Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Process
3. Results
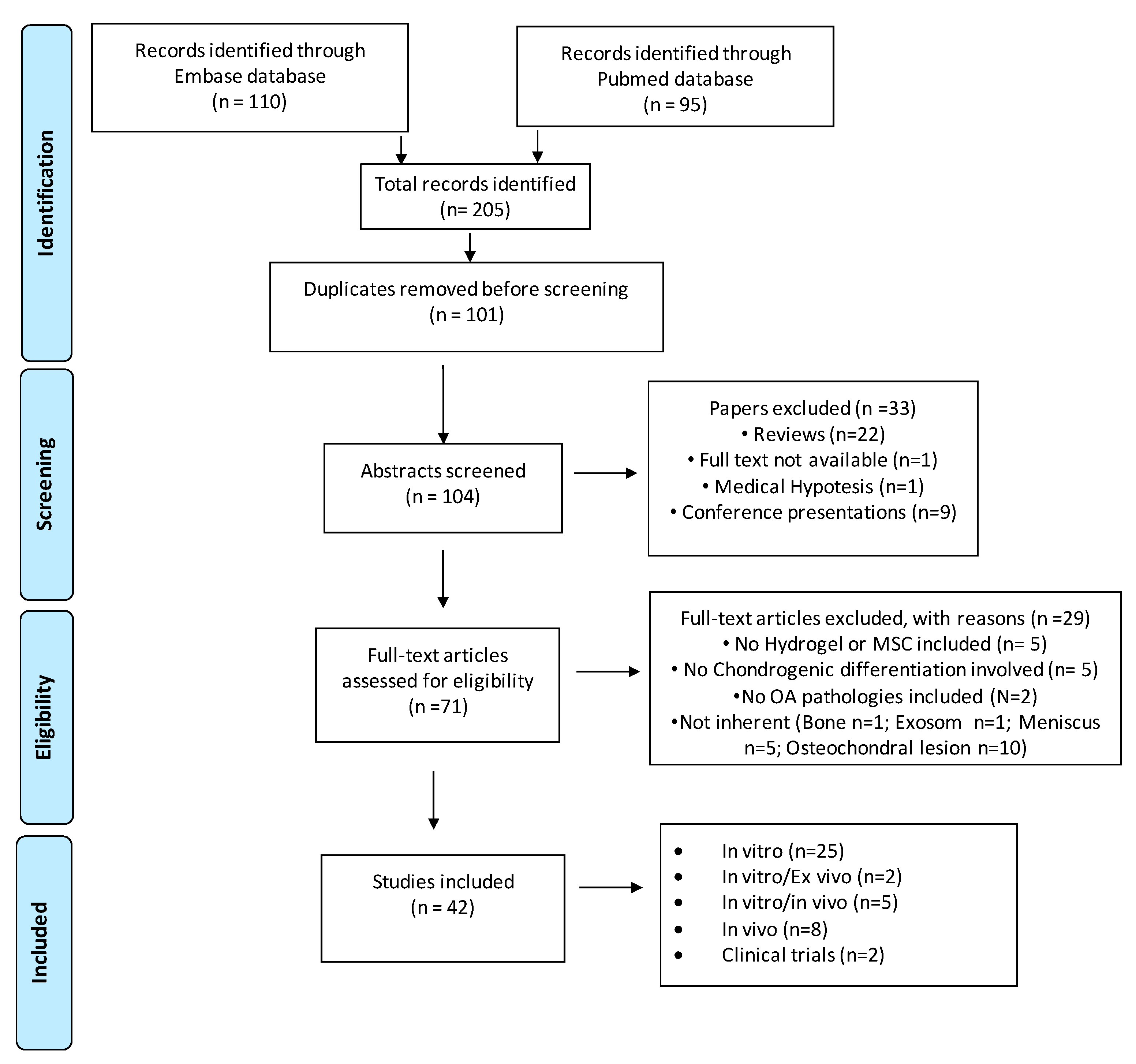
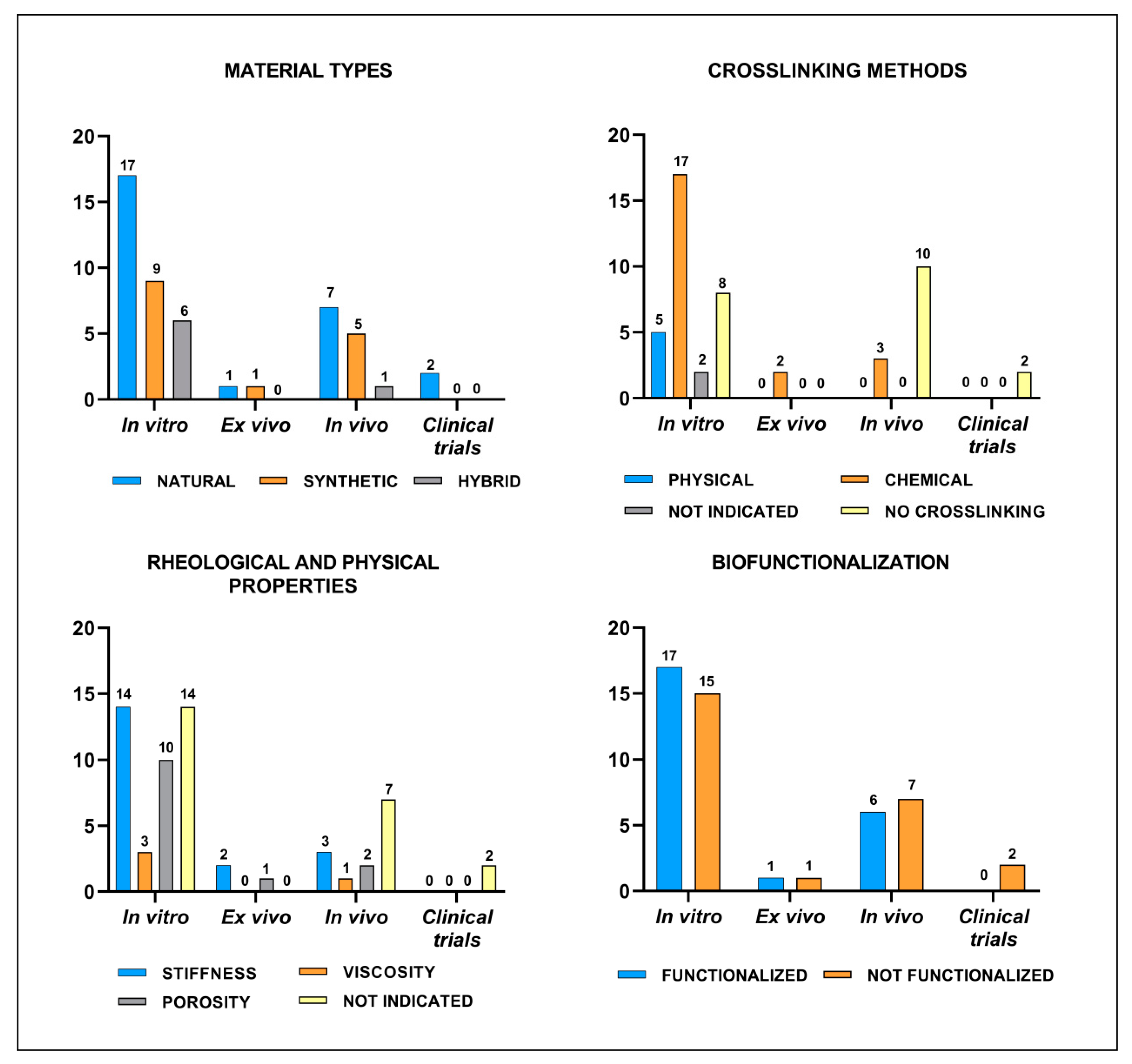
3.1. Literature Search Strategy Results
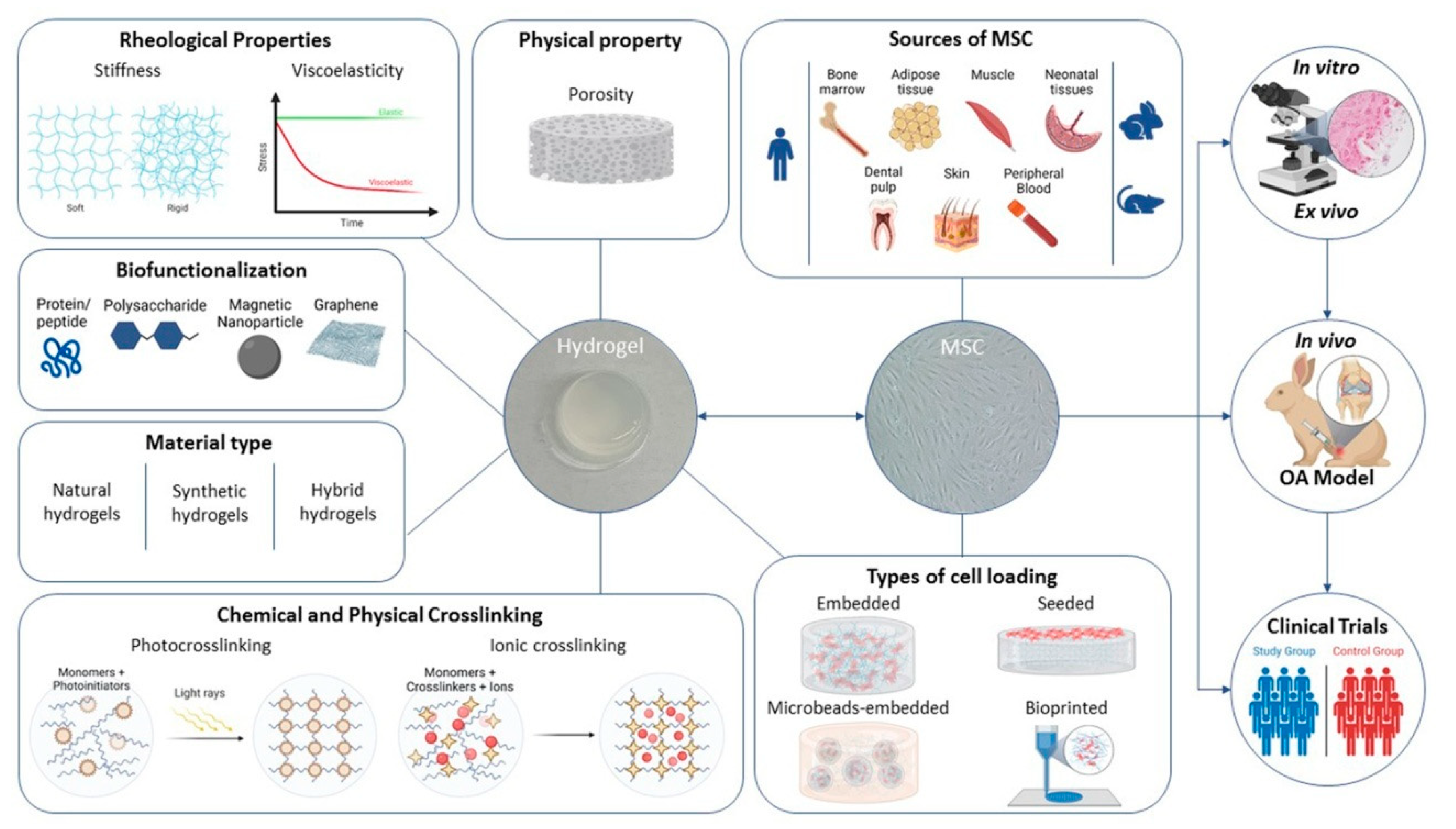
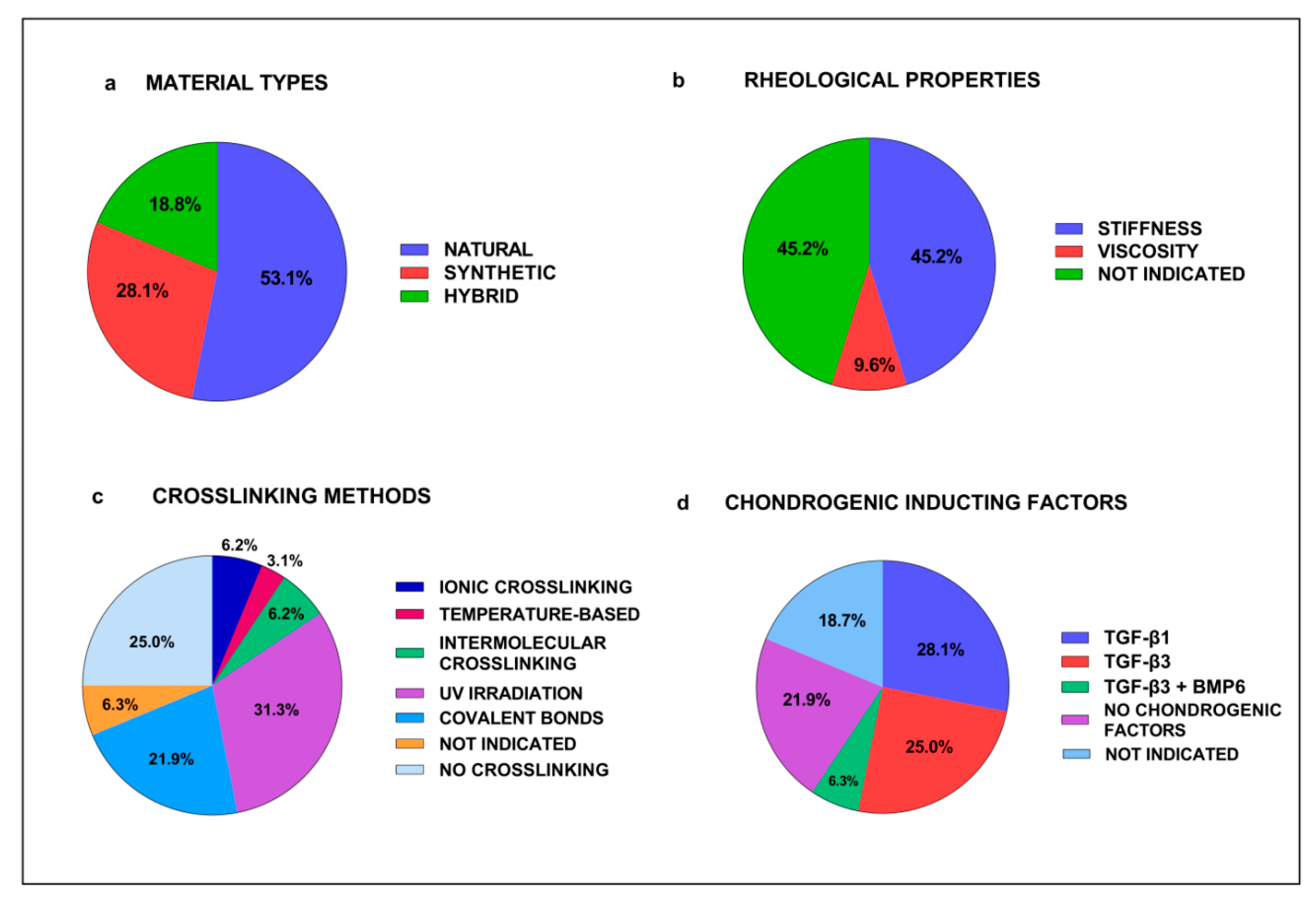
3.2. In Vitro Studies
3.3. Ex Vivo Studies
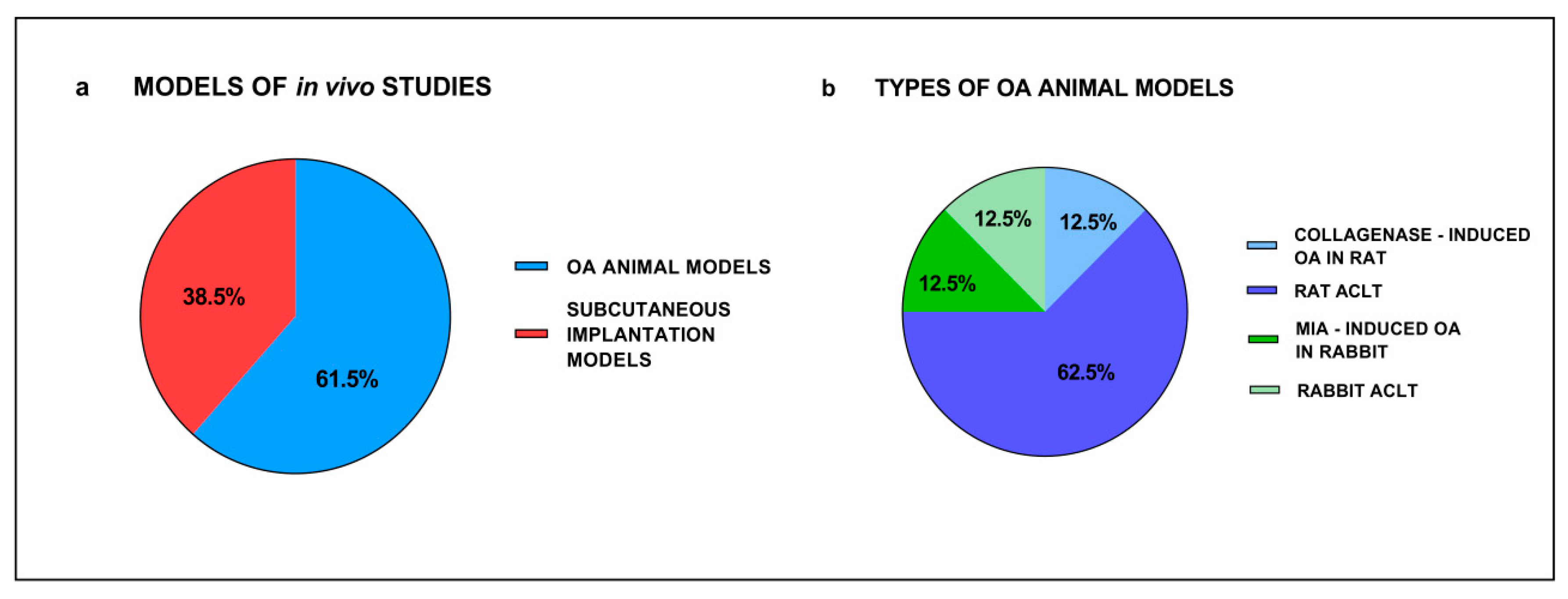
3.4. In Vivo Studies
3.5. Clinical Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| AC | articular cartilage |
| ACAN | aggrecan |
| ACLT | anterior cruciate ligament transection |
| AdBMP2 | adenoviral vector bone morphogenetic protein 2 |
| AdTGFβ1 | adenoviral vector Transforming Growth Factor Beta 1 |
| AdSOX9 | adenoviral vector SRY-Box transcription factor 9 |
| ASCs | adipose-derived stromal cells |
| BMP6 | bone morphogenetic protein 6 |
| BMSCs | bone marrow stromal cells |
| CAP | cold atmospheric plasma |
| COL2 | collagen type 2 |
| COL1 | collagen type 1 |
| COL10 | collagen type 10 |
| COMP | cartilage oligomeric matrix protein |
| CS | chondroitin sulfate |
| Dex-TA | dextran-tyramine |
| ECM | extracellular matrix |
| GAG | glycosaminoglycans |
| GelMA | methacrylated gelatin |
| HA | hyaluronic acid |
| HA-PBA | borate ester bond-based hyaluronic acid |
| HAA | Aldehyde-modified hyluronan |
| HIF1α | Hypoxia Inducible Factor 1 Subunit Alpha |
| hPL | human platelet lysate |
| HTO | high tibial osteotomy |
| hUCB | human umbilical cord blood |
| ICRS | International Cartilage Regeneration Society |
| IGF1 | Insulin-Like Growth Factor 1 |
| IKDC | International Knee Documentation Committee Score |
| IL1β | interleukin 1 beta |
| IL6 | interleukin 6 |
| iPS | induced pluripotent stem cells |
| KSS | Knee Society Score |
| Linc-Ror | long intergenic non-coding RNA regulator of reprogramming |
| MeHA | methacrylate hyaluronic acid |
| MeG | methacrylated gelatin |
| MIA | monosodium iodoacetate |
| MMP13 | matrix metalloproteinase 13 |
| MMx | medial menisectomy |
| MRI | magnetic resonance imaging |
| MSCs | mesenchymal stromal cells |
| N.I. | not indicated |
| NP | nanoparticles |
| OA | osteoarthritis |
| P | porosity |
| PEG | polyethylene glycol |
| PEG/PLGA | polyethylene glycol-poly lactic acid-co-glycolic acid |
| PEGDA | polyethylene glycol diacrylate |
| PEMF | pulsed electromagnetic field |
| PFS | peptide sequence PFSSTKT |
| PG/GC | polyglucosamine/glucosamine carbonate |
| PHA | Poloxamer 407 crosslinking hyaluronic acid |
| PVAH | hydrazide-modified polyvinyl alcohol |
| RAD | GTP-binding protein RAD |
| RGD | arginine-glycine-aspartate |
| S | stiffness |
| SAP | self-assembled peptide |
| SEM | scanning electron microscope |
| SMeHA | sulfated methacrylate hyaluronic acid |
| SOX9 | SRY-box transcription factor 9 |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| TGFβ3 | Transforming Growth Factor Beta 3 |
| ThHA | thiolated hyaluronic acid |
| ThHA-Col | thiolated hyaluronic acid hydrogel functionalized with collagen type 1 |
| TNFα | tumor necrosis factor alpha |
| UV | ultraviolet |
| V | viscosity |
| VAS | visual analogue scale |
| WOMAC | Western Ontario and McMaster University Osteoarthritis Index |
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Kisand, K.; Tamm, A.E.; Lintrop, M.; Tamm, A.O. New insights into the natural course of knee osteoarthritis: Early regulation of cytokines and growth factors, with emphasis on sex-dependent angiogenesis and tissue remodeling. A pilot study. Osteoarthr. Cartil. 2018, 26, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Trucco, D.; Vannozzi, L.; Teblum, E.; Telkhozhayeva, M.; Nessim, G.D.; Affatato, S.; Al-Haddad, H.; Lisignoli, G.; Ricotti, L. Graphene Oxide-Doped Gellan Gum-PEGDA Bilayered Hydrogel Mimicking the Mechanical and Lubrication Properties of Articular Cartilage. Adv. Healthc. Mater. 2021, 10, e2001434. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018, 40, 74–80. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Cattini, L.; Rojewski, M.; Schrezenmeier, H.; Addimanda, O.; Meliconi, R.; Lisignoli, G. Osteoarthritic Milieu Affects Adipose-Derived Mesenchymal Stromal Cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Wagenbrenner, M.; Mayer-Wagner, S.; Rudert, M.; Holzapfel, B.M.; Weissenberger, M. Combinations of Hydrogels and Mesenchymal Stromal Cells (MSCs) for Cartilage Tissue Engineering-A Review of the Literature. Gels 2021, 7, 217. [Google Scholar] [CrossRef]
