Calcium Channels, OST1 and Stomatal Defence: Current Status and Beyond
Abstract
1. Introduction
2. Mechanism of PAMPs-Triggered Stomata Closure
3. Mechanism of ABA-Triggered Stomata Closure
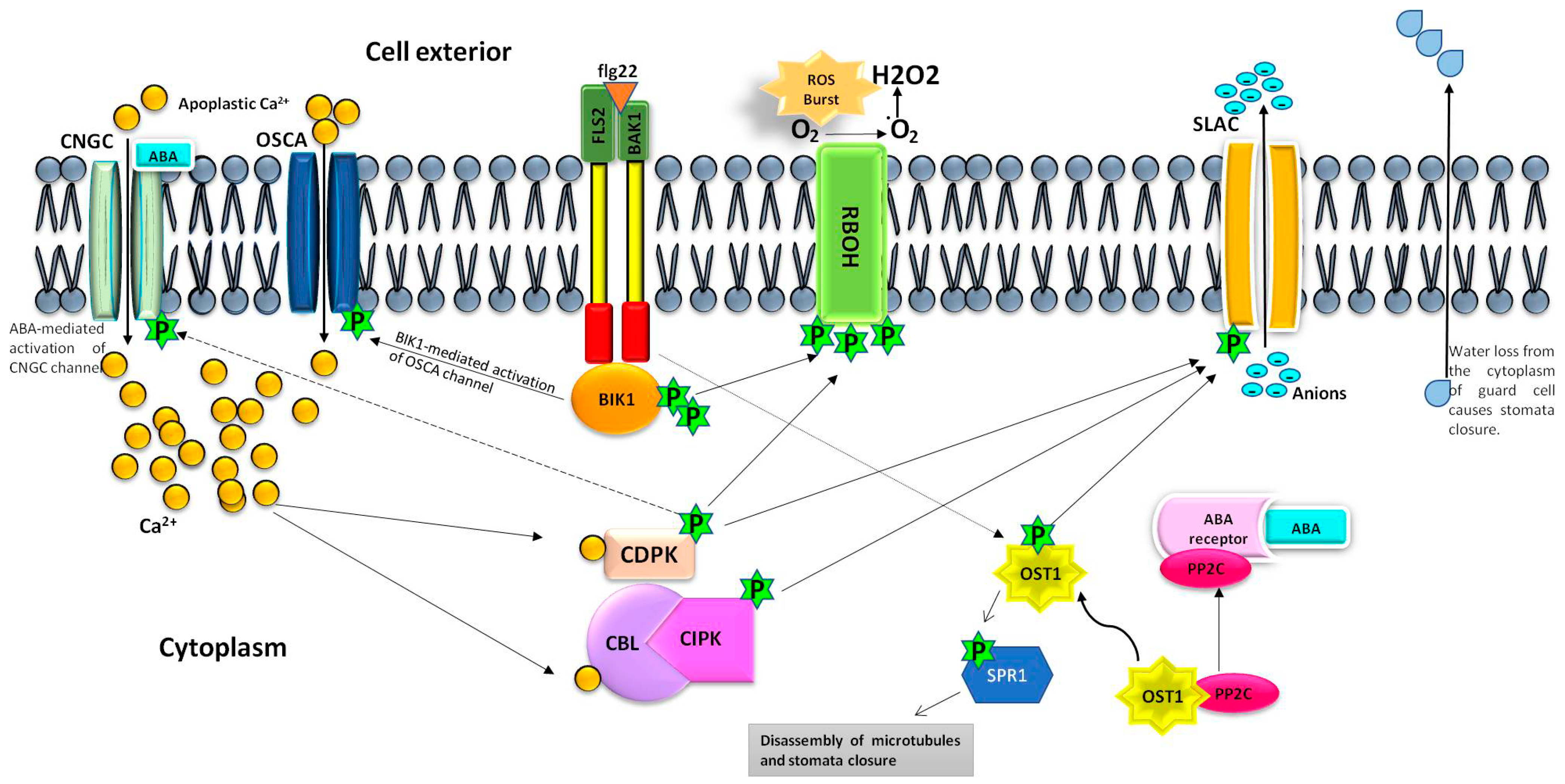
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melotto, M.; Zhang, L.; Oblessuc, P.R.; He, S.Y. Stomatal Defense a Decade Later. Plant. Physiol. 2017, 174, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, K.; Mersmann, S.; Robatzek, S.; Böhmer, M. Guarding the green: Pathways to stomatal immunity. Mol. Plant. Microbe Interact. 2013, 26, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant. Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Q.; Yang, Y.; Shen, X.; Zhu, M.; Shen, J.; Zhang, W.; Hu, H.; Wang, Y.-F. Multiple cyclic nucleotide-gated channels function as ABA-activated Ca2+ channels required for ABA-induced stomata closure in Arabidopsis. Plant Cell 2022, koac274. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitão, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qi, S.; Wang, S.; Dou, L.; Jia, M.; Mao, T.; Guo, Y.; Wang, X. The OPEN STOMATA1–SPIRAL1 module regulates microtubule stability during abscisic acid-induced stomatal closure in Arabidopsis. Plant Cell 2022, koac307. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L.; Macho, A.P.; Han, Z.; Hu, Z.; Zipfel, C.; Zhou, J.-M.; Chai, J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 2013, 342, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Dai, C.; Lee, M.M.; Kwak, J.M.; Nam, K.H. BRI1-Associated Receptor Kinase 1 Regulates Guard Cell ABA Signaling Mediated by Open Stomata 1 in Arabidopsis. Mol. Plant. 2016, 9, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y.; et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Moeder, W. Calcium channel helps shut the door on intruders. Nature 2020, 585, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Madhu; Upadhyay, S.K. Mechanosensitive ion channels in plants. In Calcium Transport Elements in Plants; Academic Press: Cambridge, MA, USA, 2021; pp. 267–279. