in Ischemia-Reperfusion Injury. Rev. Bras. Circ. Cardiovasc. 2010, 25, 575–584. [Google Scholar] [CrossRef][Green Version]
- Sezer, M.; Van Royen, N.; Umman, B.; Bugra, Z.; Bulluck, H.; Hausenloy, D.J.; Umman, S. Coronary Microvascular Injury in Reperfused Acute Myocardial Infarction: A View from an Integrative Perspective. J. Am. Heart Assoc. 2018, 7, e009949. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; Rauhalammi, S.; Clerfond, G.; Carberry, J.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; et al. Temporal Evolution of Myocardial Hemorrhage and Edema in Patients after Acute St-Segment Elevation Myocardial Infarction: Pathophysiological Insights and Clinical Implications. J. Am. Heart Assoc. 2016, 5, e002834. [Google Scholar] [CrossRef]
- Bonfig, N.L.; Soukup, C.R.; Shah, A.A.; Olet, S.; Davidson, S.J.; Schmidt, C.W.; Peterson, R.; Henry, T.D.; Traverse, J.H. Increasing Myocardial Edema Is Associated with Greater Microvascular Obstruction in ST-Segment Elevation Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H818–H824. [Google Scholar] [CrossRef]
- Gao, X.-M.; Wu, Q.-Z.; Kiriazis, H.; Su, Y.; Han, L.-P.; Todd Pearson, J.; Taylor, A.J.; Du, X.-J. Microvascular Leakage in Acute Myocardial Infarction: Characterization by Histology, Biochemistry, and Magnetic Resonance Imaging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, 1068–1075. [Google Scholar] [CrossRef]
- Huang, L.H.; Lavine, K.J.; Randolph, G.J. Cardiac Lymphatic Vessels, Transport, and Healing of the Infarcted Heart. JACC Basic Transl. Sci. 2017, 2, 477–483. [Google Scholar] [CrossRef]
- Sumii, T.; Lo, E.H. Involvement of Matrix Metalloproteinase in Thrombolysis-Associated Hemorrhagic Transformation After Embolic Focal Ischemia in Rats. Stroke 2002, 33, 831–836. [Google Scholar] [CrossRef]
- Warach, S.; Latour, L.L. Evidence of Reperfusion Injury, Exacerbated by Thrombolytic Therapy, in Human Focal Brain Ischemia Using a Novel Imaging Marker of Early Blood-Brain Barrier Disruption. Stroke 2004, 35, 2659–2661. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on Ischemia-Reperfusion Injury in Kidney Transplantation: Pathogenesis and Treatment. World J. Transplant. 2015, 5, 52. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Shah, K.A.; Fuller, B.J.; Green, C.J. Cold ischemia-induced damage to vascular endothelium results in permeability alterations in transplanted lungs. J. Thorac. Cardiovasc. Surg. 1996, 112, 1027–1035. [Google Scholar] [CrossRef]
- Pulitano, C.; Joseph, D.; Sandroussi, C.; Verran, D.; Ho, P.; Debiasio, A.; Luongo, A.; McCaughan, G.W.; Shackel, N.A.; Crawford, M. Postreperfusion Microcirculatory Derangements after Liver Transplantation: Relationship to Hemodynamics, Serum Mediators, and Outcome. Liver Transplant. 2017, 23, 527–536. [Google Scholar] [CrossRef]
- Neumar, R.W.; Nolan, J.P.; Adrie, C.; Aibiki, M.; Berg, R.A.; Böttiger, B.W.; Callaway, C.; Clark, R.S.B.; Geocadin, R.G.; Jauch, E.C.; et al. Vanden. Post-Cardiac Arrest Syndrome: Epidemiology, Pathophysiology, Treatment, and Prognostication a Consensus Statement from the International Liaison Committee on Resuscitation. Circulation 2008, 18, 2452–2483. [Google Scholar] [CrossRef]
- Kang, Y. Management of Post-Cardiac Arrest Syndrome. Acute Crit. Care 2019, 34, 173–178. [Google Scholar] [CrossRef]
- The Hypothermia after Cardia Arrest Study Group. Mild Therapeutic Hypothermia to Improve the Neurological Outcome after Cardiac Arrest. N. Eng. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef]
- Meybohm, P.; Gruenewald, M.; Albrecht, M.; Zacharowski, K.D.; Lucius, R.; Zitta, K.; Koch, A.; Tran, N.; Scholz, J.; Bein, B. Hypothermia and Postconditioning after Cardiopulmonary Resuscitation Reduce Cardiac Dysfunction by Modulating Inflammation, Apoptosis and Remodeling. PLoS ONE 2009, 4, e7588. [Google Scholar] [CrossRef]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef]
- Tiwari, A.; Elgrably, B.; Saar, G.; Vandoorne, K. Multi-Scale Imaging of Vascular Pathologies in Cardiovascular Disease. Front. Med. 2022, 9, 754369. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front. Physiol. 2015, 6, 365. [Google Scholar] [CrossRef]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Dejana, E.; Vestweber, D. The Role of VE-Cadherin in Vascular Morphogenesis and Permeability Control. Prog. Mol. Biol. Transl. Sci. 2013, 116, 119–144. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhao, J.L.; You, S.J.; Wu, Y.J.; Jing, Z.C.; Gao, R.L.; Chen, Z.J. Post-Infarction Treatment with Simvastatin Reduces Myocardial No-Reflow by Opening of the KATP Channel. Eur. J. Heart Fail. 2007, 9, 30–36. [Google Scholar] [CrossRef]
- van Buul, J.D.; Timmerman, I. Small Rho GTPase-Mediated Actin Dynamics at Endothelial Adherens Junctions. Small GTPases 2016, 7, 21–31. [Google Scholar] [CrossRef]
- Timmerman, I.; Heemskerk, N.; Kroon, J.; Schaefer, A.; van Rijssel, J.; Hoogenboezem, M.; van Unen, J.; Goedhart, J.; Gadella, T.W.J.; Yin, T.; et al. Correction to “A Local VE-Cadherin and Trio-Based Signaling Complex Stabilizes Endothelial Junctions through Rac1”. J. Cell Sci. 2015, 128, 3514. [Google Scholar] [CrossRef]