- Desando, G.; Cavallo, C.; Sartoni, F.; Martini, L.; Parrilli, A.; Veronesi, F.; Fini, M.; Giardino, R.; Facchini, A.; Grigolo, B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. Ther. 2013, 15, R22. [Google Scholar] [CrossRef]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, Y.; Li, D.; Zhang, L.; Wang, Q.; Xiao, Y.; Zhang, X. Influence of hydrogel network microstructures on mesenchymal stem cell chondrogenesis in vitro and in vivo. Acta Biomater. 2019, 91, 159–172. [Google Scholar] [CrossRef]
- Nesic, D.; Whiteside, R.; Brittberg, M.; Wendt, D.; Martin, I.; Mainil-Varlet, P. Cartilage tissue engineering for degenerative joint disease. Adv. Drug Deliv. Rev. 2006, 58, 300–322. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.T.; Li, F.; Shrestha, S.; Tuan, R.S.; Thissen, H.; Forsythe, J.S.; Frith, J.E. Cell-laden injectable microgels: Current status and future prospects for cartilage regeneration. Biomaterials 2021, 279, 121214. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10117–10122. [Google Scholar] [CrossRef]
- Hafezi, M.; Nouri Khorasani, S.; Zare, M.; Esmaeely Neisiany, R.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. [Google Scholar] [CrossRef]
- Loebel, C.; Mauck, R.L.; Burdick, J.A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 2019, 18, 883–891. [Google Scholar] [CrossRef]
- Lee, H.P.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Sartore, L.; Manferdini, C.; Saleh, Y.; Dey, K.; Gabusi, E.; Ramorino, G.; Zini, N.; Almici, C.; Re, F.; Russo, D.; et al. Polysaccharides on gelatin-based hydrogels differently affect chondrogenic differentiation of human mesenchymal stromal cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112175. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef]
- Shen, H.; Lin, H.; Sun, A.X.; Song, S.; Zhang, Z.; Dai, J.; Tuan, R.S. Chondroinductive factor-free chondrogenic differentiation of human mesenchymal stem cells in graphene oxide-incorporated hydrogels. J. Mater. Chem. B 2018, 6, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Mann, Z.; Ahlawat, S.; Mohanty, S. Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Eur. J. Pharmacol. 2022, 918, 174657. [Google Scholar] [CrossRef] [PubMed]
- Cafarelli, A.; Marino, A.; Vannozzi, L.; Puigmartí-Luis, J.; Pané, S.; Ciofani, G.; Ricotti, L. Piezoelectric Nanomaterials Activated by Ultrasound: The Pathway from Discovery to Future Clinical Adoption. ACS Nano 2021, 15, 11066–11086. [Google Scholar] [CrossRef] [PubMed]
- Trucco, D.; Riacci, L.; Vannozzi, L.; Manferdini, C.; Arrico, L.; Gabusi, E.; Lisignoli, G.; Ricotti, L. Primers for the Adhesion of Gellan Gum-Based Hydrogels to the Cartilage: A Comparative Study. Macromol. Biosci. 2022, 22, e2200096. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of mesenchymal stem cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Deng, Z.; Jin, J.; Wang, S.; Qi, F.; Chen, X.; Liu, C.; Li, Y.; Ma, Y.; Lyu, F.; Zheng, Q. Narrative review of the choices of stem cell sources and hydrogels for cartilage tissue engineering. Ann. Transl. Med. 2020, 8, 1598. [Google Scholar] [CrossRef]
- Lai, J.H.; Rogan, H.; Kajiyama, G.; Goodman, S.B.; Smith, R.L.; Maloney, W.; Yang, F. Interaction between osteoarthritic chondrocytes and adipose-derived stem cells is dependent on cell distribution in three-dimension and transforming growth factor-β3 induction. Tissue Eng. Part A 2015, 21, 992–1002. [Google Scholar] [CrossRef]
