Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Doxorubicin and NMN Administration
2.3. Body Composition
2.4. Transthoracic Echocardiography
2.5. Treadmill Exhaustion Test
2.6. Rotarod Test
2.7. Liquid Chromatography Mass Spectrometry (LC-MS) Analysis (NAD+ Metabolome)
2.8. Troponin-1, Lactate Dehydrogenase, 4-Hydroxynonenal and Cytokines and Chemokines Analysis
2.9. RNA-seq Analysis in Heart Tissue
2.10. Quantitative RT-PCR (RT-qPCR)
2.11. Statistical Analysis
3. Results
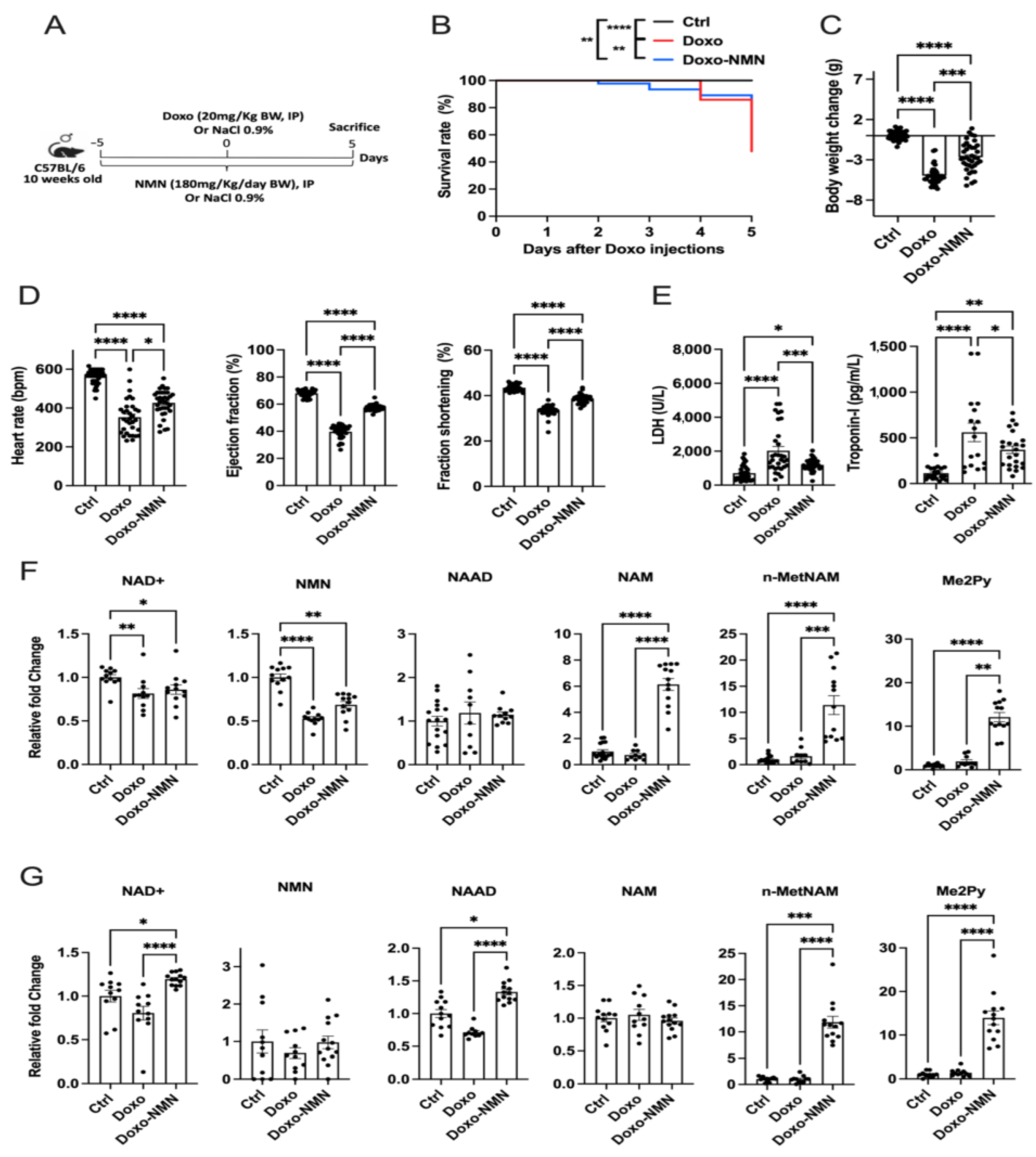
3.1. NMN Improves Survival, Body Weight Loss and Cardiac Function Decline Induced by the Acute Doxo Treatment in Mice
3.2. NMN Affects the NAD+ Metabolome in Mice Receiving Acute Doxo Treatment
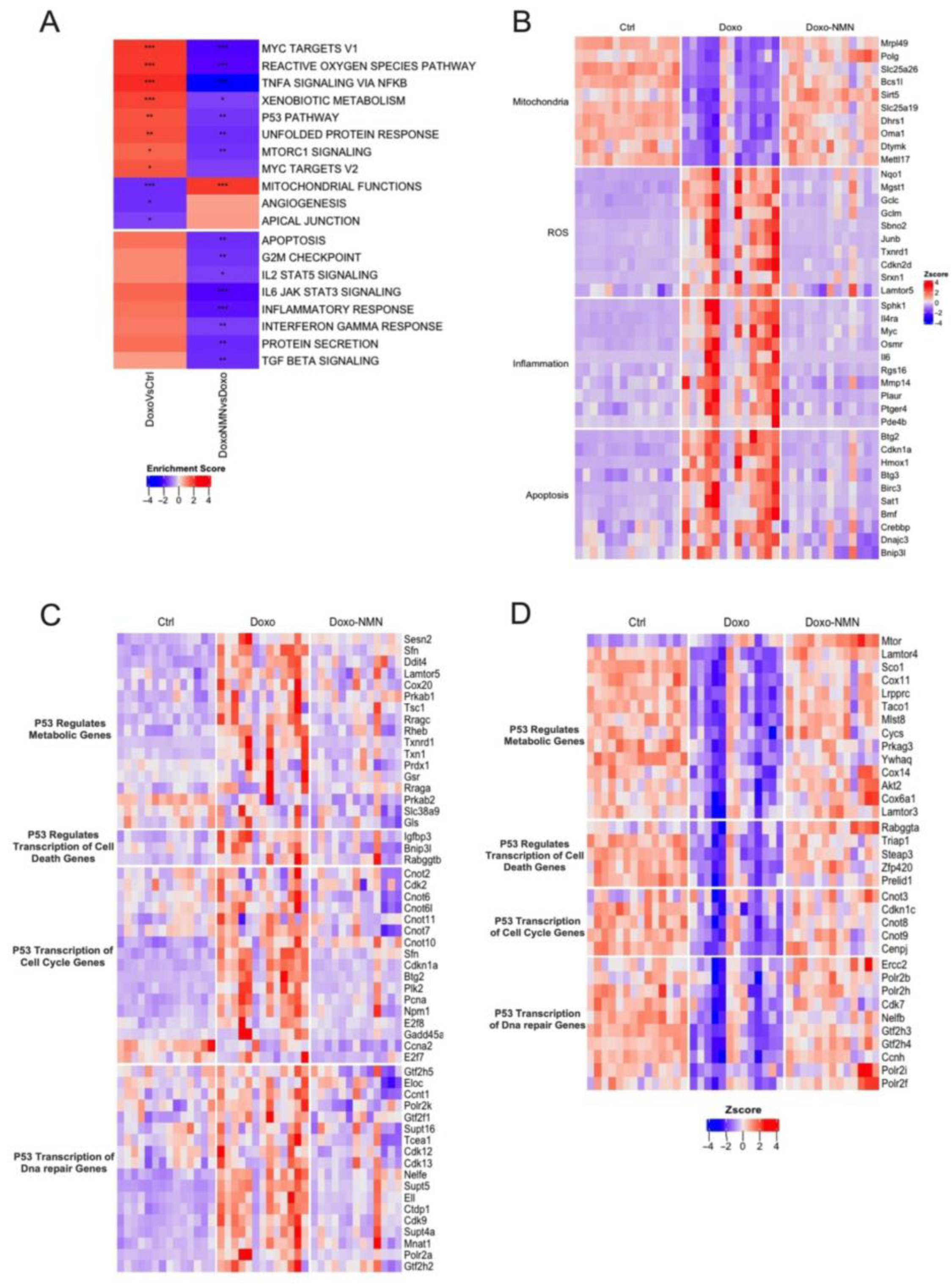
3.3. Treatment with NMN Reduces the Expression of Genes Associated with Mitochondrial Damages, Oxidative Stress, Inflammation, and Apoptosis Induced by the Acute Doxo Treatment
3.4. NMN Administration Is a Key Modulator of p53 Pathway Transcriptomic Changes Induced by Doxo Acute Treatment
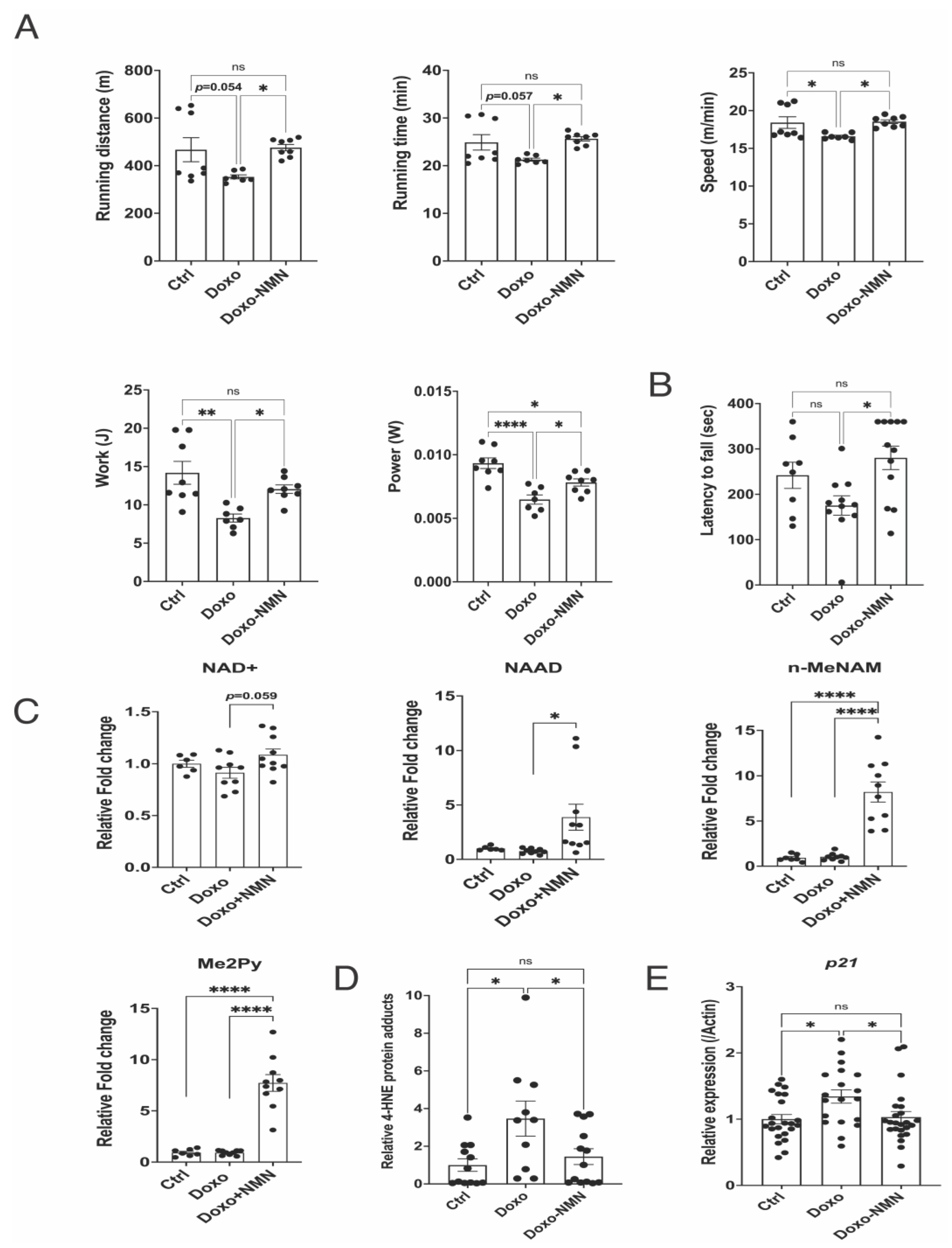
3.5. Orally Administered NMN Is Bioavailable and Improves Survival and Bodyweight Loss Induced by Doxo
3.6. The Doxo-Induced Alteration in the Expression of Genes Related to Mitochondrial Functions, DNA Damage and Apoptosis Was Improved by the Oral NMN Treatment in the Heart
3.7. The Doxo-Induced Loss of Physical Activity Is Prevented by Oral NMN Administration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Robin Yabroff, K.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA 2013, 309, 2371. [Google Scholar] [CrossRef] [PubMed]
- Sanoff, H.K.; Deal, A.M.; Krishnamurthy, J.; Torrice, C.; Dillon, P.; Sorrentino, J.; Ibrahim, J.G.; Jolly, T.A.; Williams, G.; Carey, L.A.; et al. Effect of Cytotoxic Chemotherapy on Markers of Molecular Age in Patients with Breast Cancer. J. Natl. Cancer Inst. 2014, 106, dju057. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, S.; Mueller, H.; Goergen, H.; Mayer, A.; Brillant, C.; Behringer, K.; Halbsguth, T.V.; Hitz, F.; Soekler, M.; Shonukan, O.; et al. Cancer-Related Fatigue in Patients with and Survivors of Hodgkin’s Lymphoma: A Longitudinal Study of the German Hodgkin Study Group. Lancet Oncol. 2016, 17, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Kirkland, J.L.; Gramatges, M.M.; Wang, Z.; Kundu, M.; McCastlain, K.; Li-Harms, X.; Zhang, J.; Tchkonia, T.; Pluijm, S.M.F.; et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Gibson, C.J.; Rockne, R.C.; Ness, K.K. Premature Aging in Young Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Hydock, D.S.; Lien, C.