Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success
Abstract
1. Evolutionary Dynamics Shaped the Decidual Response to Progesterone
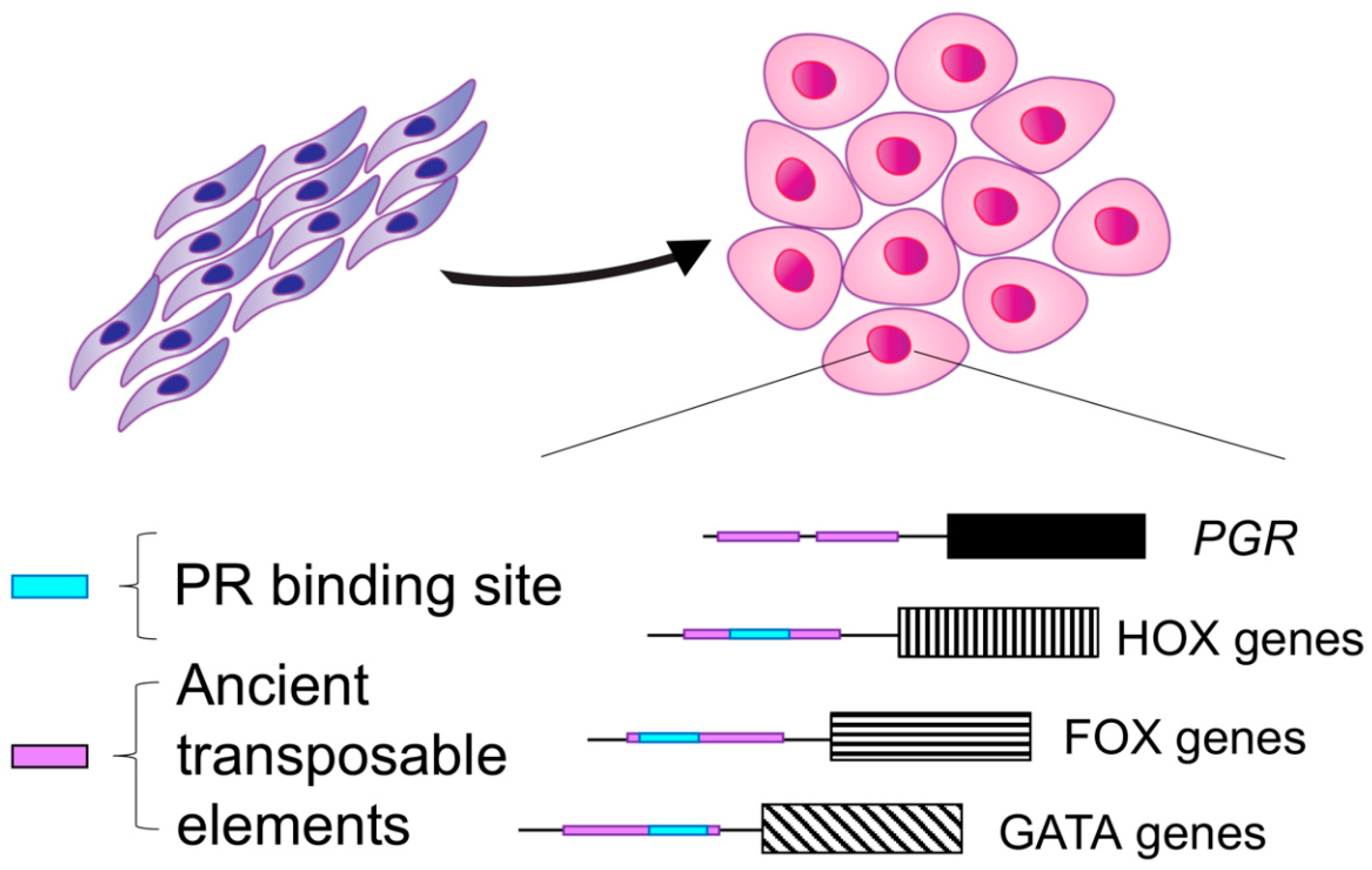
1.1. Decidualization Is a Critical Process in Placental Mammals
1.2. Evolution of Progesterone Receptor Action in the Decidua
1.3. Progesterone Receptor Variants Are Associated with Adverse Pregnancy Outcomes
2. Progesterone Receptor Action Controls Decidualization and Is Required for the Successful Establishment and Outcome of a Pregnancy
2.1. Alternative Splicing Results in Two PR Isoforms with Distinct Uterine Roles
2.2. Progesterone Receptor Knockout Mouse Model
2.3. The Genomic Landscape of the Progesterone Receptor in Reproductive Tissues
2.4. PR Binding during Endometrial Stromal Cell Decidualization
2.5. Altered PR Function Is Associated with Preeclampsia and Recurrent Pregnancy Loss
3. Progesterone Withdrawal and the Onset Parturition in Women
3.1. Characteristics of Labor Onset in Women
3.2. Myometrial PR-B Suppresses Myometrial Contractility
3.3. P4 as a Treatment for the Prevention of Preterm Labor
3.4. Upregulation of PR-A during Labor
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renfree, M.B.; Renfree, M. Monotreme and marsupial reproduction. Reprod. Fertil. Dev. 1995, 7, 1003–1020. [Google Scholar] [CrossRef] [PubMed]
- Guernsey, M.W.; Chuong, E.B.; Cornelis, G.; Renfree, M.B.; Baker, J.C. Molecular conservation of marsupial and eutherian placentation and lactation. eLife 2017, 6, e27450. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.P.; Kin, K.; Muglia, L.; Pavlicev, M. Evolution of mammalian pregnancy and the origin of the decidual stromal cell. Int. J. Dev. Biol. 2014, 58, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mossman, H.W. Structural changes in vertebrate fetal membranes associated with the adoption of viviparity. Obstet. Gynecol. Annu. 1974, 3, 7–32. [Google Scholar] [PubMed]
- Ng, S.-W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef]
- Dambaeva, S.; Bilal, M.; Schneiderman, S.; Germain, A.; Fernandez, E.; Kwak-Kim, J.; Beaman, K.; Coulam, C. Decidualization score identifies an endometrial dysregulation in samples from women with recurrent pregnancy losses and unexplained infertility. F S Rep. 2020, 2, 95–103. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Dominguez, F.; Quiñonero, A.; Diaz-Gimeno, P.; Kapidzic, M.; Gormley, M.; Ona, K.; Padilla-Iserte, P.; McMaster, M.; Genbacev, O.; et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. USA 2017, 114, E8468–E8477. [Google Scholar] [CrossRef]
- Lynch, V.J.; Nnamani, M.C.; Kapusta, A.; Brayer, K.; Plaza, S.L.; Mazur, E.C.; Emera, D.; Sheikh, S.Z.; Grutzner, F.; Bauersachs, S.; et al. Ancient Transposable Elements Transformed the Uterine Regulatory Landscape and Transcriptome during the Evolution of Mammalian Pregnancy. Cell Rep. 2015, 10, 551–561. [Google Scholar] [CrossRef]