- Wallez, Y.; Cand, F.; Cruzalegui, F.; Wernstedt, C.; Souchenalytskyi, S.; Vilgrain, I.; Huber, P. Src Kinase Phosphorylates Vascular Endothelial-Cadherin in Response to Vascular Endothelial Growth Factor: Identification of Tyrosine 685 as the Unique Target Site. Oncogene 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Nawroth, R.; Poell, G.; Ranft, A.; Kloep, S.; Samulowitz, U.; Fachinger, G.; Golding, M.; Shima, D.T.; Deutsch, U.; Vestweber, D. VE-PTP and VE-Cadherin Ectodomains Interact to Facilitate Regulation of Phosphorylation and Cell Contacts. EMBO J. 2002, 21, 4885–4895. [Google Scholar] [CrossRef]
- Hilbert, T.; Klaschik, S. The Angiopoietin/TIE Receptor System: Focusing Its Role for Ischemia-Reperfusion Injury. Cytokine Growth Factor Rev. 2015, 26, 281–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Kontos, C.D.; Annex, B.H.; Popel, A.S. Angiopoietin-Tie Signaling Pathway in Endothelial Cells: A Computational Model. iScience 2019, 20, 497–511. [Google Scholar] [CrossRef]
- Lee, S.W.; Won, J.Y.; Lee, H.Y.; Lee, H.J.; Youn, S.W.; Lee, J.Y.; Cho, C.H.; Cho, H.J.; Oh, S.; Chae, I.H.; et al. Angiopoietin-1 Protects Heart against Ischemia/Reperfusion Injury through VE-Cadherin Dephosphorylation and Myocardiac Integrin-Β1/ERK/Caspase-9 Phosphorylation Cascade. Mol. Med. 2011, 17, 1095–1106. [Google Scholar] [CrossRef]
- Patel, J.v.; Lim, H.S.; Varughese, G.I.; Hughes, E.A.; Lip, G.Y.H. Angiopoietin-2 Levels as a Biomarker of Cardiovascular Risk in Patients with Hypertension. Ann. Med. 2008, 40, 215–222. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, C.K.; Kang, S.; Park, I.; Kim, Y.H.; Kim, S.K.; Hong, S.P.; Bae, H.; He, Y.; Kubota, Y.; et al. Angiopoietin-2 Exacerbates Cardiac Hypoxia and Inflammation after Myocardial Infarction. J. Clin. Investig. 2018, 128, 5018–5033. [Google Scholar] [CrossRef]
- Shen, J.; Frye, M.; Lee, B.L.; Reinardy, J.L.; McClung, J.M.; Ding, K.; Kojima, M.; Xia, H.; Seidel, C.; Lima E Silva, R.; et al. Targeting VE-PTP Activates TIE2 and Stabilizes the Ocular Vasculature. J. Clin. Investig. 2014, 124, 4564–4576. [Google Scholar] [CrossRef]
- Souma, T.; Thomson, B.R.; Heinen, S.; Carota, I.A.; Yamaguchi, S.; Onay, T.; Liu, P.; Ghosh, A.K.; Li, C.; Eremina, V.; et al. Context-Dependent Functions of Angiopoietin 2 Are Determined by the Endothelial Phosphatase VEPTP. Proc. Natl. Acad. Sci. USA 2018, 115, 1298–1303. [Google Scholar] [CrossRef]
- Raza, Z.; Saleem, U.; Naureen, Z. Sphingosine 1-Phosphate Signaling in Ischemia and Reperfusion Injury. Prostaglandins Other Lipid Mediat. 2020, 149, 106436. [Google Scholar] [CrossRef]
- Wilkerson, B.A.; Argraves, K.M. The Role of Sphingosine-1-Phosphate in Endothelial Barrier Function. Biochim. Biophys. Acta 2014, 1841, 1403–1412. [Google Scholar] [CrossRef]
- Morel, S.; Christoffersen, C.; Axelsen, L.N.; Montecucco, F.; Rochemont, V.; Frias, M.A.; Mach, F.; James, R.W.; Naus, C.C.; Chanson, M.; et al. Sphingosine-1-Phosphate Reduces Ischaemia-Reperfusion Injury by Phosphorylating the Gap Junction Protein Connexin43. Cardiovasc. Res. 2016, 109, 385–396. [Google Scholar] [CrossRef]
- Nitzsche, A.; Poittevin, M.; Benarab, A.; Bonnin, P.; Faraco, G.; Uchida, H.; Favre, J.; Garcia-Bonilla, L.; Garcia, M.C.L.; Léger, P.L.; et al. Endothelial S1P1 Signaling Counteracts Infarct Expansion in Ischemic Stroke. Circ. Res. 2021, 128, 363–382. [Google Scholar] [CrossRef]
- Kong, Q.; Dai, L.; Wang, Y.; Zhang, X.; Li, C.; Jiang, S.; Li, Y.; Ding, Z.; Liu, L. HSPA12B Attenuated Acute Myocardial Ischemia/Reperfusion Injury via Maintaining Endothelial Integrity in a PI3K/Akt/MTOR-Dependent Mechanism. Sci. Rep. 2016, 6, 33636. [Google Scholar] [CrossRef]
- Andrés-Villarreal, M.; Barba, I.; Poncelas, M.; Inserte, J.; Rodriguez-Palomares, J.; Pineda, V.; Garcia-Dorado, D. Measuring Water Distribution in the Heart: Preventing Edema Reduces Ischemia-Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003843. [Google Scholar] [CrossRef]
- Ng, F.C.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Thijs, V.; Wu, T.Y.; Shah, D.G.; Dewey, H.M.; Sharma, G.; Desmond, P.M.; et al. Microvascular Dysfunction in Blood-Brain Barrier Disruption and Hypoperfusion Within the Infarct Posttreatment Are Associated with Cerebral Edema. Stroke 2022, 53, 1597–1605. [Google Scholar] [CrossRef]
- Sutton, T.A. Alteration of Microvascular Permeability in Acute Kidney Injury. Microvasc. Res. 2009, 77, 4–7. [Google Scholar] [CrossRef]
- Heusch Gerd. Treatment of Myocardial Ischemia/Reperfusion Injury by Ischemic and Pharmacological Postconditioning. Compr. Physiol. 2015, 5, 1123–1145. [Google Scholar]
- Hausenloy, D.J.; Yellon, D.M. New Directions for Protecting the Heart against Ischaemia-Reperfusion Injury: Targeting the Reperfusion Injury Salvage Kinase (RISK)-Pathway. Cardiovasc. Res. 2004, 61, 448–460. [Google Scholar] [CrossRef]
- Hadebe, N.; Cour, M.; Lecour, S. The SAFE Pathway for Cardioprotection: Is This a Promising Target? Basic Res. Cardiol. 2018, 113, 9. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Y.; Luo, X.; Wang, J.; Lan, X.; Liu, P.; Feng, Y.; Jian, W. Myocardial Ischemia-Reperfusion Injury: Therapeutics from a Mitochondria-Centric Perspective. Cardiology 2021, 146, 781–792. [Google Scholar] [CrossRef]
- Kosieradzki, M.; Pratschke, J.; Kupiec-Węgliński, J.; Rowiński, W. Ischemia/Reperfusion Injury, Its Mechanisms, and Prevention. J. Transplant. 2012, 2012, 610370. [Google Scholar] [CrossRef]