-Y.; Jensen, B.T.; Parry, T.L.; Schneider, C.M.; Hayward, R. Effects of In Vivo Doxorubicin Treatment on Skeletal Muscle Function. Med. Sci. Sport. Exerc. 2011, 43, 903. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-Related Fatigue—Mechanisms, Risk Factors, and Treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular Mechanism of Doxorubicin-Induced Cardiomyopathy—An Update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Stěrba, M.; Popelová, O.; Vávrová, A.; Jirkovský, E.; Kovaříková, P.; Geršl, V.; Simůnek, T. Oxidative Stress, Redox Signaling, and Metal Chelation in Anthracycline Cardiotoxicity and Pharmacological Cardioprotection. Antioxid. Redox Signal. 2013, 18, 899–929. [Google Scholar] [CrossRef] [PubMed]
- Fabris, S.; MacLean, D.A. Skeletal Muscle an Active Compartment in the Sequestering and Metabolism of Doxorubicin Chemotherapy. PLoS ONE 2015, 10, e0139070. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- Imai, S.-I.; Guarente, L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.-I. NAD Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Li, J.; Ying, T.; Zhang, B.; Lu, J. Nicotinamide Mononucleotide (NMN) as an Anti-Aging Health Product—Promises and Safety Concerns. J. Adv. Res. 2022, 37, 267–278. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.-R.; Huang, X.-Z.; Xie, Q.-H.; Xu, Y.-Y.; Shang, D.; Hao, C.-M. Nicotinamide Mononucleotide, an NAD Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1–Dependent Manner. J. Am. Soc. Nephrol. 2017, 28, 2337–2352. [Google Scholar] [CrossRef]
- Zhan, T.; Xiong, H.; Pang, J.; Zhang, W.; Ye, Y.; Liang, Z.; Huang, X.; He, F.; Jian, B.; He, W.; et al. Modulation of NAD Biosynthesis Activates SIRT1 and Resists Cisplatin-Induced Ototoxicity. Toxicol. Lett. 2021, 349, 115–123. [Google Scholar] [CrossRef]
- Hosseini, L.; Vafaee, M.S.; Badalzadeh, R. Melatonin and Nicotinamide Mononucleotide Attenuate Myocardial Ischemia/Reperfusion Injury via Modulation of Mitochondrial Function and Hemodynamic Parameters in Aged Rats. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 240–250. [Google Scholar] [CrossRef]