- Marinić, M.; Mika, K.; Chigurupati, S.; Lynch, V.J. Evolutionary transcriptomics implicates HAND2 in the origins of implantation and regulation of gestation length. eLife 2021, 10, e61257. [Google Scholar] [CrossRef]
- Senft, A.D.; Macfarlan, T.S. Transposable elements shape the evolution of mammalian development. Nat. Rev. Genet. 2021, 22, 691–711. [Google Scholar] [CrossRef]
- Stadtmauer, D.J.; Wagner, G.P. Single-cell analysis of prostaglandin E2-induced human decidual cell in vitro differentiation: A minimal ancestral deciduogenic signal. Biol. Reprod. 2021, 106, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Coughlan, S.J.; McKenna, N.; Garrett, E.; Kieback, D.G.; Carney, D.N.; Headon, D.R. Ovarian carcinoma-associated TaqI restriction fragment length polymorphism in intron G of the progesterone receptor gene is due to an Alu sequence insertion. Cancer Res. 1995, 55, 2743–2745. [Google Scholar] [PubMed]
- Deininger, P.L. Alu elements: Know the SINEs. Genome Biol. 2011, 12, 236. [Google Scholar] [CrossRef]
- Agoulnik, I.; Tong, X.-W.; Fischer, D.-C.; Korner, K.; Atkinson, N.E.; Edwards, D.P.; Headon, D.R.; Weigel, N.L.; Kieback, D.G. A Germline Variation in the Progesterone Receptor Gene Increases Transcriptional Activity and May Modify Ovarian Cancer Risk. J. Clin. Endocrinol. Metab. 2004, 89, 6340–6347. [Google Scholar] [CrossRef]
- Romano, A.; Delvoux, B.; Fischer, D.-C.; Groothuis, P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J. Mol. Endocrinol. 2007, 38, 331–350. [Google Scholar] [CrossRef]
- Langmia, I.M.; Apalasamy, Y.D.; Omar, S.Z.; Mohamed, Z. Progesterone Receptor (PGR) gene polymorphism is associated with susceptibility to preterm birth. BMC Med. Genet. 2015, 16, 63. [Google Scholar] [CrossRef]
- Manuck, T.A.; Major, H.D.; Varner, M.W.; Chettier, R.; Nelson, L.; Esplin, M.S. Progesterone Receptor Genotype, Family History, and Spontaneous Preterm Birth. Obstet. Gynecol. 2010, 115, 765–770. [Google Scholar] [CrossRef]
- Zeberg, H.; Kelso, J.; Pääbo, S. The Neandertal Progesterone Receptor. Mol. Biol. Evol. 2020, 37, 2655–2660. [Google Scholar] [CrossRef]
- Li, J.; Hong, X.; Mesiano, S.; Muglia, L.J.; Wang, X.; Snyder, M.; Stevenson, D.K.; Shaw, G.M. Natural Selection Has Differentiated the Progesterone Receptor among Human Populations. Am. J. Hum. Genet. 2018, 103, 45–57. [Google Scholar] [CrossRef]
- Conneely, O.M.; Mulac-Jericevic, B.; Lydon, J.P.; De Mayo, F.J. Reproductive functions of the progesterone receptor isoforms: Lessons from knock-out mice. Mol. Cell. Endocrinol. 2001, 179, 97–103. [Google Scholar] [CrossRef]
- Wen, D.X.; Xu, Y.F.; Mais, D.E.; Goldman, M.E.; McDonnell, D.P. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol. Cell. Biol. 1994, 14, 8356–8364. [Google Scholar] [CrossRef]