- Bai, J.; Lyden, P.D. Revisiting Cerebral Postischemic Reperfusion Injury: New Insights in Understanding Reperfusion Failure, Hemorrhage, and Edema. Int. J. Stroke 2015, 10, 143–152. [Google Scholar] [CrossRef]
- Pawitan, J.A. Potential Agents against Plasma Leakage. ISRN Pharmacol. 2011, 2011, 975048. [Google Scholar] [CrossRef]
- Ahrens, I.; Peter, K. FX-06, a Fibrin-Derived Bβ15-42 Peptide for the Potential Treatment of Reperfusion Injury Following Myocardial Infarction. Curr. Opin. Investig. Drugs 2009, 10, 997–1003. [Google Scholar]
- Henning, R.; Zacharowski, K.; Petzelbauer, P. FX06 (Fibrin-Derived Peptide Bbeta15-42)-A Potential Candidate for Myocardial Reperfusion Therapy. Drugs Future 2006, 31, 811. [Google Scholar] [CrossRef]
- Jennewein, C.; Tran, N.; Paulus, P.; Ellinghaus, P.; Eble, J.A.; Zacharowski, K. Novel Aspects of Fibrin(Ogen) Fragments during Inflammation. Mol. Med. 2011, 17, 568–573. [Google Scholar] [CrossRef]
- Sörensen, I.; Rong, S.; Susnik, N.; Gueler, F.; Shushakova, N.; Albrecht, M.; Dittrich, A.-M.; von Vietinghoff, S.; Becker, J.U.; Melk, A.; et al. Bβ15–42 Attenuates the Effect of Ischemia-Reperfusion Injury in Renal Transplantation. J. Am. Soc. Nephrol. 2011, 22, 1887–1896. [Google Scholar] [CrossRef][Green Version]
- Tian, Z.; Dong, B.; Blackwell, J.W.; Stewart, P.W.; Egan, T.M. Effect of a Vascular Endothelial Cadherin Antagonist in a Rat Lung Transplant Model. Ann. Thorac. Surg. 2013, 95, 1028–1033. [Google Scholar] [CrossRef]
- Liu, A.; Fang, H.; Yang, Y.; Sun, J.; Fan, H.; Liu, S.; Dirsch, O.; Dahmen, U. The Fibrin-Derived Peptide Bβ15-42 Attenuates Liver Damage in a Rat Model of Liver Ischemia/Reperfusion Injury. Shock 2013, 39, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, D.; Schneeberger, S.; Friedl, P.; Zacharowski, K.; Wick, N.; Boesch, F.; Margreiter, R.; Laufer, G.; Petzelbauer, P.; Semsroth, S. The Fibrin-Derived Peptide Bβ15-42 Significantly Attenuates Ischemia-Reperfusion Injury in a Cardiac Transplant Model. Transplantation 2010, 89, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Petzelbauer, P.; Zacharowski, P.A.; Miyazaki, Y.; Friedl, P.; Wickenhauser, G.; Castellino, F.J.; Gröger, M.; Wolff, K.; Zacharowski, K. The Fibrin-Derived Peptide Bβ15–42 Protects the Myocardium against Ischemia-Reperfusion Injury. Nat. Med. 2005, 11, 298–304. [Google Scholar] [CrossRef]
- Zacharowski, K.; Zacharowski, P.A.; Friedl, P.; Mastan, P.; Koch, A.; Boehm, O.; Rother, R.P.; Reingruber, S.; Henning, R.; Emeis, J.J.; et al. The Effects of the Fibrin-Derived Peptide Bβ15-42 in Acute and Chronic Rodent Models of Myocardial Ischemia-Reperfusion. Shock 2007, 27, 631–637. [Google Scholar] [CrossRef]
- Roesner, J.P.; Petzelbauer, P.; Koch, A.; Mersmann, J.; Zacharowski, P.A.; Boehm, O.; Reingruber, S.; Pasteiner, W.; Mascher, D.; Wolzt, M.; et al. The Fibrin-Derived Peptide Bβ15–42 Is Cardioprotective in a Pig Model of Myocardial Ischemia-Reperfusion Injury. Crit. Care Med. 2007, 35, 1730–1735. [Google Scholar] [CrossRef]
- Roesner, J.P.; Petzelbauer, P.; Koch, A.; Tran, N.; Iber, T.; Mutz, C.; Vollmar, B.; Nöldge-Schomburg, G.E.F.; Zacharowski, K. A Double Blind, Single Centre, Sub-Chronic Reperfusion Trial Evaluating FX06 Following Haemorrhagic Shock in Pigs. Resuscitation 2009, 80, P264–P271. [Google Scholar] [CrossRef]
- Roesner, J.P.; Petzelbauer, P.; Koch, A.; Tran, N.; Iber, T.; Vagts, D.A.; Scheeren, T.W.; Vollmar, B.; Nöldge-Schomburg, G.E.; Zacharowski, K. Bβ15-42 (FX06) Reduces Pulmonary, Myocardial, Liver, and Small Intestine Damage in a Pig Model of Hemorrhagic Shock and Reperfusion. Crit. Care Med. 2009, 37, 598–605. [Google Scholar] [CrossRef]
- Bergt, S.; Gruenewald, M.; Beltschany, C.; Grub, A.; Neumann, T.; Albrecht, M.; Vollmar, B.; Zacharowski, K.; Roesner, J.P.; Meybohm, P. The Fibrin-Derived Peptide Bβ15–42 (FX06) Ameliorates Vascular Leakage and Improves Survival and Neurocognitive Recovery: Implications From Two Animal Models of Cardiopulmonary Resuscitation. Crit. Care Med. 2016, 44, e988–e995. [Google Scholar] [CrossRef]
- Urbschat, A.; Rupprecht, K.; Zacharowski, K.; Obermüller, N.; Scheller, B.; Holfeld, J.; Tepeköylü, C.; Hofmann, R.; Paulus, P. Combined Peri-Ischemic Administration of Bβ15–42 in Treating Ischemia Reperfusion Injury of the Mouse Kidney. Microvasc. Res. 2015, 101, 48–54. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Ajay, A.K.; Hoffmann, D.; Kim, T.M.; Ramirez, V.; Campanholle, G.; Bobadilla, N.A.; Waikar, S.S.; Vaidya, V.S. Fibrinogen β-Derived Bβ15-42 Peptide Protects against Kidney Ischemia/Reperfusion Injury. Blood 2011, 118, 1934–1942. [Google Scholar] [CrossRef]
- Urbschat, A.; Zacharowski, K.; Obermüller, N.; Rupprecht, K.; Penzkofer, D.; Jennewein, C.; Tran, N.; Scheller, B.; Dimmeler, S.; Paulus, P. The Small Fibrinopeptide Bβ15-42 as Renoprotective Agent Preserving the Endothelial and Vascular Integrity in Early Ischemia Reperfusion Injury in the Mouse Kidney. PLoS ONE 2014, 9, e84432. [Google Scholar] [CrossRef]
- Jennewein, C.; Mehring, M.; Tran, N.; Paulus, P.; Ockelmann, P.A.; Habeck, K.; Latsch, K.; Scheller, B.; Zacharowski, K.; Mutlak, H. The Fibrinopeptide Lβ15-42 Reduces Inflammation in Mice Subjected to Polymicrobial Sepsis. Shock 2012, 38, 275–280. [Google Scholar] [CrossRef]