- Lu, J.; Shen, X.; Sun, X.; Yin, H.; Yang, S.; Lu, C.; Wang, Y.; Liu, Y.; Huang, Y.; Yang, Z.; et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics 2018, 8, 5039–5058. [Google Scholar] [CrossRef]
- Lima, V.P.; Tobin, G.C.; de Jesus Pereira, M.R.; Silveira, M.D.; Witz, M.I.; Nardi, N.B. Chondrogenic effect of liquid and gelled platelet lysate on canine adipose-derived mesenchymal stromal cells. Res. Vet. Sci. 2019, 124, 393–398. [Google Scholar] [CrossRef]
- Tiruvannamalai Annamalai, R.; Mertz, D.R.; Daley, E.L.; Stegemann, J.P. Collagen Type II enhances chondrogenic differentiation in agarose-based modular microtissues. Cytotherapy 2016, 18, 263–277. [Google Scholar] [CrossRef]
- Weißenberger, M.; Weißenberger, M.H.; Wagenbrenner, M.; Heinz, T.; Reboredo, J.; Holzapfel, B.M.; Rudert, M.; Groll, J.; Evans, C.H.; Steinert, A.F. Different types of cartilage neotissue fabricated from collagen hydrogels and mesenchymal stromal cells via SOX9, TGFB1 or BMP2 gene transfer. PLoS ONE 2020, 15, e0237479. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Trucco, D.; Saleh, Y.; Gabusi, E.; Dolzani, P.; Lenzi, E.; Vannozzi, L.; Ricotti, L.; Lisignoli, G. RGD-Functionalized Hydrogel Supports the Chondrogenic Commitment of Adipose Mesenchymal Stromal Cells. Gels 2022, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Lin, J.; Liu, Q. Dual Delivery of TGF-β3 and Ghrelin in Microsphere/Hydrogel Systems for Cartilage Regeneration. Molecules 2021, 26, 5732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Valot, L.; Maumus, M.; Brunel, L.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Gels 2021, 7, 73. [Google Scholar] [CrossRef]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Kim, S.; Jung, Y.C.; Wang, Y.; Kang, B.J.; Kim, K. Dual delivery of stem cells and insulin-like growth factor-1 in coacervate-embedded composite hydrogels for enhanced cartilage regeneration in osteochondral defects. J. Control. Release Off. J. Control. Release Soc. 2020, 327, 284–295. [Google Scholar] [CrossRef]
- He, C.; Clark, K.L.; Tan, J.; Zhou, H.; Tuan, R.S.; Lin, H.; Wu, S.; Alexander, P.G. Modeling early changes associated with cartilage trauma using human-cell-laden hydrogel cartilage models. Stem Cell Res. Ther. 2022, 13, 400. [Google Scholar] [CrossRef]
- Behan, K.; Dufour, A.; Garcia, O.; Kelly, D. Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering. Biomolecules 2022, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, C.E.; Walimbe, T.; Panitch, A.; Liu, J.C. Incorporation of a Collagen-Binding Chondroitin Sulfate Molecule to a Collagen Type I and II Blend Hydrogel for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Sivasubramaniyan, K.; Vainieri, M.L.; Oieni, J.; Kops, N.; Yayon, A.; van Osch, G. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J. Control. Release Off. J. Control. Release Soc. 2019, 309, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yan, D.; Zhou, X.; Cui, H.; Esworthy, T.; Hann, S.Y.; Keidar, M.; Zhang, L.G. Integrating cold atmospheric plasma with 3D printed bioactive nanocomposite scaffold for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110844. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, F.; Bingham, C.O., 3rd; Elisseeff, J.H. Matrix metalloproteinases and inhibitors in cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2012, 6, 144–154. [Google Scholar] [CrossRef]
- Castro, N.J.; Patel, R.; Zhang, L.G. Design of a Novel 3D Printed Bioactive Nanocomposite Scaffold for Improved Osteochondral Regeneration. Cell. Mol. Bioeng. 2015, 8, 416–432. [Google Scholar] [CrossRef]
- Zheng, D.; Dan, Y.; Yang, S.H.; Liu, G.H.; Shao, Z.W.; Yang, C.; Xiao, B.J.; Liu, X.; Wu, S.; Zhang, T.; et al. Controlled chondrogenesis from adipose-derived stem cells by recombinant transforming growth factor-β3 fusion protein in peptide scaffolds. Acta Biomater. 2015, 11, 191–203. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, S.; Du, Y.; Zannettino, A.C.W.; Zhang, H. Fabrication of a Cartilage Patch by Fusing Hydrogel-Derived Cell Aggregates onto Electrospun Film. Tissue Eng. Part A 2020, 26, 863–871. [Google Scholar] [CrossRef]