- Sartorius, C.A.; Melville, M.Y.; Hovland, A.R.; Takimoto, G.S.; Horwitz, K.B.; Tung, L. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol. Endocrinol. 1994, 8, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Mulac-Jericevic, B.; Mullinax, R.A.; DeMayo, F.J.; Lydon, J.P.; Conneely, O.M. Subgroup of Reproductive Functions of Progesterone Mediated by Progesterone Receptor-B Isoform. Science 2000, 289, 1751–1754. [Google Scholar] [CrossRef]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.J.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744–9749. [Google Scholar] [CrossRef]
- Wetendorf, M.; Wu, S.-P.; Wang, X.; Creighton, C.J.; Wang, T.; Lanz, R.B.; Blok, L.; Tsai, S.Y.; Tsai, M.-J.; Lydon, J.P.; et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment†. Biol. Reprod. 2017, 96, 313–326. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Huang, Z.; Balaji, J.; Kim, T.H.; Wang, T.; Zhou, L.; Deleon, A.; Cook, M.E.; Marbrey, M.W.; et al. The role of epithelial progesterone receptor isoforms in embryo implantation. iScience 2021, 24, 103487. [Google Scholar] [CrossRef]
- Wetendorf, M.; Li, R.; Wu, S.-P.; Liu, J.; Creighton, C.J.; Wang, T.; Janardhan, K.S.; Willson, C.J.; Lanz, R.B.; Murphy, B.D.; et al. Constitutive expression of progesterone receptor isoforms promotes the development of hormone-dependent ovarian neoplasms. Sci. Signal. 2020, 13, eaaz9646. [Google Scholar] [CrossRef]
- Peavey, M.C.; Wu, S.-P.; Li, R.; Liu, J.; Emery, O.M.; Wang, T.; Zhou, L.; Wetendorf, M.; Yallampalli, C.; Gibbons, W.E.; et al. Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility. Proc. Natl. Acad. Sci. USA 2021, 118, e2011643118. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.-P.A.; Wang, T.; Adams, N.; Wu, S.-P.; Young, S.L.; Spencer, T.E.; DeMayo, F. Human Endometrial Transcriptome and Progesterone Receptor Cistrome Reveal Important Pathways and Epithelial Regulators. J. Clin. Endocrinol. Metab. 2019, 105, e1419–e1439. [Google Scholar] [CrossRef]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Franco, H.L.; Jeong, J.-W.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin. Cell Dev. Biol. 2008, 19, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, T.A.; DeMayo, F.; Rich, S.; Conneely, O.M.; O’Malley, B.W. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc. Natl. Acad. Sci. USA 1999, 96, 12021–12026. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Lai, P.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Paolino, M.; Koglgruber, R.; Cronin, S.J.F.; Uribesalgo, I.; Rauscher, E.; Harreiter, J.; Schuster, M.; Bancher-Todesca, D.; Pranjic, B.; Novatchkova, M.; et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature 2020, 589, 442–447. [Google Scholar] [CrossRef]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Updat. 2014, 21, 155–173. [Google Scholar] [CrossRef]