- Tang, H.; Abouleila, Y.; Mashaghi, A. Lassa Hemorrhagic Shock Syndrome-on-a-Chip. Biotechnol. Bioeng. 2021, 118, 1405–1410. [Google Scholar] [CrossRef]
- Westover, J.B.; Hickerson, B.T.; van Wettere, A.J.; Hurst, B.L.; Kurz, J.P.; Dagley, A.; Wülfroth, P.; Komeno, T.; Furuta, Y.; Steiner, T.; et al. Vascular Leak and Hypercytokinemia Associated with Severe Fever with Thrombocytopenia Syndrome Virus Infection in Mice. Pathogens 2019, 8, 158. [Google Scholar] [CrossRef]
- Gröger, M.; Pasteiner, W.; Ignatyev, G.; Matt, U.; Knapp, S.; Atrasheuskaya, A.; Bukin, E.; Freidl, P.; Zinkl, D.; Hofer-Warbinek, R.; et al. Peptide Bβ15-42 Preserves Endothelial Barrier Function in Shock. PLoS ONE 2009, 4, e5391. [Google Scholar] [CrossRef]
- Yakovlev, S.; Cao, C.; Galisteo, R.; Zhang, L.; Strickland, D.K.; Medved, L. Fibrin-VLDL Receptor-Dependent Pathway Promotes Leukocyte Transmigration by Inhibiting Src Kinase Fyn and Is a Target for Fibrin Β15-42 Peptide. Thromb. Haemost. 2019, 119, 1816–1826. [Google Scholar] [CrossRef]
- Ludwig, N.; Rossaint, J. Fibrin Degradation Product Β15-42-New Insights in an Old Pathway. Thromb. Haemost. 2019, 119, 1719. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, H.; Park, B.W.; Noh, M.; Kim, Y.; Park, J.; Park, J.H.; Kim, J.J.; Sim, W.S.; Ban, K.; et al. CU06-1004 Enhances Vascular Integrity and Improves Cardiac Remodeling by Suppressing Edema and Inflammation in Myocardial Ischemia–Reperfusion Injury. Exp. Mol. Med. 2022, 54, 23–34. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.H.; Maharjan, S.; Park, J.A.; Choi, K.-S.; Park, H.; Jeong, Y.; Ahn, J.H.; Kim, I.H.; Lee, J.-C.; et al. Sac-1004, a Vascular Leakage Blocker, Reduces Cerebral Ischemia—Reperfusion Injury by Suppressing Blood–Brain Barrier Disruption and Inflammation. J. Neuroinflamm. 2017, 14, 122. [Google Scholar] [CrossRef]
- Kim, D.Y.; Zhang, H.; Park, S.; Kim, Y.; Bae, C.R.; Kim, Y.M.; Kwon, Y.G. CU06-1004 (Endothelial Dysfunction Blocker) Ameliorates Astrocyte End-Feet Swelling by Stabilizing Endothelial Cell Junctions in Cerebral Ischemia/Reperfusion Injury. J Mol. Med. 2020, 98, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Zhang, H.; Lee, S.; Park, S.; Noh, M.; Kim, Y.M.; Kwon, Y.G. CU06-1004 Alleviates Experimental Colitis by Modulating Colonic Vessel Dysfunction. Front. Pharmacol. 2020, 11, 571266. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Lee, S.; Agrawal, V.; Choi, H.J.; Maeng, Y.S.; Kim, K.; Kim, N.J.; Suh, Y.G.; Kwon, Y.G. Sac-0601 Prevents Retinal Vascular Leakage in a Mouse Model of Diabetic Retinopathy. Eur. J. Pharmacol. 2011, 657, 35–40. [Google Scholar] [CrossRef]
- Noh, M.; Kim, Y.; Zhang, H.; Kim, H.; Bae, C.R.; Lee, S.; Kwon, Y.G. Oral Administration of CU06-1004 Attenuates Vascular Permeability and Stabilizes Neovascularization in Retinal Vascular Diseases. Eur. J. Pharmacol. 2023, 939, 175427. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, J.H.; Park, D.J.; Zhang, H.; Noh, M.; Kim, Y.; Kim, Y.S.; Kim, H.; Kim, Y.M.; Ha, S.J.; et al. CU06-1004-Induced Vascular Normalization Improves Immunotherapy by Modulating Tumor Microenvironment via Cytotoxic T Cells. Front. Immunol. 2021, 11, 620111. [Google Scholar] [CrossRef]
- Maharjan, S.; Kim, K.; Agrawal, V.; Choi, H.J.; Kim, N.J.; Kim, Y.M.; Suh, Y.G.; Kwon, Y.G. Sac-1004, a Novel Vascular Leakage Blocker, Enhances Endothelial Barrier through the CAMP/Rac/Cortactin Pathway. Biochem. Biophys. Res. Commun. 2013, 435, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Galaup, A.; Gomez, E.; Souktani, R.; Durand, M.; Cazes, A.; Monnot, C.; Teillon, J.; Le Jan, S.; Bouleti, C.; Briois, G.; et al. Protection Against Myocardial Infarction and No-Reflow Through Preservation of Vascular Integrity by Angiopoietin-Like 4. Circulation 2012, 125, 140–149. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lin, J.Y.; Feng, Y.R.; Liu, D.S.; Zhao, X.Z.; Li, T.; Li, S.Y.; Sun, J.C.; Li, S.F.; Jia, W.Y.; et al. Recombinant Angiopoietin-like Protein 4 Attenuates Intestinal Barrier Structure and Function Injury after Ischemia/Reperfusion. World J. Gastroenterol. 2021, 27, 5404–5423. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Li, X.; Geng, Y.; Cui, H.; Jin, C.; Wang, P.; Li, Y.; Yang, Y. Tongxinluo Attenuates Reperfusion Injury in Diabetic Hearts by Angiopoietin-like 4-Mediated Protection of Endothelial Barrier Integrity via PPAR-α Pathway. PLoS ONE 2018, 13, e0198403. [Google Scholar] [CrossRef]
- Rübig, E.; Stypmann, J.; van Slyke, P.; Dumont, D.J.; Spieker, T.; Buscher, K.; Reuter, S.; Goerge, T.; Pavenstädt, H.; Kümpers, P. The Synthetic Tie2 Agonist Peptide Vasculotide Protects Renal Vascular Barrier Function in Experimental Acute Kidney Injury. Sci. Rep. 2016, 6, 22111. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, D.H.; Lee, A.S.; Lee, S.; Kang, K.P.; Lee, S.Y.; Jang, K.Y.; Sung, M.J.; Park, S.K.; Kim, W. Peritubular Capillary Preservation with COMP-Angiopoietin-1 Decreases Ischemia-Reperfusion-Induced Acute Kidney Injury. Am. J. Physiol. Renal. Physiol 2009, 297, F952-60. [Google Scholar] [CrossRef] [PubMed]