- Rajabi, M.; Vafaee, M.S.; Hosseini, L.; Badalzadeh, R. Pretreatment with Nicotinamide Mononucleotide Increases the Effect of Ischaemic Postconditioning on Cardioprotection and Mitochondrial Function Following Ex Vivo Myocardial Reperfusion Injury in Aged Rats. Clin. Exp. Pharmacol. Physiol. 2022, 49, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Tang, J.J.; Rashid, M.A.; Cho, C.H.; Corujo-Ramirez, A.; Choi, J.; Bae, M.G.; Brogren, D.; Hawse, J.R.; Hou, X.; et al. Nicotinamide Mononucleotide Prevents Cisplatin-Induced Cognitive Impairments. Cancer Res. 2021, 81, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.J.; Mokhtari, B.; Badalzadeh, R. Combination of Nicotinamide Mononucleotide and Troxerutin Induces Full Protection against Doxorubicin-Induced Cardiotoxicity by Modulating Mitochondrial Biogenesis and Inflammatory Response. Mol. Biol. Rep. 2022, 49, 8209–8218. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, N.; Shishido, T.; Takeishi, Y.; Kubota, I. Modulation of Doxorubicin-Induced Cardiac Dysfunction in Toll-Like Receptor-2–Knockout Mice. Circulation 2004, 110, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Limbad, C.; Doi, R.; McGirr, J.; Ciotlos, S.; Perez, K.; Clayton, Z.S.; Daya, R.; Seals, D.R.; Campisi, J.; Melov, S. Senolysis Induced by 25-Hydroxycholesterol Targets CRYAB in Multiple Cell Types. iScience 2022, 25, 103848. [Google Scholar] [CrossRef]
- Nie, Y.; Sato, Y.; Wang, C.; Yue, F.; Kuang, S.; Gavin, T.P. Impaired Exercise Tolerance, Mitochondrial Biogenesis, and Muscle Fiber Maintenance in miR-133a-Deficient Mice. FASEB J. 2016, 30, 3745–3758. [Google Scholar] [CrossRef]
- Cros, C.; Margier, M.; Cannelle, H.; Charmetant, J.; Hulo, N.; Laganier, L.; Grozio, A.; Canault, M. Nicotinamide Mononucleotide Administration Triggers Macrophages Reprogramming and Alleviates Inflammation During Sepsis Induced by Experimental Peritonitis. Front. Mol. Biosci. 2022, 9, 895028. [Google Scholar] [CrossRef]
- Demarest, T.G.; Truong, G.T.D.; Lovett, J.; Mohanty, J.G.; Mattison, J.A.; Mattson, M.P.; Ferrucci, L.; Bohr, V.A.; Moaddel, R. Assessment of NADmetabolism in Human Cell Cultures, Erythrocytes, Cerebrospinal Fluid and Primate Skeletal Muscle. Anal. Biochem. 2019, 572, 1–8. [Google Scholar] [CrossRef]
- Trammell, S.A.; Brenner, C. Targeted, LCMS-Based Metabolomics for Quantitative Measurement of NAD(+) Metabolites. Comput. Struct. Biotechnol. J. 2013, 4, e201301012. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. p53-Dependent Apoptosis Pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Hiensch, A.E.; Bolam, K.A.; Mijwel, S.; Jeneson, J.A.L.; Huitema, A.D.R.; Kranenburg, O.; van der Wall, E.; Rundqvist, H.; Wengstrom, Y.; May, A.M. Doxorubicin-Induced Skeletal Muscle Atrophy: Elucidating the Underlying Molecular Pathways. Acta Physiol. 2020, 229, e13400. [Google Scholar] [CrossRef]