- Rubel, C.A.; Lanz, R.B.; Kommagani, R.; Franco, H.L.; Lydon, J.P.; DeMayo, F.J. Research Resource: Genome-Wide Profiling of Progesterone Receptor Binding in the Mouse Uterus. Mol. Endocrinol. 2012, 26, 1428–1442. [Google Scholar] [CrossRef]
- Yin, P.; Roqueiro, D.; Huang, L.; Owen, J.K.; Xie, A.; Navarro, A.; Monsivais, D.; Coon, V.J.S.; Kim, J.J.; Dai, Y.; et al. Genome-Wide Progesterone Receptor Binding: Cell Type-Specific and Shared Mechanisms in T47D Breast Cancer Cells and Primary Leiomyoma Cells. PLoS ONE 2012, 7, e29021. [Google Scholar] [CrossRef]
- Liu, S.; Yin, P.; Xu, J.; Dotts, A.J.; Kujawa, S.A.; Coon, V.J.S.; Zhao, H.; Dai, Y.; Bulun, S.E. Progesterone receptor-DNA methylation crosstalk regulates depletion of uterine leiomyoma stem cells: A potential therapeutic target. Stem Cell Rep. 2021, 16, 2099–2106. [Google Scholar] [CrossRef]
- Liu, S.; Yin, P.; Xu, J.; Dotts, A.J.; Kujawa, S.A.; Coon, V.J.S.; Zhao, H.; Shilatifard, A.; Dai, Y.; Bulun, S.E. Targeting DNA Methylation Depletes Uterine Leiomyoma Stem Cell–enriched Population by Stimulating Their Differentiation. Endocrinology 2020, 161, bqaa143. [Google Scholar] [CrossRef]
- Vaiman, D. Towards an Epigenetic Treatment of Leiomyomas? Endocrinology 2020, 161, bqaa172. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Hayashi, N.; White, J.O. Progesterone Receptor Regulates Decidual Prolactin Expression in Differentiating Human Endometrial Stromal Cells1. Endocrinology 1999, 140, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.S.; Hantak, A.M.; Stubbs, L.J.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Roles of Progesterone Receptor A and B Isoforms during Human Endometrial Decidualization. Mol. Endocrinol. 2015, 29, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Mazur, E.C.; Vasquez, Y.; Lichun, J.; Kommagani, R.; Jiang, L.; Chen, R.; Lanz, R.B.; Kovanci, E.; Gibbons, W.E.; DeMayo, F.J. Progesterone Receptor Transcriptome and Cistrome in Decidualized Human Endometrial Stromal Cells. Endocrinology 2015, 156, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, A.; Sison, C.A.M.; Yilmaz, B.D.; Coon, V.J.S.; Dyson, M.; E Bulun, S. GATA2 and Progesterone Receptor Interaction in Endometrial Stromal Cells Undergoing Decidualization. Endocrinology 2020, 161, bqaa070. [Google Scholar] [CrossRef]
- Jones, M.C.; Fusi, L.; Higham, J.H.; Abdel-Hafiz, H.; Horwitz, K.B.; Lam, E.W.-F.; Brosens, J.J. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc. Natl. Acad. Sci. USA 2006, 103, 16272–16277. [Google Scholar] [CrossRef]
- Li, X.; O’Malley, B.W. Unfolding the Action of Progesterone Receptors. J. Biol. Chem. 2003, 278, 39261–39264. [Google Scholar] [CrossRef]
- Abdel-Hafiz, H.; Dudevoir, M.L.; Horwitz, K.B. Mechanisms Underlying the Control of Progesterone Receptor Transcriptional Activity by SUMOylation. J. Biol. Chem. 2009, 284, 9099–9108. [Google Scholar] [CrossRef]