- Syrjälä, S.O.; Nykänen, A.I.; Tuuminen, R.; Raissadati, A.; Keränen, M.A.I.; Arnaudova, R.; Krebs, R.; Koh, G.Y.; Alitalo, K.; Lemström, K.B. Donor Heart Treatment with COMP-Ang1 Limits Ischemia-Reperfusion Injury and Rejection of Cardiac Allografts. Am. J. Transplant. 2015, 15, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Higashikawa, K.; Yasui, H.; Kuge, Y.; Ohno, Y.; Kihara, A.; Midori, Y.A.; Houkin, K.; Kawabori, M. FTY720 Protects Against Ischemia–Reperfusion Injury by Preventing the Redistribution of Tight Junction Proteins and Decreases Inflammation in the Subacute Phase in an Experimental Stroke Model. Transl. Stroke Res. 2020, 11, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Poirier, B.; Briand, V.; Kadereit, D.; Schäfer, M.; Wohlfart, P.; Philippo, M.-C.; Caillaud, D.; Gouraud, L.; Grailhe, P.; Bidouard, J.-P.; et al. A G Protein–Biased S1P 1 Agonist, SAR247799, Protects Endothelial Cells without Affecting Lymphocyte Numbers. Sci. Signal. 2020, 13, eaax8050. [Google Scholar] [CrossRef]
- Li, X.; Gui, Z.; Liu, H.; Qian, S.; Jia, Y.; Luo, X. Remifentanil Pretreatment Ameliorates H/R-Induced Cardiac Microvascular Endothelial Cell Dysfunction by Regulating the PI3K/Akt/HIF-1α Signaling Pathway. Bioengineered 2021, 12, 7872–7881. [Google Scholar] [CrossRef]
- Gao, X.-M.; Su, Y.; Moore, S.; Han, L.-P.; Kiriazis, H.; Lu, Q.; Zhao, W.-B.; Ruze, A.; Fang, B.-B.; Duan, M.-J.; et al. Relaxin Mitigates Microvascular Damage and Inflammation Following Cardiac Ischemia-Reperfusion. Basic Res. Cardiol. 2019, 114, 30. [Google Scholar] [CrossRef]
- Devarakonda, T.; Mauro, A.G.; Guzman, G.; Hovsepian, S.; Cain, C.; Das, A.; Praveen, P.; Hossain, M.A.; Salloum, F.N. B7-33, a Functionally Selective Relaxin Receptor 1 Agonist, Attenuates Myocardial Infarction–Related Adverse Cardiac Remodeling in Mice. J. Am. Heart Assoc. 2020, 9, e015748. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Luiken, T.T.J.; Veltien, A.; Uthman, L.; Kuster, C.T.A.; Rodwell, L.; de Waard, G.A.; Kea-te Lindert, M.; Akiva, A.; Thijssen, D.H.J.; et al. Imatinib Attenuates Reperfusion Injury in a Rat Model of Acute Myocardial Infarction. Basic Res. Cardiol. 2023, 118, 2. [Google Scholar] [CrossRef]
- Mondal, N.K.; Behera, J.; Kelly, K.E.; George, A.K.; Tyagi, P.K.; Tyagi, N. Tetrahydrocurcumin Epigenetically Mitigates Mitochondrial Dysfunction in Brain Vasculature during Ischemic Stroke. Neurochem. Int. 2019, 122, 120–138. [Google Scholar] [CrossRef]
- Walsh, S.R.; Tang, T.Y.; Kullar, P.; Jenkins, D.P.; Dutka, D.P.; Gaunt, M.E. Ischaemic Preconditioning during Cardiac Surgery: Systematic Review and Meta-Analysis of Perioperative Outcomes in Randomised Clinical Trials. Eur. J. Cardio-Thorac. Surg. 2008, 34, 985–994. [Google Scholar] [CrossRef]
- Thielmann, M.; Kottenberg, E.; Kleinbongard, P.; Wendt, D.; Gedik, N.; Pasa, S.; Price, V.; Tsagakis, K.; Neuhäuser, M.; Peters, J.; et al. Cardioprotective and Prognostic Effects of Remote Ischaemic Preconditioning in Patients Undergoing Coronary Artery Bypass Surgery: A Single-Centre Randomised, Double-Blind, Controlled Trial. Lancet 2013, 382, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.-P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of Myocardial Injury by Ischemic Postconditioning during Reperfusion: Comparison with Ischemic Preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H579–H588. [Google Scholar] [CrossRef] [PubMed]
- Halkos, M.E.; Kerendi, F.; Corvera, J.S.; Wang, N.-P.; Kin, H.; Payne, C.S.; Sun, H.-Y.; Guyton, R.A.; Vinten-Johansen, J.; Zhao, Z.-Q. Myocardial Protection with Postconditioning Is Not Enhanced by Ischemic Preconditioning. Ann. Thorac. Surg. 2004, 78, 961–969. [Google Scholar] [CrossRef]
- Yang, X.M.; Proctor, J.B.; Cui, L.; Krieg, T.; Downey, J.M.; Cohen, M.V. Multiple, Brief Coronary Occlusions during Early Reperfusion Protect Rabbit Hearts by Targeting Cell Signaling Pathways. J. Am. Coll. Cardiol. 2004, 44, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Vinten-Johansen, J. Postconditioning: Reduction of Reperfusion-Induced Injury. Cardiovasc. Res. 2006, 70, 200–211. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic Conditioning and Targeting Reperfusion Injury: A 30 Year Voyage of Discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef]
- Xue, F.; Yang, X.; Zhang, B.; Zhao, C.; Song, J.; Jiang, T.; Jiang, W. Postconditioning the Human Heart in Percutaneous Coronary Intervention. Clin. Cardiol. 2010, 33, 439–444. [Google Scholar] [CrossRef]
- Staat, P.; Rioufol, G.; Piot, C.; Cottin, Y.; Cung, T.T.; L’Huillier, I.; Aupetit, J.-F.; Bonnefoy, E.; Finet, G.; André-Fouët, X.; et al. Postconditioning the Human Heart. Circulation 2005, 112, 2143–2148. [Google Scholar] [CrossRef]
- Mukherjee, P.; Jain, M. Effect of Ischemic Postconditioning during Primary Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction: A Single-Center Cross-Sectional Study. Ann. Card. Anaesth. 2019, 22, 347. [Google Scholar] [CrossRef]
- Hahn, J.-Y.; Song, Y.B.; Kim, E.K.; Yu, C.W.; Bae, J.-W.; Chung, W.-Y.; Choi, S.-H.; Choi, J.-H.; Bae, J.-H.; An, K.J.; et al. Ischemic Postconditioning During Primary Percutaneous Coronary Intervention. Circulation 2013, 128, 1889–1896. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Clemmensen, P.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamaki, K.; et al. Effect of Ischemic Postconditioning during Primary Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 490–497. [Google Scholar] [CrossRef]