- Cros, C.; Cannelle, H.; Laganier, L.; Grozio, A.; Canault, M. Safety Evaluation after Acute and Sub-Chronic Oral Administration of High Purity Nicotinamide Mononucleotide (NMN-C®) in Sprague-Dawley Rats. Food Chem. Toxicol. 2021, 150, 112060. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S.-I. Nicotinamide Mononucleotide, a Key NAD(+) Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef]
- Irie, J.; Inagaki, E.; Fujita, M.; Nakaya, H.; Mitsuishi, M.; Yamaguchi, S.; Yamashita, K.; Shigaki, S.; Ono, T.; Yukioka, H.; et al. Effect of Oral Administration of Nicotinamide Mononucleotide on Clinical Parameters and Nicotinamide Metabolite Levels in Healthy Japanese Men. Endocr. J. 2020, 67, 153–160. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Süle, Z.; et al. Nicotinamide Mononucleotide (NMN) Supplementation Rescues Cerebromicrovascular Endothelial Function and Neurovascular Coupling Responses and Improves Cognitive Function in Aged Mice. Redox Biol. 2019, 24, 101192. [Google Scholar] [CrossRef]
- You, Y.; Gao, Y.; Wang, H.; Li, J.; Zhang, X.; Zhu, Z.; Liu, N. Subacute Toxicity Study of Nicotinamide Mononucleotide via Oral Administration. Front. Pharmacol. 2020, 11, 604404. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.S.; Parry, T.L.; Brown, D.I.; Mota, R.I.; Huang, W.; Beak, J.Y.; Sola, M.; Zhou, C.; Hicks, S.T.; Caughey, M.C.; et al. Doxorubicin Exposure Causes Subacute Cardiac Atrophy Dependent on the Striated Muscle-Specific Ubiquitin Ligase MuRF1. Circ. Heart Fail. 2019, 12, e005234. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.A.A.; Lark, D.S.; Reese, L.R.; Torres, M.J.; Ryan, T.E.; Lin, C.-T.; Cathey, B.L.; Neufer, P.D. Targeted Overexpression of Mitochondrial Catalase Protects against Cancer Chemotherapy-Induced Skeletal Muscle Dysfunction. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E293–E301. [Google Scholar] [CrossRef]
- Trammell, S.A.J.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide Riboside Is Uniquely and Orally Bioavailable in Mice and Humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View From the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Qi, H.; Wang, C.; Wang, C.; Zhang, J.; Dong, L. Doxorubicin-Induced Systemic Inflammation Is Driven by Upregulation of Toll-Like Receptor TLR4 and Endotoxin Leakage. Cancer Res. 2016, 76, 6631–6642. [Google Scholar] [CrossRef] [PubMed]