- Rodriguez, A.; Briley, S.M.; Patton, B.; Tripurani, S.K.; Rajapakshe, K.; Coarfa, C.; Rajkovic, A.; Andrieux, A.; Dejean, A.; Pangas, S.A. Loss of the E2 SUMO-conjugating enzyme Ube2i in oocytes during ovarian folliculogenesis causes infertility in mice. Development 2019, 146, 176701. [Google Scholar] [CrossRef]
- Rodriguez, A.; Pangas, S.A. Regulation of germ cell function by SUMOylation. Cell Tissue Res. 2015, 363, 47–55. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Castillo-Marco, N.; Clemente-Ciscar, M.; Cordero, T.; Muñoz-Blat, I.; Amadoz, A.; Jimenez-Almazan, J.; Monfort-Ortiz, R.; Climent, R.; Perales-Marin, A.; et al. Disrupted PGR-B and ESR1 signaling underlies defective decidualization linked to severe preeclampsia. eLife 2021, 10, e70753. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2013, 99, 63. [Google Scholar] [CrossRef] [PubMed]
- Kuon, R.; Strowitzki, T.; Sohn, C.; Daniel, V.; Toth, B. Immune profiling in patients with recurrent miscarriage. J. Reprod. Immunol. 2015, 108, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bahia, W.; Finan, R.; Al-Mutawa, M.; Haddad, A.; Soua, A.; Janhani, F.; Mahjoub, T.; Almawi, W. Genetic variation in the progesterone receptor gene and susceptibility to recurrent pregnancy loss: A case-control study. BJOG Int. J. Obstet. Gynaecol. 2017, 125, 729–735. [Google Scholar] [CrossRef]
- Aruna, M.; Nagaraja, T.; Andal, S.; Tarakeswari, S.; Sirisha, P.V.S.; Reddy, A.G.; Thangaraj, K.; Singh, L.; Reddy, B.M. Role of Progesterone Receptor Polymorphisms in the Recurrent Spontaneous Abortions: Indian Case. PLoS ONE 2010, 5, e8712. [Google Scholar] [CrossRef]
- Su, M.-T.; Lee, I.-W.; Chen, Y.-C.; Kuo, P.-L. Association of progesterone receptor polymorphism with idiopathic recurrent pregnancy loss in Taiwanese Han population. J. Assist. Reprod. Genet. 2010, 28, 239–243. [Google Scholar] [CrossRef][Green Version]
- Cramer, D.W.; Hornstein, M.D.; McShane, P.; Powers, R.; Lescault, P.J.; Vitonis, A.F.; De Vivo, I. Human progesterone receptor polymorphisms and implantation failure during in vitro fertilization. Am. J. Obstet. Gynecol. 2003, 189, 1085–1092. [Google Scholar] [CrossRef]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef]
- Csapo, A.I.; Pinto-Dantas, C.A. The effect of progesterone on the human uterus. Proc. Natl. Acad. Sci. USA 1965, 54, 1069–1076. [Google Scholar] [CrossRef]
- O’Malley, B.W.; Sherman, M.R.; Toft, D.O. Progesterone “Receptors” in the Cytoplasm and Nucleus of Chick Oviduct Target Tissue. Proc. Natl. Acad. Sci. USA 1970, 67, 501–508. [Google Scholar] [CrossRef]