- Yao, H.; Xue, J.; Wang, Q.; Xie, R.; Li, W.; Liu, S.; Cai, J.; Qin, D.; Wang, D.A.; Ren, L. Glucosamine-modified polyethylene glycol hydrogel-mediated chondrogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 661–670. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Leijten, J.C.; Wennink, J.W.; Chatterjea, A.G.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. The effect of platelet lysate supplementation of a dextran-based hydrogel on cartilage formation. Biomaterials 2012, 33, 3651–3661. [Google Scholar] [CrossRef]
- Yan, X.; Yang, B.; Chen, Y.; Song, Y.; Ye, J.; Pan, Y.; Zhou, B.; Wang, Y.; Mao, F.; Dong, Y.; et al. Anti-Friction MSCs Delivery System Improves the Therapy for Severe Osteoarthritis. Adv. Mater. 2021, 33, e2104758. [Google Scholar] [CrossRef] [PubMed]
- Aulin, C.; Bergman, K.; Jensen-Waern, M.; Hedenqvist, P.; Hilborn, J.; Engstrand, T. In situ cross-linkable hyaluronan hydrogel enhances chondrogenesis. J. Tissue Eng. Regen. Med. 2011, 5, e188–e196. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Y.; Huang, Z.; Zhao, P.; Liang, Q.; Liu, Y.; Duan, L.; Liu, W.; Zhu, F.; Bian, L.; et al. Magnetic Enhancement of Chondrogenic Differentiation of Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, D.J.; Frith, J.E.; Frith, T.J.; Ovchinnikov, D.A.; Cooper-White, J.J.; Wolvetang, E.J. Derivation of mesenchymal stromal cells from canine induced pluripotent stem cells by inhibition of the TGFβ/activin signaling pathway. Stem Cells Dev. 2014, 23, 3021–3033. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fang, F.; Kong, Y.; Greer, S.E.; Kuss, M.; Liu, B.; Xue, W.; Jiang, X.; Lovell, P.; Mohs, A.M.; et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication 2021, 14, 014107. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, L.; Cai, X.; Li, Z.; Fan, Y.; Yang, F. Icariin-Loaded Hydrogel Regulates Bone Marrow Mesenchymal Stem Cell Chondrogenic Differentiation and Promotes Cartilage Repair in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 755260. [Google Scholar] [CrossRef] [PubMed]
- Pipino, G.; Risitano, S.; Alviano, F.; Wu, E.J.; Bonsi, L.; Vaccarisi, D.C.; Indelli, P.F. Microfractures and hydrogel scaffolds in the treatment of osteochondral knee defects: A clinical and histological evaluation. J. Clin. Orthop. Trauma 2019, 10, 67–75. [Google Scholar] [CrossRef]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Escobar Ivirico, J.L.; Kan, H.M.; Shah, S.; Otsuka, T.; Bordett, R.; Barajaa, M.; Nagiah, N.; Pandey, R.; Nair, L.S.; et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc. Natl. Acad. Sci. USA 2022, 119, e2120968119. [Google Scholar] [CrossRef]
- Yu, W.; Hu, B.; Boakye-Yiadom, K.O.; Ho, W.; Chen, Q.; Xu, X.; Zhang, X.Q. Injectable hydrogel mediated delivery of gene-engineered adipose-derived stem cells for enhanced osteoarthritis treatment. Biomater. Sci. 2021, 9, 7603–7616. [Google Scholar] [CrossRef]
- Xing, D.; Liu, W.; Wang, B.; Li, J.J.; Zhao, Y.; Li, H.; Liu, A.; Du, Y.; Lin, J. Intra-articular Injection of Cell-laden 3D Microcryogels Empower Low-dose Cell Therapy for Osteoarthritis in a Rat Model. Cell Transplant. 2020, 29, 963689720932142. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, Z.M.; Li, Y.C.; Wang, H.X.; Lo, J.H.T.; Zhang, X.T.; Li, G. Linc-ROR promotes mesenchymal stem cells chondrogenesis and cartilage formation via regulating SOX9 expression. Osteoarthr. Cartil. 2021, 29, 568–578. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Y.; Chi, Y.; Liu, D.; Yang, S.; Han, Z.; Li, Z. Two-step generation of mesenchymal stem/stromal cells from human pluripotent stem cells with reinforced efficacy upon osteoarthritis rabbits by HA hydrogel. Cell Biosci. 2021, 11, 6. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, T.H.; Kang, D.L.; Baek, S.Y.; Lee, Y.; Koh, Y.G.; Kim, Y.I. Chondrogenic differentiation of human ASCs by stiffness control in 3D fibrin hydrogel. Biochem. Biophys. Res. Commun. 