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, A.; Madhu; Dixit, S.; Singh, K.; Upadhyay, S.K. OSCA Genes in Bread Wheat: Molecular Characterization, Expression Profiling, and Interaction Analyses Indicated Their Diverse Roles during Development and Stress Response. Int. J. Mol. Sci. 2022, 23, 14867. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Sun, L. Structure of the hyperosmolality-gated calcium-permeable channel OSCA1.2. Nat. Commun. 2018, 9, 5060. [Google Scholar] [CrossRef] [PubMed]
- Guzel Deger, A.; Scherzer, S.; Nuhkat, M.; Kedzierska, J.; Kollist, H.; Brosché, M.; Unyayar, S.; Boudsocq, M.; Hedrich, R.; Roelfsema, M.R. Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 2015, 208, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Alema’n, F.; et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 2015, 4, e03599. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Moeder, W.; Yoshioka, K. Plant Cyclic Nucleotide-Gated Channels: New Insights on Their Functions and Regulation. Plant. Physiol. 2020, 184, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; He, K.; Peng, J.; Wang, X.; Mao, T. The E3 ligase MREL57 modulates microtubule stability and stomatal closure in response to ABA. Nat. Commun. 2021, 12, 2181. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Waadt, R.; Nuhkat, M.; Kollist, H.; Hedrich, R.; Roelfsema, M.R.G. Calcium signals in guard cells enhance the efficiency by which abscisic acid triggers stomatal closure. New Phytol. 2019, 224, 177–187. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, S.K. Calcium Channels, OST1 and Stomatal Defence: Current Status and Beyond. Cells 2023, 12, 127. https://doi.org/10.3390/cells12010127
Upadhyay SK. Calcium Channels, OST1 and Stomatal Defence: Current Status and Beyond. Cells. 2023; 12(1):127. https://doi.org/10.3390/cells12010127
Chicago/Turabian StyleUpadhyay, Santosh Kumar. 2023. "Calcium Channels, OST1 and Stomatal Defence: Current Status and Beyond" Cells 12, no. 1: 127. https://doi.org/10.3390/cells12010127
APA StyleUpadhyay, S. K. (2023). Calcium Channels, OST1 and Stomatal Defence: Current Status and Beyond. Cells, 12(1), 127. https://doi.org/10.3390/cells12010127