- Sauter, K.A.D.; Wood, L.J.; Wong, J.; Iordanov, M.; Magun, B.E. Doxorubicin and Daunorubicin Induce Processing and Release of Interleukin-1β through Activation of the NLRP3 Inflammasome. Cancer Biol. Ther. 2011, 11, 1008–1016. [Google Scholar] [CrossRef]
- Litmanovich, A.; Khazim, K.; Cohen, I. The Role of Interleukin-1 in the Pathogenesis of Cancer and Its Potential as a Therapeutic Target in Clinical Practice. Oncol. Ther. 2018, 6, 109–127. [Google Scholar] [CrossRef]
- Ying, L.; Zhang, Q.; Yang, Y.-M.; Zhou, J.-Y. A Combination of Serum Biomarkers in Elderly Patients with Sarcopenia: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2022, 2022, 4026940. [Google Scholar] [CrossRef]
- Lavine, K.J.; Pinto, A.R.; Epelman, S.; Kopecky, B.J.; Clemente-Casares, X.; Godwin, J.; Rosenthal, N.; Kovacic, J.C. The Macrophage in Cardiac Homeostasis and Disease: JACC Macrophage in CVD Series (Part 4). J. Am. Coll. Cardiol. 2018, 72, 2213–2230. [Google Scholar] [CrossRef]
- Hydock, D.S.; Lien, C.-Y.; Jensen, B.T.; Schneider, C.M.; Hayward, R. Characterization of the Effect of in Vivo Doxorubicin Treatment on Skeletal Muscle Function in the Rat. Anticancer Res. 2011, 31, 2023–2028. [Google Scholar] [PubMed]
- Bonifati, D.M.; Ori, C.; Rossi, C.R.; Caira, S.; Fanin, M.; Angelini, C. Neuromuscular Damage after Hyperthermic Isolated Limb Perfusion in Patients with Melanoma or Sarcoma Treated with Chemotherapeutic Agents. Cancer Chemother. Pharmacol. 2000, 46, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kwon, O.-S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.-K.; Hwang, M.-H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased Mitochondrial Emission of Reactive Oxygen Species and Calpain Activation Are Required for Doxorubicin-Induced Cardiac and Skeletal Muscle Myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef]
- Smuder, A.J.; Kavazis, A.N.; Min, K.; Powers, S.K. Exercise Protects against Doxorubicin-Induced Oxidative Stress and Proteolysis in Skeletal Muscle. J. Appl. Physiol. 2011, 110, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-Induced Cardiotoxicity: Mechanisms of Action, Incidence, Risk Factors, Prevention, and Treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-Induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef]