- Thuny, F.; Lairez, O.; Roubille, F.; Mewton, N.; Rioufol, G.; Sportouch, C.; Sanchez, I.; Bergerot, C.; Thibault, H.; Cung, T.T.; et al. Post-Conditioning Reduces Infarct Size and Edema in Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Jensen, J.O.; Rommel, K.P.; De Waha-Thiele, S.; Fuernau, G.; Desch, S.; Thiele, H.; Eitel, I. Combined Intrahospital Remote Ischemic Perconditioning and Postconditioning Improves Clinical Outcome in St-Elevation Myocardial Infarction: Long-Term Results of the Lipsia Conditioning Trial. Circ. Res. 2019, 124, 1482–1491. [Google Scholar] [CrossRef]
- Heusch, G.; Rassaf, T. Time to Give Up on Cardioprotection?: A Critical Appraisal of Clinical Studies on Ischemic Pre-, Post-, and Remote Conditioning∗. Circ. Res. 2016, 119, 676–695. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N. Eng. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Böning, A.; Niemann, B.; et al. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N. Eng. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of Remote Ischaemic Conditioning on Clinical Outcomes in Patients with Acute Myocardial Infarction (CONDI-2/ERIC-PPCI): A Single-Blind Randomised Controlled Trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Ottani, F.; Latini, R.; Staszewsky, L.; Vecchia, L.L.; Locuratolo, N.; Sicuro, M.; Masson, S.; Barlera, S.; Milani, V.; Lombardi, M.; et al. Cyclosporine A in Reperfused Myocardial Infarction The Multicenter, Controlled, Open-Label CYCLE Trial. J. Am. Coll. Cardiol. 2016, 67, 1415–1427. [Google Scholar]
- Cung, T.-T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Eng. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- Atar, D.; Arheden, H.; Berdeaux, A.; Bonnet, J.L.; Carlsson, M.; Clemmensen, P.; Cuvier, V.; Danchin, N.; Dubois-Randé, J.L.; Engblom, H.; et al. Effect of Intravenous TRO40303 as an Adjunct to Primary Percutaneous Coronary Intervention for Acute ST-Elevation Myocardial Infarction: MITOCARE Study Results. Eur. Heart J. 2015, 36, 112–119. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Puma, J.A.; Barbagelata, N.A.; Dicarli, M.F.; Leesar, M.A.; Browne, K.F.; Eisenberg, P.R.; Bolli, R.; Casas, A.C.; Molina-Viamonte, V.; et al. Adenosine as an Adjunct to Thrombolytic Therapy for Acute Myocardial Infarction-Results of a Multicenter, Randomized, Placebo-Controlled Trial: The Acute Myocardial Infarction STudy of ADenosine (AMISTAD) Trial. J. Am. Coll. Cardiol. 1999, 34, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W. A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial of Adenosine as an Adjunct to Reperfusion in the Treatment of Acute Myocardial Infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Armstrong, P.W.; The APEX Investigators. Pexelizumab for Acute ST-Elevation Myocardial Infarction in Patients Undergoing Primary Percutaneous Coronary Intervention A Randomized Controlled Trial. JAMA 2007, 297, 43–51. [Google Scholar]
- Lincoff, A.M.; Roe, M.; Aylward, P.; Galla, J.; Rynkiewicz, A.; Guetta, V.; Zelizko, M.; Kleiman, N.; White, H.; McErlean, E.; et al. Inhibition of Delta-Protein Kinase C by Delcasertib as an Adjunct to Primary Percutaneous Coronary Intervention for Acute Anterior ST-Segment Elevation Myocardial Infarction: Results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014, 35, 2516–2523. [Google Scholar] [CrossRef]
- Bainey, K.R.; Armstrong, P.W. Ameliorating Reperfusion Injury in STEMI: Dead or Alive? Eur. Heart J. 2014, 35, 2504–2506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thiele, H.; Wöhrle, J.; Hambrecht, R.; Rittger, H.; Birkemeyer, R.; Lauer, B.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Wiemer, M.; et al. Intracoronary versus Intravenous Bolus Abciximab during Primary Percutaneous Coronary Intervention in Patients with Acute ST-Elevation Myocardial Infarction: A Randomised Trial. Lancet 2012, 379, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Belonje, A.M.S.; Zijlstra, F.; Hillege, H.L.; Anker, S.D.; Slart, R.H.J.A.; Tio, R.A.; van ’T Hof, A.; Jukema, J.W.; Peels, H.O.J.; et al. A Single Dose of Erythropoietin in ST-Elevation Myocardial Infarction. Eur. Heart J. 2010, 31, 2593–2600. [Google Scholar] [CrossRef]
- Ponikowski, P.; Jankowska, E.A. EPO’s Rescue Mission in Acute Myocardial Infarction: Still More Hopes than Evidence. Eur. Heart J. 2010, 31, 2577–2579. [Google Scholar] [CrossRef]
- Heusch, G. Critical Issues for the Translation of Cardioprotection. Circ. Res. 2017, 120, 1477–1486. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. Is Cardioprotection Salvageable? Circulation 2020, 141, 415–417. [Google Scholar] [CrossRef]
- Heusch, G.; Kleinbongard, P.; Rassaf, T. Cardioprotection beyond Infarct Size Reduction. Circ. Res. 2019, 122, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A. Current State of Clinical Translation of Cardioprotective Agents for Acute Myocardial Infarction. Circ. Res. 2013, 113, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion-from Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; Rodríguez-Sinovas, A.; Ruiz-Meana, M.; Inserte, J. Protection Against Myocardial Ischemia-Reperfusion Injury in Clinical Practice. Rev. Española Cardiol. 2014, 67, 394–404. [Google Scholar] [CrossRef]