2020, 522, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.E.; Kim, S.H.; Kim, S.J.; Jeon, S.J.; Kim, S.H.; Jung, Y. Therapeutic effects of neuropeptide substance P coupled with self-assembled peptide nanofibers on the progression of osteoarthritis in a rat model. Biomaterials 2016, 74, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, S.M.; Kim, S.H.; Tatman, P.; Gee, A.O.; Kim, D.H.; Lee, K.E.; Jung, Y.; Kim, S.J. Effect of self-assembled peptide-mesenchymal stem cell complex on the progression of osteoarthritis in a rat model. Int. J. Nanomed. 2014, 9 (Suppl. 1), 141–157. [Google Scholar] [CrossRef]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Na, S.M.; Ahn, H.W.; Kang, J.K.; Seon, J.K.; Song, E.K. Allogenic Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Are More Effective Than Bone Marrow Aspiration Concentrate for Cartilage Regeneration After High Tibial Osteotomy in Medial Unicompartmental Osteoarthritis of Knee. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2021, 37, 2521–2530. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
| Hydrogel Type | Porosity (P), Stiffness (S) and Viscosity (V) | Functionalized with | Crosslinked | Cell Type and Loading | Chondrogenic Inducting Factors | Main Results | Reference |
|---|---|---|---|---|---|---|---|
| Fibrin/hyaluronan hydrogel (RegenoGel™) clinical approved as medical device | PSV—N.I. | No | No | Femoral-derived human bone marrow SCs (BMSCs) (embedded in hydrogel) | TGFβ1 | ↑of COL2, ACAN and GAG at day 28 | Lolli et al. [52] |
| Aldehyde-modified hyaluronan (HAA) and hydrazide-modified polyvinyl alcohol (PVAH) | PV—N.I. S reported | No | Yes | Murine cell line W20-17 MSCs (embedded in hydrogel) | N.I. | ↑of COL2, ACAN, GAG and proteoglycan at day 28 | Aulin et al. [62] |
| Decellularized cartilage from porcine condyle | PSV—N.I. | Self-assembling peptides (RAD, PFS and RAD/PFS) | N.I. | Rabbit MSCs (seeded on hydrogel) | TGFβ3 | ↑of COL2, ACAN, SOX9 and COL1 at day 14 in hydrogel with RAD/PFS | Lu et al. [38] |
| 40% polyethylene glycol (PEG)/60% polyethylene glycol diacrylate (PEGDA) printed hydrogel with a cold atmospheric plasma (CAP) treatment | P reported SV—N.I. | TGFβ1 loaded nanoparticles (NP) | Yes | Human MSCs (seeded on hydrogel) | No | ↑of COL2, ACAN and SOX9 at day 21 | S.J Lee et al. [54] |
| 7% PEGDA-3% condroitin sulfate (CS)-methacrylate | PSV—N.I. | No | Yes | Human ASCs and osteoarthritic (OA) chondrocytes (C) mixed at different ratios (embedded in hydrogel) | TGFβ3 | ↑of COL2, ACAN, GAG and COL1 at day 21 in mixed culture (25C:75ASCs) | Lai et al. [37] |
| 50% gelatin-50% beta-cyclodextrin | PSV—N.I. | Magnetic nanoparticles | Yes | Rat BMSCs (seeded on hydrogel) | TGFβ1 | ↑of COL2, ACAN and SOX9 at day 14 mainly after magnetic field treatment | Huang et al. [63] |
| 10% PEGDA | PSV—N.I. | No | Yes | Goat BMSCs (embedded in hydrogel) | TGFβ1 | ↑of COL2 and GAG at day 21 | Li et al. [55] |
| Polyglucosamine/glucosamine carbonate (PG/GC) (JointRep™ Oligo Medic INC.) clinical approved as medical device | PSV—N.I. | No | N.I. | Human ASCs (embedded in hydrogel) | N.I. | ↑of COL2, proteoglycan and GAG at day 21 | Pipino et al. [67] |
| Sulfated (S) methacrylate hyaluronic acid (MeHA) | PSV—N.I. | TGFβ1 | Yes | Human MSCs (embedded in hydrogel) | TGFβ1 | ↑of COL2, ACAN and GAG ↓ of COL1 and COL10 at day 28 in low and high SMeHA | Feng Q. et al. [47] |
| Dextran-tyramine (Dex-TA) | PS reported V—N.I. | Incorporated human platelet lysate (hPL) | Yes | Human MSCs (embedded in hydrogel) | TGFβ3 + BMP-6 | ↑of COL2, GAG and proteoglycan and ↓of COL1 at day 21 | Moreira Teixeira et al. [60] |
| Diacrylate PEG-DA (MWn = 700): PEG (MW = 300) (60% wt/wt) | P reported SV—N.I. | Nanocrystalline hydroxyapatite and TGFβ1 | Yes | Human MSCs (seeded on hydrogel) | No | ↑of COL2 and GAG at day 21 | Castro et al. [56] |