- Mesiano, S. Myometrial Progesterone responsiveness and the Control of Human Parturition. J. Soc. Gynecol. Investig. 2004, 11, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-P.; DeMayo, F.J. Progesterone Receptor Signaling in Uterine Myometrial Physiology and Preterm Birth. Curr. Top Dev. Biol. 2017, 125, 171–190. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2018, 7, e37–e46. [Google Scholar] [CrossRef]
- Tan, H.; Yi, L.; Rote, N.S.; Hurd, W.W.; Mesiano, S. Progesterone Receptor-A and -B Have Opposite Effects on Proinflammatory Gene Expression in Human Myometrial Cells: Implications for Progesterone Actions in Human Pregnancy and Parturition. J. Clin. Endocrinol. Metab. 2012, 97, E719–E730. [Google Scholar] [CrossRef] [PubMed]
- Sykes, L.; Bennett, P.R. Efficacy of progesterone for prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 126–136. [Google Scholar] [CrossRef]
- Romero, R.; Stanczyk, F.Z. Progesterone is not the same as 17α-hydroxyprogesterone caproate: Implications for obstetrical practice. Am. J. Obstet. Gynecol. 2013, 208, 421–426. [Google Scholar] [CrossRef]
- Shennan, A.; Suff, N.; Simpson, J.L.; Jacobsson, B.; Mol, B.W.; Grobman, W.A.; Norman, J.; Bianchi, A.; Munjanja, S.; González, C.M.V.; et al. FIGO good practice recommendations on progestogens for prevention of preterm delivery. Int. J. Gynecol. Obstet. 2021, 155, 16–18. [Google Scholar] [CrossRef]
- Meis, P.J.; Klebanoff, M.; Thom, E.; Dombrowski, M.P.; Sibai, B.; Moawad, A.H.; Spong, C.Y.; Hauth, J.C.; Miodovnik, M.; Varner, M.; et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-Hydroxyprogesterone Caproate. N. Engl. J. Med. 2003, 348, 2379–2385. [Google Scholar] [CrossRef]
- Blackwell, S.C.; Gyamfi-Bannerman, C.; Biggio, J.R., Jr.; Chauhan, S.P.; Hughes, B.L.; Louis, J.M.; Manuck, T.A.; Miller, H.S.; Das, A.F.; Saade, G.R.; et al. 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): A Multicenter, International, Randomized Double-Blind Trial. Am. J. Perinatol. 2020, 37, 127–136. [Google Scholar] [CrossRef]
- Norman, J.E.; Marlow, N.; McConnachie, A.; Petrou, S.; Sebire, N.J.; Lavender, T.; Whyte, S.; Norrie, J.; Messow, C.-M.; Shennan, A.; et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): A multicentre, randomised, double-blind trial. Lancet 2016, 387, 2106–2116. [Google Scholar] [CrossRef]
- Norman, J. Progesterone and preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Jones, L.; Flenady, V.; Cincotta, R.; Crowther, C.A. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst. Rev. 2013, 7, CD004947. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.E.; Mackenzie, F.; Owen, P.; Mactier, H.; Hanretty, K.; Cooper, S.; Calder, A.; Mires, G.; Danielian, P.; Sturgiss, S.; et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): A randomised, double-blind, placebo-controlled study and meta-analysis. Lancet 2009, 373, 2034–2040. [Google Scholar] [CrossRef]