- Nebigil, C.G.; Désaubry, L. Updates in Anthracycline-Mediated Cardiotoxicity. Front. Pharmacol. 2018, 9, 1262. [Google Scholar] [CrossRef]
- Gilliam, L.A.A.; St Clair, D.K. Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid. Redox Signal. 2011, 15, 2543–2563. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Jang, M.K.; Park, C.; Hong, S.; Li, H.; Rhee, E.; Doorenbos, A.Z. Skeletal Muscle Mass Change During Chemotherapy: A Systematic Review and Meta-Analysis. Anticancer Res. 2020, 40, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Broder, H.; Gottlieb, R.A.; Lepor, N.E. Chemotherapy and Cardiotoxicity. Rev. Cardiovasc. Med. 2008, 9, 75–83. [Google Scholar] [PubMed]
- Hamada, J.; Baasanjav, A.; Ono, N.; Murata, K.; Kako, K.; Ishida, J.; Fukamizu, A. Possible Involvement of Downregulation of the Apelin-APJ System in Doxorubicin-Induced Cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H931–H941. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Qian, B.; Min, X.; Gao, X.; Li, C.; Cheng, Y.; Huang, J. Over-Expression of Heat Shock Protein 27 Attenuates Doxorubicin-Induced Cardiac Dysfunction in Mice. Eur. J. Heart Fail. 2007, 9, 762–769. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhu, Z.; Liu, H.; Guo, H.; Xiong, C.; Xie, K.; Zhang, X.; Su, S. Protective Effect of Berberine on Doxorubicin-induced Acute Hepatorenal Toxicity in Rats. Mol. Med. Rep. 2016, 13, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shen, Y.; Zhou, L.; Sangwung, P.; Fujioka, H.; Zhang, L.; Liao, X. Short-Term Administration of Nicotinamide Mononucleotide Preserves Cardiac Mitochondrial Homeostasis and Prevents Heart Failure. J. Mol. Cell. Cardiol. 2017, 112, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, B.; Lin, Q.; Xu, W.; Zuo, W.; Li, J.; Liu, N.; Tu, T.; Zhang, B.; Xiao, Y.; et al. Nicotinamide Mononucleotide Attenuates Isoproterenol-Induced Cardiac Fibrosis by Regulating Oxidative Stress and Smad3 Acetylation. Life Sci. 2021, 274, 119299. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; He, B.; Zhu, D.; Wang, L.; Huang, R.; Zhu, J.; Wang, C.; Gao, F. Nicotinamide Mononucleotide Attenuates Doxorubicin-Induced Cardiotoxicity by Reducing Oxidative Stress, Inflammation and Apoptosis in Rats. Arch. Biochem. Biophys. 2021, 712, 109050. [Google Scholar] [CrossRef]