| Silylated collagen | P reported SV—N.I. | Mimetic synthetic peptides | No | Human MSCs (embedded in hydrogel) | TGFβ3 | ↑of COL2, SOX9, ACAN and COL10 at day 21 | Valot et al. [45] |
| Fibrin MeHA | PS reported V—N.I. | No | Yes | Human MSCs (embedded in hydrogel) | N.I. | ↑of SOX9 (in presence of platelet lysate) at day 12 | Snyder et al. [46] |
| DNA supramolecular | PV—N.I. S reported | No | No | Rabbit BMSCs (embedded in hydrogel) | N.I. | ↑of COL2, SOX9 and ACAN and ↓ of COL1 and COL10 at day 14 | Yan et al. [61] |
| Bacterial cellulose | P reported SV—N.I. | No | No | Equine MSCs (seeded on hydrogel) | TGFβ1 | ↑of proteoglycan and GAG at 7 and 14 days | Favi et al. [12] |
| Gelled platelet lysate | PSV—N.I. | No | No | Canine ASCs (embedded in hydrogel) | TGFβ1 | ↑of proteoglycan and GAG at day 28 | Lima et al. [39] |
| Microbeads of agarose | PSV—N.I. | Different % of collagen type 2 | No | Human MSCs (embedded in microbeads) | TGFβ1 | ↑of soluble GAG at day 21 | Tiruvannamalai Annamalai et al. [40] |
| Self-assembled synthetic peptides | PSV—N.I. | Arginine-glycine-aspartate (RGD) | No | Rabbit ASCs infected with lentivirus-mature TGFβ3 (embedded in hydrogel) | TGFβ3 | ↑of COL2, ACAN and SOX9 at day 21 | Zheng et al. [57] |
| PEG-hyaluronic acid (HA) | PSV—N.I. | Pentosan polysulfate | N.I. | Canine MSCs from induced pluripotent stem cells by inhibition the TGFβ/Activin signaling pathway (embedded in hydrogel) | TGFβ3 | ↑of proteoglycan and GAG at day 21 | Whitworth et al. [64] |
| Methacrylated gelatin: HA (MeG:HA) ratios | PV—N.I. S reported | No | Yes | Human BMSCs (embedded in hydrogel) | TGFβ3 | ↑of COL2, SOX9 and ACAN and ↓ of COL10 at 56 days in 9:1 MeG:HA hydrogel ratio | Lin H et al. [48] |
| Collagen type 1 | PSV—N.I. | No | No | Human BMSCs infected with adenoviral vector-(Ad)-SOX9, AdTGFβ1 and AdBMP2 (embedded in hydrogel) | No | ↑of COL2, GAG and condroitin sulfate in all transduced hBMSCs at day 21; ↓of COL10 only in AdSOX9 transduced at day 21 | Weißenberger et al. [41] |
| Thiolated gelatin (gelatin-SH)/PEGDA | PV—N.I. S reported | Insulin-Like Growth Factor (IGF)-1 cargo | Yes | Human ASCs (embedded in hydrogel) | N.I. | ↑of COL2, ACAN, SOX9 and GAG and ↓ of COL1 at day 21 | Cho et al. [53] |
| MeHA | PSV—N.I. | Microbeads of PEG/poly lactic acid-co-glycolic acid (PLGA) containing different concentrations of TGFβ3 or ghrelin | Yes | Human BMSCs (microsphere) | No | ↑of COL2, ACAN and SOX9 and ↓of COL1 at day 10, in microbeads with 10 ng/mL TGFβ and 0.1 nM ghrelin | Lin J et al. [43] |
| Poly (N-isopropylacrylamide-co-acrylic acid (p(NIPAAm-AA) thermosensitive | SV—N.I. P reported | No | Yes | Immortalized human MSCs (UE7T-13) (embedded in hydrogel) | N.I. | ↑of COL2, ACAN and SOX9 at day 28 and 35 and ↓of COL1 at day 42 | Zhang J et al. [58] |
| Polyethylene glycol (PEG) PEGDA | PV—N.I. S reported | Glucosamine (10 mM) | Yes | Human BMSCs (embedded in hydrogel) | TGFβ1 | ↑of COL2, ACAN and SOX9 and ↓ of COL1, COL10 and MMP13 at days 21 and 42 | Yao et al. [59] |
| Collagen type 1 | PSV—N.I. | Graphene oxide adsorbed TGFβ3 | No | Human BMSCs from OA patients (embedded in hydrogel) | No | ↑of COL2, ACAN, SOX9 and GAG at day 28 | Zhou et al. [44] |
| Thiolated gelatin crosslinked borate ester bond-based HA (HA-PBA) | PSV reported | No | Yes | Rabbit ASCs (embedded in hydrogel) | TGFβ1 | ↑of COL2, ACAN and SOX9 and ↓ of COL1, COL10 and MMP13 at days 14 and 28 | Shi et al. [65] |
| Poloxamer 407 crosslinking of HA (PHA) | PS reported V—N.I. | Icariin | Yes | Rat BMSCs (embedded in hydrogel) | TGFβ3 | ↑of COL2, ACAN, SOX9, proteoglycan and Hypoxia Inducible Factor 1 Subunit α (HIF1α) at day 12 in icariin-embedded hydrogel | Zhu et al. [66] |
| VitroGel® | P—N.I. SV reported | RGD | Yes | Human ASCs (embedded in hydrogel) | TGFβ3 + BMP-6 | ↑of COL2, ACAN, SOX9, GAG and cartilage oligomeric matrix protein (COMP) and ↓of COL1 at day 28 | Manferdini et al. [42] |