- Lehr, H.-A.; Menger, M.D.; Granger, D.N. Ischemia-Reperfusion Injury: Enthusiasm in Laboratory Research but Dilemma in Clinical Trials? Circulation 1994, 90, 1580. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, M.T.; Laarman, G.J.; Simoons, M.L.; Duncker, D.J.G.M. Reperfusion Injury in Humans: A Review of Clinical Trials on Reperfusion Injury Inhibitory Strategies. Cardiovasc. Res. 2007, 74, 343–355. [Google Scholar] [CrossRef]
- Spath, N.B.; Mills, N.L.; Cruden, N.L. Novel Cardioprotective and Regenerative Therapies in Acute Myocardial Infarction: A Review of Recent and Ongoing Clinical Trials. Fut Cardiol. 2016, 12, 655–672. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Li, G. Preclinical Multi-Target Strategies for Myocardial Ischemia-Reperfusion Injury. Front. Cardiovasc. Med. 2022, 9, 967115. [Google Scholar] [CrossRef]
- Lejay, A.; Fang, F.; John, R.; Van, J.A.D.; Barr, M.; Thaveau, F.; Chakfe, N.; Geny, B.; Scholey, J.W. Ischemia Reperfusion Injury, Ischemic Conditioning and Diabetes Mellitus. J. Mol. Cell Cardiol. 2016, 91, 11–22. [Google Scholar] [CrossRef]
- Balakumar, P.; Singh, H.; Singh, M.; Anand-Srivastava, M.B. The Impairment of Preconditioning-Mediated Cardioprotection in Pathological Conditions. Pharmacol. Res. 2009, 60, 18–23. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Schulz, R.; Baxter, G.F. Interaction of Cardiovascular Risk Factors with Myocardial Ischemia/Reperfusion Injury, Preconditioning, and Postconditioning. Pharmacol. Rev. 2007, 59, 418–458. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Schulz, R.; Heusch, G. Loss of Cardioprotection with Ageing. Cardiovasc. Res. 2009, 83, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Querio, G.; Geddo, F.; Antoniotti, S.; Gallo, M.P.; Penna, C. Sex and Response to Cardioprotective Conditioning Maneuvers. Front. Physiol. 2021, 12, 667961. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Yellon, D.M. Cardioprotection: The Disconnect between Bench and Bedside. Circulation 2016, 134, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Engelman, R.M.; Otani, H.; Maulik, N.; Rousou, J.A.; Flack, J.E.; Deaton, D.W.; Das, D.K. Myocardial Protection by Preconditioning of Heart With Losartan, an Angiotensin II Type 1–Receptor Blocker. Circulation 2000, 102 (Suppl. S3), Iii-346–Iii-351. [Google Scholar] [CrossRef]
- Hu, J.; Lu, Y. Statin and Myocardial Ischemia-Reperfusion Injury. Int. J. Cardiol. 2016, 222, 988. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, L.A.; Maluenda, A.; McFadyen, J.D.; Searle, A.K.; Yu, E.; Haller, C.; Chaikof, E.L.; Peter, K.; Wang, X. Combined Antiplatelet/Anticoagulant Drug for Cardiac Ischemia/Reperfusion Injury. Circ. Res. 2020, 127, 1211–1213. [Google Scholar] [CrossRef]
- Wu, J.; Cai, W.; Du, R.; Li, H.; Wang, B.; Zhou, Y.; Shen, D.; Shen, H.; Lan, Y.; Chen, L.; et al. Sevoflurane Alleviates Myocardial Ischemia Reperfusion Injury by Inhibiting P2X7-NLRP3 Mediated Pyroptosis. Front. Mol. Biosci. 2021, 8, 768594. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Liao, J.; Zhang, S.; Liu, H.; Yang, C.; Dong, Q.; Zhao, N.; Huang, Z.; Guo, K.; et al. Propofol Induces Cardioprotection against Ischemia-Reperfusion Injury via Suppression of Transient Receptor Potential Vanilloid 4 Channel. Front. Pharmacol. 2019, 10, 1150. [Google Scholar] [CrossRef]
- Fordyce, C.B.; Gersh, B.J.; Stone, G.W.; Granger, C.B. Novel Therapeutics in Myocardial Infarction: Targeting Microvascular Dysfunction and Reperfusion Injury. Trends Pharmacol. Sci. 2015, 36, 605–616. [Google Scholar] [CrossRef]
- Zicola, E.; Arrigo, E.; Mancardi, D. H2S Pretreatment Is Promigratory and Decreases Ischemia/Reperfusion Injury in Human Microvascular Endothelial Cells. Oxid. Med. Cell Longev. 2021, 2021, 8886666. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.; Liles, W.C. Emerging Therapeutic Strategies to Prevent Infection-Related Microvascular Endothelial Activation and Dysfunction. Virulence 2013, 4, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. The Coronary Circulation as a Target of Cardioprotection. Circ. Res. 2016, 118, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; Van Royen, N.; Schulz, R.; Heusch, G. The Coronary Circulation in Acute Myocardial Ischaemia/Reperfusion Injury: A Target for Cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Atar, D.; Petzelbauer, P.; Schwitter, J.; Huber, K.; Rensing, B.; Kasprzak, J.D.; Butter, C.; Grip, L.; Hansen, P.R.; Süselbeck, T.; et al. Effect of Intravenous FX06 as an Adjunct to Primary Percutaneous Coronary Intervention for Acute ST-Segment Elevation Myocardial Infarction. Results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) Trial. J. Am. Coll. Cardiol. 2009, 53, 720–729. [Google Scholar] [CrossRef]
- Hallén, J.; Petzelbauer, P.; Schwitter, J.; Geudelin, B.; Buser, P.; Atar, D. Impact of Time to Therapy and Presence of Collaterals on the Efficacy of FX06 in Acute ST Elevation Myocardial Infarction: A Substudy of the F.I.R.E., the Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury Trial. EuroIntervention 2010, 5, 946–952. [Google Scholar] [CrossRef]