- Dolinsky, V.W. The Role of Sirtuins in Mitochondrial Function and Doxorubicin-Induced Cardiac Dysfunction. Biol. Chem. 2017, 398, 955–974. [Google Scholar] [CrossRef]
- Danz, E.D.B.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol Prevents Doxorubicin Cardiotoxicity through Mitochondrial Stabilization and the Sirt1 Pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef]
- Cappetta, D.; Esposito, G.; Piegari, E.; Russo, R.; Ciuffreda, L.P.; Rivellino, A.; Berrino, L.; Rossi, F.; De Angelis, A.; Urbanek, K. SIRT1 Activation Attenuates Diastolic Dysfunction by Reducing Cardiac Fibrosis in a Model of Anthracycline Cardiomyopathy. Int. J. Cardiol. 2016, 205, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Ma, Z.-G.; Zhang, X.; Xu, S.-C.; Zeng, X.-F.; Yang, Z.; Deng, W.; Tang, Q.-Z. CTRP3 Protected against Doxorubicin-Induced Cardiac Dysfunction, Inflammation and Cell Death via Activation of Sirt1. J. Mol. Cell. Cardiol. 2018, 114, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, Y.; Zheng, M.; Cao, T.; Wang, G.; Zhang, L.; Ni, R.; Brockman, J.; Zhong, H.; Fan, G.-C.; et al. Nicotinamide Riboside Promotes Autolysosome Clearance in Preventing Doxorubicin-Induced Cardiotoxicity. Clin. Sci. 2019, 133, 1505–1521. [Google Scholar] [CrossRef]
- Zhu, W.; Soonpaa, M.H.; Chen, H.; Shen, W.; Payne, R.M.; Liechty, E.A.; Caldwell, R.L.; Shou, W.; Field, L.J. Acute Doxorubicin Cardiotoxicity Is Associated with p53-Induced Inhibition of the Mammalian Target of Rapamycin Pathway. Circulation 2009, 119, 99–106. [Google Scholar] [CrossRef] [PubMed]
- McLure, K.G.; Takagi, M.; Kastan, M.B. NAD+ Modulates p53 DNA Binding Specificity and Function. Mol. Cell. Biol. 2004, 24, 9958–9967. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Sruthy, K.B.; Parthiban, S.; Sugunapriyadharshini, S.; George, A.; Tirupathi Pichiah, P.B.; Suman, S.; Abilash, V.G.; Arunachalam, S. Elevated Lipolysis in Adipose Tissue by Doxorubicin via PPARα Activation Associated with Hepatic Steatosis and Insulin Resistance. Eur. J. Pharmacol. 2019, 843, 162–176. [Google Scholar] [CrossRef]
- Biondo, L.A.; Junior, E.A.L.; Souza, C.O.; Cruz, M.M.; Cunha, R.D.C.; Alonso-Vale, M.I.; Oyama, L.M.; Oller Nascimento, C.M.; Pimentel, G.D.; dos Santos, R.V.T.; et al. Impact of Doxorubicin Treatment on the Physiological Functions of White Adipose Tissue. PLoS ONE 2016, 11, e0151548. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.-J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD-HS Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2019, 176, 944–945. [Google Scholar] [CrossRef]