- Rehal, A.; Benkő, Z.; Matallana, C.D.P.; Syngelaki, A.; Janga, D.; Cicero, S.; Akolekar, R.; Singh, M.; Chaveeva, P.; Burgos, J.; et al. Early vaginal progesterone versus placebo in twin pregnancies for the prevention of spontaneous preterm birth: A randomized, double-blind trial. Am. J. Obstet. Gynecol. 2020, 224, 86.e1–86.e19. [Google Scholar] [CrossRef] [PubMed]
- Rode, L.; Klein, K.; Nicolaides, K.; Krampl-Bettelheim, E.; Tabor, A.; The PREDICT Group. Prevention of preterm delivery in twin gestations (PREDICT): A multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet. Gynecol. 2011, 38, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Thekkeveedu, R.K.; Dankhara, N.; Desai, J.; Klar, A.L.; Patel, J. Outcomes of multiple gestation births compared to singleton: Analysis of multicenter KID database. Matern. Health Neonatol. Perinatol. 2021, 7, 15. [Google Scholar] [CrossRef]
- Ke, W.; Chen, C.; Luo, H.; Tang, J.; Zhang, Y.; Gao, W.; Yang, X.; Tian, Z.; Chang, Q.; Liang, Z. Histone Deacetylase 1 Regulates the Expression of Progesterone Receptor A During Human Parturition by Occupying the Progesterone Receptor A Promoter. Reprod. Sci. 2016, 23, 955–964. [Google Scholar] [CrossRef]
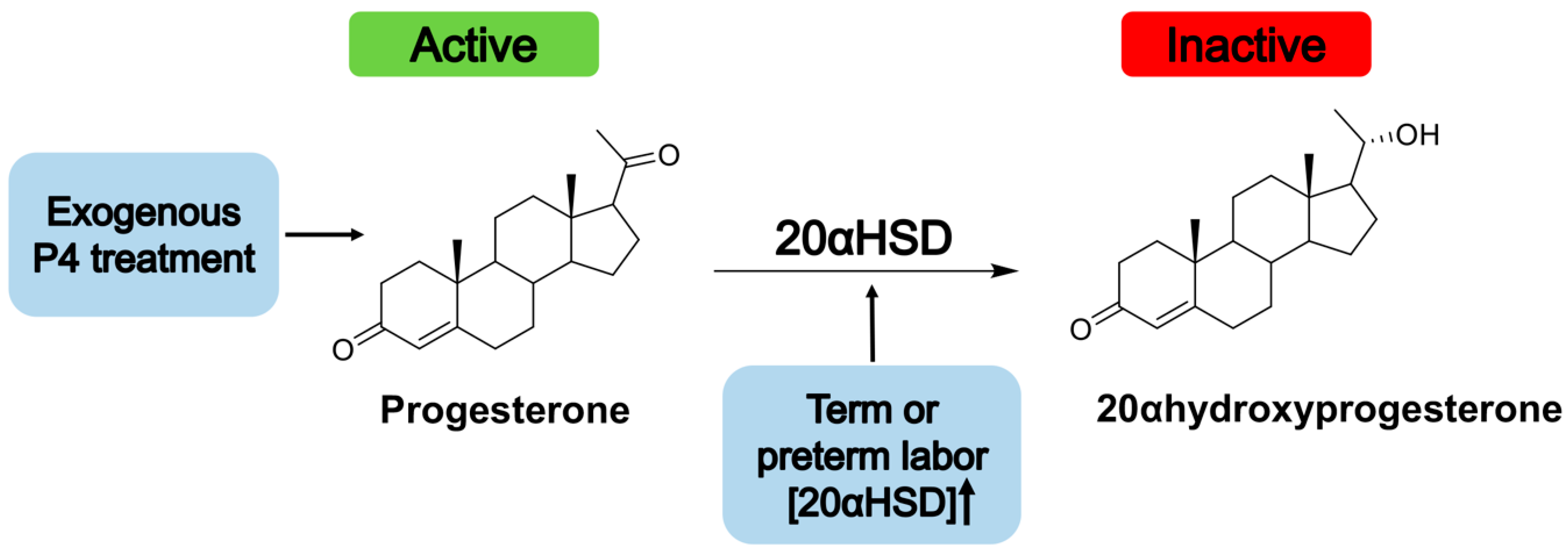
- Piekorz, R.P.; Gingras, S.; Hoffmeyer, A.; Ihle, J.N.; Weinstein, Y. Regulation of Progesterone Levels during Pregnancy and Parturition by Signal Transducer and Activator of Transcription 5 and 20α-Hydroxysteroid Dehydrogenase. Mol. Endocrinol. 2005, 19, 431–440. [Google Scholar] [CrossRef]
- Williams, K.C.; Renthal, N.E.; Condon, J.C.; Gerard, R.D.; Mendelson, C.R. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc. Natl. Acad. Sci. USA 2012, 109, 7529–7534. [Google Scholar] [CrossRef]
- Nadeem, L.; Balendran, R.; Dorogin, A.; Mesiano, S.; Shynlova, O.; Lye, S.J. Pro-inflammatory signals induce 20α-HSD expression in myometrial cells: A key mechanism for local progesterone withdrawal. J. Cell. Mol. Med. 2021, 25, 6773–6785. [Google Scholar] [CrossRef]
- Shynlova, O.; Nadeem, L.; Dorogin, A.; Mesiano, S.; Lye, S.J. The selective progesterone receptor modulator-promegestone-delays term parturition and prevents systemic inflammation-mediated preterm birth in mice. Am. J. Obstet. Gynecol. 2021, 226, 249.e1–249.e21. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cope, D.I.; Monsivais, D. Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success. Cells 2022, 11, 1474. https://doi.org/10.3390/cells11091474
Cope DI, Monsivais D. Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success. Cells. 2022; 11(9):1474. https://doi.org/10.3390/cells11091474
Chicago/Turabian StyleCope, Dominique I., and Diana Monsivais. 2022. "Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success" Cells 11, no. 9: 1474. https://doi.org/10.3390/cells11091474
APA StyleCope, D. I., & Monsivais, D. (2022). Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success. Cells, 11(9), 1474. https://doi.org/10.3390/cells11091474