| Methacrylated gelatin (GelMA) | PV—N.I. S reported | No | Yes | Human BMSCs (embedded in hydrogel) | TGFβ3 | ↑of COL2, ACAN, SOX9 and proteoglycan at day 28 | He et al. [49] |
| Methacrylated porcine decellularized cartilage ECM | P—N.I. SV reported | No | Yes | Goat MSCs (embedded in hydrogel) | TGFβ1 | ↑of COL2, GAG and ↓ of COL1 at day 21 | Behan et al. [50] |
| Chondroitin sulfate (CS) | PS reported V—N.I. | Collagen type 1 and 2 blend | No | Rabbit BMSCs (embedded in hydrogel) | TGFβ3 | ↑of GAG and COL2 at day 28 | Kilmer et al. [51] |
| Hydrogel Type | Porosity (P), Stiffness (S) and Viscosity (V) | Functionalized with | Crosslinked | Ex Vivo Model | Cell Type and Loading | Chondrogenic Inducting Factors | Main Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Dex-TA | PS reported V—N.I. | Incorporated hPL | Yes | Hydrogel adhesion to OA human cartilage | Human MSCs embedded in Dex-TA with and without PL chondrogenic induced for 8 days | TGFβ3 + BMP6 | Dex-TA hydrogels/OA cartilage interface showed close interactions | Moreira Teixeira et al. [60] |
| GelMA | S reported PV—N.I. | No | Yes | Engineered cartilage construct subject to an impactor system | GelMA hydrogel-BMSCs chondrogenically induced for 28 days (engineered construct) | TGFβ3 | Traumatic impact on engineered-construct-induced changes in cartilage genes and induction of chondrocyte catabolic genes | He et al. [49] |
| Hydrogel Type | Porosity (P), Stiffness (S) and Viscosity (V) | Functionalized with | Crosslinked | Animal Model (Time to Develop OA or Time of Subcutaneous Implantation) | Cell type/ Hydrogel | Chondrogenic Inducting Factors | Gauge In Vivo Injection | Main Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Sulfated (S) MeHA, two sulfate concentrations (low and high) tested | PSV—N.I. | TGFβ1 | Yes | Subcutaneous implantation in nude mice (4 weeks) Rat anterior cruciate ligament transection (ACLT) and medial resection (4 weeks) | Human MSCs (embedded in hydrogel) preconditioned for 14 days in vitro before implantation | No | N.I. | ↑COL2 and ACAN and ↓ COL10 and MMP13 at 4 weeks in high sulphate concentration | Feng Q et al. [47] |
| Decellularized human amnion | PV—N.I. S reported | No | No | OA model: rat collagenase-induced (1 week) | Knee joint injected with rat ASCs (embedded in hydrogel) | No | 29 G | ↓ of inflammatory factors, ↑of GAG at 4 weeks | Bhattacharjee et al. [69] |
| Thiolated-HA (ThHA) | PSV reported | Collagen type 1 | No | OA model: rat ACLT and medial meniscectomy (4 weeks) | Knee joint injected with rat ASCs overexpressing TGFβ1 (embedded in hydrogel) | No | 25 G | ↓ of inflammatory factors and osteophytes, ↑of GAG and COL2 at 4 weeks | Yu et al. [70] |
| Gelatin-based 3D microgel | PSV—N.I. | No | No | OA model: rat ACLT (4 weeks) | Knee joint injected with human umbilical cord (UC)-MSCs (seeded in microgel) | N.I. | N.I. | ↑of proteoglycan, COL1 and COL2; ↓of osteophytes both at 4 and 8 weeks | Xing et al. [71] |
| MeHA | PSV—N.I. | No | Yes | Subcutaneous: implantation in nude mice (2 weeks) | Human BMSCs overexpressing Linc-ROR (embedded in hydrogel) preconditioned for 2 weeks in vitro | N.I. | N.I. | ↑of COL 2, SOX9 and ACAN, ↓ of MMP13 and COL10 at 2 weeks | Feng L et al. [72] |
| Self-assembled peptide (SAP) | PSV—N.I. | Neuropeptide (SP) different concentrations | No | OA model: rat ACL and medial collateral transections (2 weeks) | Knee joint injected with rat MSCs embedded in hydrogel | No | 26 G | ↓of inflammatory factors and bone density, ↑of SOX9 and COL 2 at 6 weeks; similar result with only SAP-SP | S.J Kim et al. [75] |
| 1% Hyaluronan | PSV—N.I. | No | No | OA model: monosodium iodoacetate (MIA)-induced rabbit (2 weeks) | Knee joint injected three times (once every 3 weeks) with human embryonic stem cell-MSCs (embedded in hydrogel) | TGFβ | N.I. | ↑of GAG and proteoglycan at 9 weeks | Zhang L et al. [73] |