- Liao, B.; Zhao, Y.; Wang, D.; Zhang, X.; Hao, X.; Hu, M. Nicotinamide Mononucleotide Supplementation Enhances Aerobic Capacity in Amateur Runners: A Randomized, Double-Blind Study. J. Int. Soc. Sport. Nutr. 2021, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Seol, J.; Sato, T.; Fukamizu, Y.; Sakurai, T.; Okura, T. Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2022, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, E.; Yu, N.N.; Vsevolod, V.A.; Mukhametdinova, D.; Goncharova, I.; Zelinskaya, I.; Sviridov, E.; Martynov, M.; Osipova, S.; Toropova, Y. Intravenous Nicotinamide Riboside Administration Has a Cardioprotective Effect in Chronic Doxorubicin-Induced Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 13096. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Liang, B.; Gao, Y.; Ye, T.; Li, M.; Zhang, Y.; Lu, Q.; Hu, X.; Li, H.; Yuan, Y.; et al. Nicotinic Acid Riboside Regulates Nrf-2/P62-Related Oxidative Stress and Autophagy to Attenuate Doxorubicin-Induced Cardiomyocyte Injury. Biomed Res. Int. 2022, 2022, 6293329. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell Death and Diseases Related to Oxidative stress:4-Hydroxynonenal (HNE) in the Balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margier, M.; Kuehnemann, C.; Hulo, N.; Morales, J.; Ashok Kumaar, P.V.; Cros, C.; Cannelle, H.; Charmetant, J.; Verdin, E.; Canault, M.; et al. Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice. Cells 2023, 12, 108. https://doi.org/10.3390/cells12010108
Margier M, Kuehnemann C, Hulo N, Morales J, Ashok Kumaar PV, Cros C, Cannelle H, Charmetant J, Verdin E, Canault M, et al. Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice. Cells. 2023; 12(1):108. https://doi.org/10.3390/cells12010108
Chicago/Turabian StyleMargier, Marielle, Chisaka Kuehnemann, Nicolas Hulo, Jazmin Morales, Prasanna Vadhana Ashok Kumaar, Cecile Cros, Helene Cannelle, Julie Charmetant, Eric Verdin, Matthias Canault, and et al. 2023. "Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice" Cells 12, no. 1: 108. https://doi.org/10.3390/cells12010108
APA StyleMargier, M., Kuehnemann, C., Hulo, N., Morales, J., Ashok Kumaar, P. V., Cros, C., Cannelle, H., Charmetant, J., Verdin, E., Canault, M., & Grozio, A. (2023). Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice. Cells, 12(1), 108. https://doi.org/10.3390/cells12010108