- Wolf, T.; Kann, G.; Becker, S.; Stephan, C.; Brodt, H.R.; de Leuw, P.; Grünewald, T.; Vogl, T.; Kempf, V.A.J.; Keppler, O.T.; et al. Severe Ebola Virus Disease with Vascular Leakage and Multiorgan Failure: Treatment of a Patient in Intensive Care. Lancet 2015, 385, 1428–1435. [Google Scholar] [CrossRef]
- Adam, E.H.; Schmid, B.; Sonntagbauer, M.; Kranke, P.; Zacharowski, K.; Meybohm, P. Fibrin-Derived Peptide Bβ15-42 (FX06) as Salvage Treatment in Critically Ill Patients with COVID-19-Associated Acute Respiratory Distress Syndrome. Crit. Care 2020, 24, 574. [Google Scholar] [CrossRef]
- Kloka, J.; Friedrichson, B.; Dauth, S.; Foldenauer, A.C.; Bulczak-Schadendorf, A.; Vehreschild, M.J.G.T.; Matos, F.M.; Riera-Mestre, A.; van Asselt, A.D.I.; de Robertis, E.; et al. Potential of FX06 to Prevent Disease Progression in Hospitalized Non-Intubated COVID-19 Patients-the Randomized, EU-Wide, Placebo-Controlled, Phase II Study Design of IXION. Trials 2022, 23, 688. [Google Scholar] [CrossRef]
- Bergougnan, L.; Armani, S.; Golor, G.; Tardat, A.; Vitse, O.; Hurbin, F.; Scemama, M.; Poitiers, F.; Radzik, D.; Gaudin, C.; et al. First-in-Human Study of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Single and Multiple Oral Doses of SAR247799, a Selective G-Protein-Biased Sphingosine-1 Phosphate Receptor-1 Agonist for Endothelial Protection. Br. J. Clin. Pharmacol. 2021, 87, 598–611. [Google Scholar] [CrossRef]
- Bell, R.M.; Bøtker, H.E.; Carr, R.D.; Davidson, S.M.; Downey, J.M.; Dutka, D.P.; Heusch, G.; Ibanez, B.; Macallister, R.; Stoppe, C.; et al. 9th Hatter Biannual Meeting: Position Document on Ischaemia/Reperfusion Injury, Conditioning and the Ten Commandments of Cardioprotection. Basic Res. Cardiol. 2016, 111, 41. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Bellis, A.; di Gioia, G.; Mauro, C.; Mancusi, C.; Barbato, E.; Izzo, R.; Trimarco, B.; Morisco, C. Reducing Cardiac Injury during St-Elevation Myocardial Infarction: A Reasoned Approach to a Multitarget Therapeutic Strategy. J. Clin. Med. 2021, 10, 2968. [Google Scholar] [CrossRef] [PubMed]

| Study Name | Type of Intervention | Number of Patients | Primary Endpoint And Outcome | Reference |
|---|---|---|---|---|
| ERICCA | Remote ischemic preconditioning in coronary artery bypass graft surgery | 1612 | MACCE Negative | [135] |
| RIPHeart | Remote ischemic preconditioning for heart surgery | 1403 | MACCE Negative | [136] |
| CONDI 2- ERIC-PPCI | Ischemic conditioning in STEMI patients undergoing PCI | 5401 | Combined cardiac death of HF hospitalization 12 months Negative Combined cardiac death of HF hospitalization 30 days Negative | [137] |
| DANAMI-3- iPOST | Ischemic Postconditioning During PCI for Patients With STEMI | 1234 | MACE Negative | [131] |
| CYCLE | Intravenous Bolus of Cyclosporin A before PCI in patients with STEMI | 410 | 6-month composite (all-cause mortality, HF, cardiogenic shock) Negative, (more events in CsA group) | [138] |
| CIRCUS | Intravenous Bolus of Cyclosporin A before PCI in patients with STEMI | 970 | Composite of death or rehospitalization or worsening of HF, adverse LV remodeling at 1 year Negative | [139] |
| MITOCARE | Intravenous bolus of MPTP blocker TRO40303 before PCI in patients with STEMI | 168 | Infarct size (CK, TnI) Negative (CK AUC0–72 h) TnI AUC0–72 h) | [140] |
| AMISTAD | Adenosine infusion during and after PCI for patients with STEMI | 236 | Infarct size by SPECT imaging at 5–12 days IS reduction by 31% | [141] |
| AMISTAD-II | Adenosine infusion during and after PCI for patients with STEMI | 2118 | MACE at 6 months Negative | [142] |
| APEX-AMI | Bolus injection of anti-C5 antibody pexelizumab prior to PCI in patients with STEMI | 5745 | 30-day mortality Negative MACE at 90 days Negative | [143] |
| PROTECTION-AMI | Infusion of delta-PKC inhibitor delcasertib during and after PCI in patients with STEMI | 1010 | Infarct size (CK-MB AUC) Negative (no difference in all dose groups) No difference in composite of death, HF and serious ventricular arrhythmias at 3 months | [144,145] |
| AIDA STEMI | Infusion of GpIIb/IIIa MAb abciximab in patients with STEMI undergoing PCI | 2065 | MACE at 90 days Negative | [146] |
| HEBE III | Single dose of erythropoietin within 3 hrs after PCI in patients with STEMI | 529 | Mean LVEF after 6 weeks Negative | [147,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloka, J.A.; Friedrichson, B.; Wülfroth, P.; Henning, R.; Zacharowski, K. Microvascular Leakage as Therapeutic Target for Ischemia and Reperfusion Injury. Cells 2023, 12, 1345. https://doi.org/10.3390/cells12101345
Kloka JA, Friedrichson B, Wülfroth P, Henning R, Zacharowski K. Microvascular Leakage as Therapeutic Target for Ischemia and Reperfusion Injury. Cells. 2023; 12(10):1345. https://doi.org/10.3390/cells12101345
Chicago/Turabian StyleKloka, Jan Andreas, Benjamin Friedrichson, Petra Wülfroth, Rainer Henning, and Kai Zacharowski. 2023. "Microvascular Leakage as Therapeutic Target for Ischemia and Reperfusion Injury" Cells 12, no. 10: 1345. https://doi.org/10.3390/cells12101345
APA StyleKloka, J. A., Friedrichson, B., Wülfroth, P., Henning, R., & Zacharowski, K. (2023). Microvascular Leakage as Therapeutic Target for Ischemia and Reperfusion Injury. Cells, 12(10), 1345. https://doi.org/10.3390/cells12101345