| DNA supramolecular | PV—N.I. S reported | No | No | OA model: rabbit ACLT and medial meniscectomy (MMx) | Knee joint injected three times (once a week for 3 weeks) with rabbit MSCs embedded in hydrogel | N.I. | N.I. | ↑of COL 2, GAG and proteoglycan at 6 weeks | Yan et al. [61] |
| Self-assembled synthetic peptides | PSV—N.I. | RGD | No | Subcutaneous: implantation in nude mice (6 weeks) | Rabbit ASCs preconditioned for 3 weeks in vitro before implantation (embedded in hydrogel) | TGF-β3 | N.I. | ↑of COL 2, ACAN, proteoglycan and GAG at 4 and 6 weeks | Zheng et al. [57] |
| Polyethylene glycol (PEG) PEGDA | PV—N.I. S reported | Glucosamine (10 mM) | Yes | Subcutaneous: implantation in nude mice (8 weeks) | Human BMSCs (embedded in hydrogel) preconditioned for 12 h in vitro before implantation | TGFβ1 | N.I. | ↑of COL2 and GAG at 8 weeks | Yao et al. [59] |
| Fibrinogen:trombin different ratios | PV—N.I. S reported | No | No | Subcutaneous: implantation in rat (1 and 4 weeks) | Human ASCs (embedded in hydrogel) | N.I. | N.I. | ↑COL2 and GAG at 4 weeks in hydrogel with fibrinogen 30mg/mL: trombin 100IU/mL ratio | Kim J.S et al. [74] |
| SAP | PSV-N.I. | No | No | OA model: rat ACLT, medial collateral transection and removal of medial meniscus (3 weeks) | Knee joint injected rat MSCs (embedded in hydrogel) | No | 26 G | ↓ of inflammatory factors and apoptosis and ↑of COL2 2 at 6 weeks | Kim J.E et al. [76] |
| Poloxamer 407 crosslinking HA (PHA) | PS reported V—N.I. | Icariin | Yes | OA model: rat destabilization of medial meniscus by medial collateral transection (2 weeks) | Knee joint injected rat MSCs (embedded in hydrogel) | TGFβ3 | N.I. | ↓ of inflammatory factors and ↑of COL 2, SOX9, GAG and proteoglycan at 12 weeks | Zhu et al. [66] |
| Hydrogel Type | Porosity (P), Stiffness (S) and Viscosity (V) | Functionalized with | Crosslinked | Study Design | Cells/ Hydrogel | Chondrogenic Inducting Factors | Patients Evaluated | Main Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| HA (Caristem®) clinically approved as medical device | PSV—N.I. | No | No | Phase I/II clinical trial in patients with moderate knee OA and painful full-thickness cartilage defects treated with multiple drill holes and divided in two groups:
| Human UCB-MSCs (Caristem®) | N.I. | At 24 weeks and 7 years by means of physical examination, VAS score for pain, IKDC, MRI and histological evaluations | The treatment had an acceptable efficacy and safety profile without undesired effects at 7 years | Park et al. [77] |
| HA (Caristem®) clinically approved as medical device | PSV—N.I. | No | No | High tibial osteotomy (HTO) for medial unicompartmental OA treated with multiple drill holes and divided in two groups:
| Human UCB-MSCs (Caristem®) | N.I. | At 1 year follow-up by means of IKDC, WOMAC and KSS pain and function scores | At 1 year no significant differences between the two groups; second-look arthroscopy after 1 year showed by ICRS grade a better regeneration of the cartilage in hUCB-MSCs-HA group | Lee N.H et al. [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manferdini, C.; Gabusi, E.; Saleh, Y.; Lenzi, E.; D’Atri, G.; Ricotti, L.; Lisignoli, G. Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications. Cells 2022, 11, 3969. https://doi.org/10.3390/cells11243969
Manferdini C, Gabusi E, Saleh Y, Lenzi E, D’Atri G, Ricotti L, Lisignoli G. Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications. Cells. 2022; 11(24):3969. https://doi.org/10.3390/cells11243969
Chicago/Turabian StyleManferdini, Cristina, Elena Gabusi, Yasmin Saleh, Enrico Lenzi, Giovanni D’Atri, Leonardo Ricotti, and Gina Lisignoli. 2022. "Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications" Cells 11, no. 24: 3969. https://doi.org/10.3390/cells11243969
APA StyleManferdini, C., Gabusi, E., Saleh, Y., Lenzi, E., D’Atri, G., Ricotti, L., & Lisignoli, G. (2022). Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications. Cells, 11(24), 3969. https://doi.org/10.3390/cells11243969






