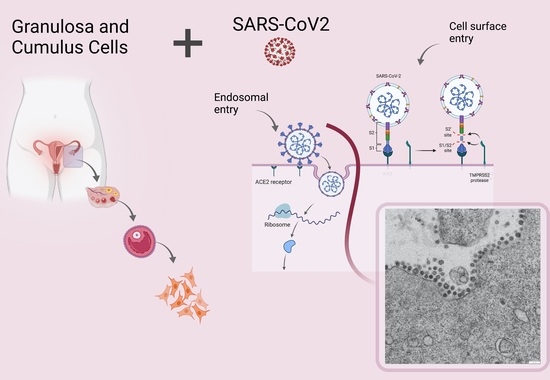
SARS-CoV-2 Infection of Human Ovarian Cells: A Potential Negative Impact on Female Fertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Human Biological Sample Collection
2.2. Granulosa and Cumulus Cells Isolation
2.3. SARS-CoV-2 Culture and In Vitro Infection
2.4. Infection of Granulosa and Cumulus Cells
2.5. Culture Supernatant Titration by TCID50 Assay
2.6. Detection of SARS-CoV-2 RNA
2.7. qRT-PCR
2.8. Immunofluorescence
2.9. Western Blotting
2.10. Transmission (TEM) and Immuno-Electron Microscopy (IMEM)
2.11. Statistical Analysis
3. Results
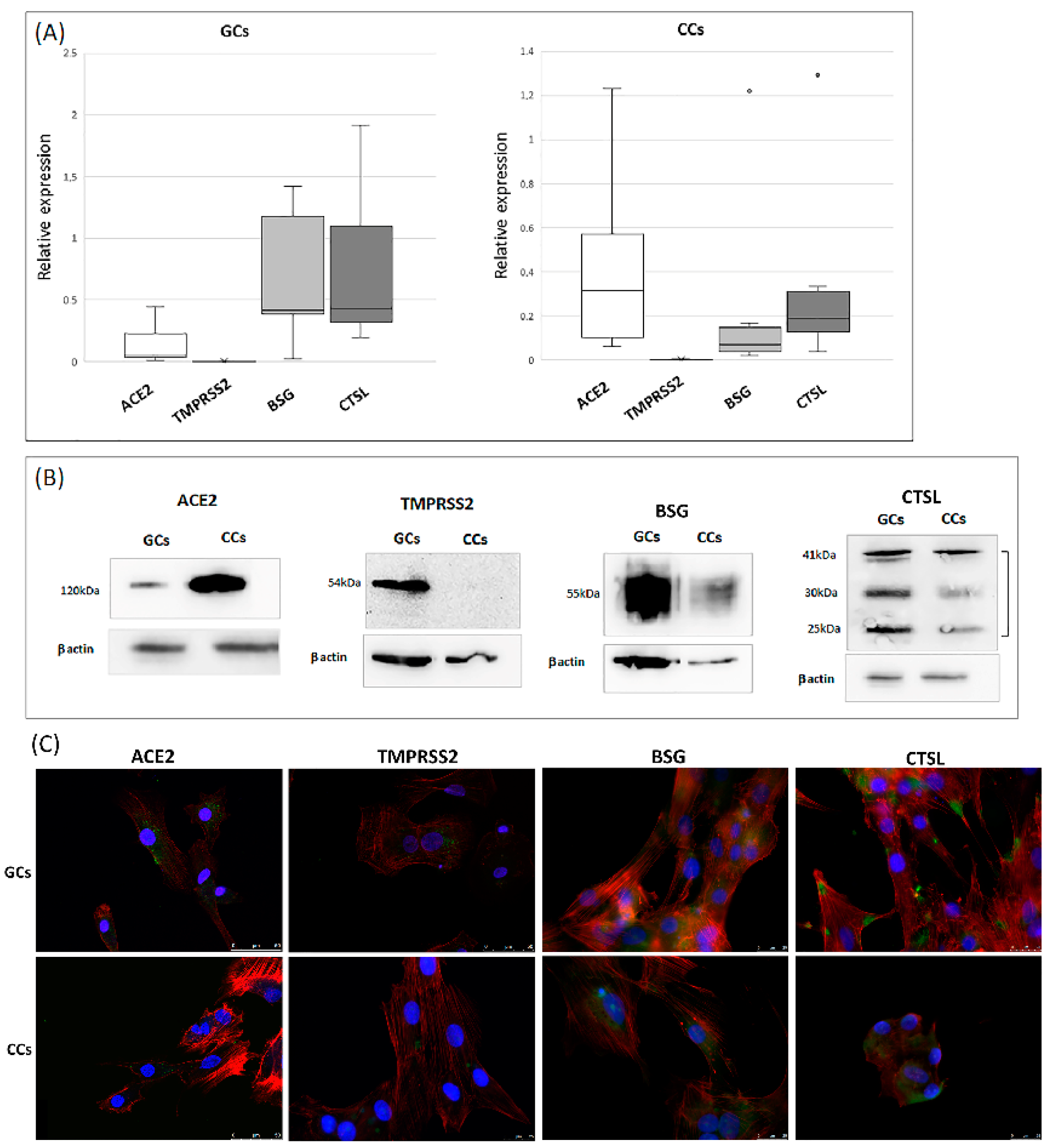
3.1. ACE2 Receptor and TMPRSS2, BSG, CTSL Accessory Proteases Are Expressed in Human Granulosa and Cumulus Cells
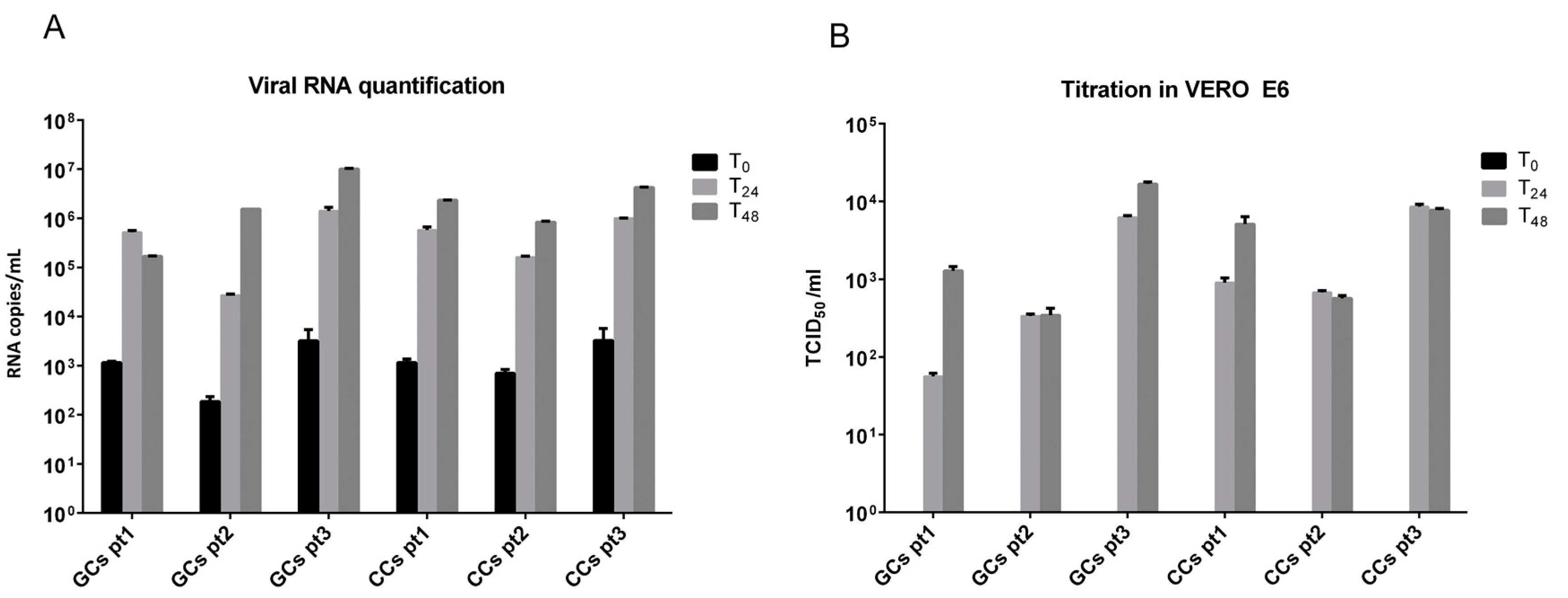
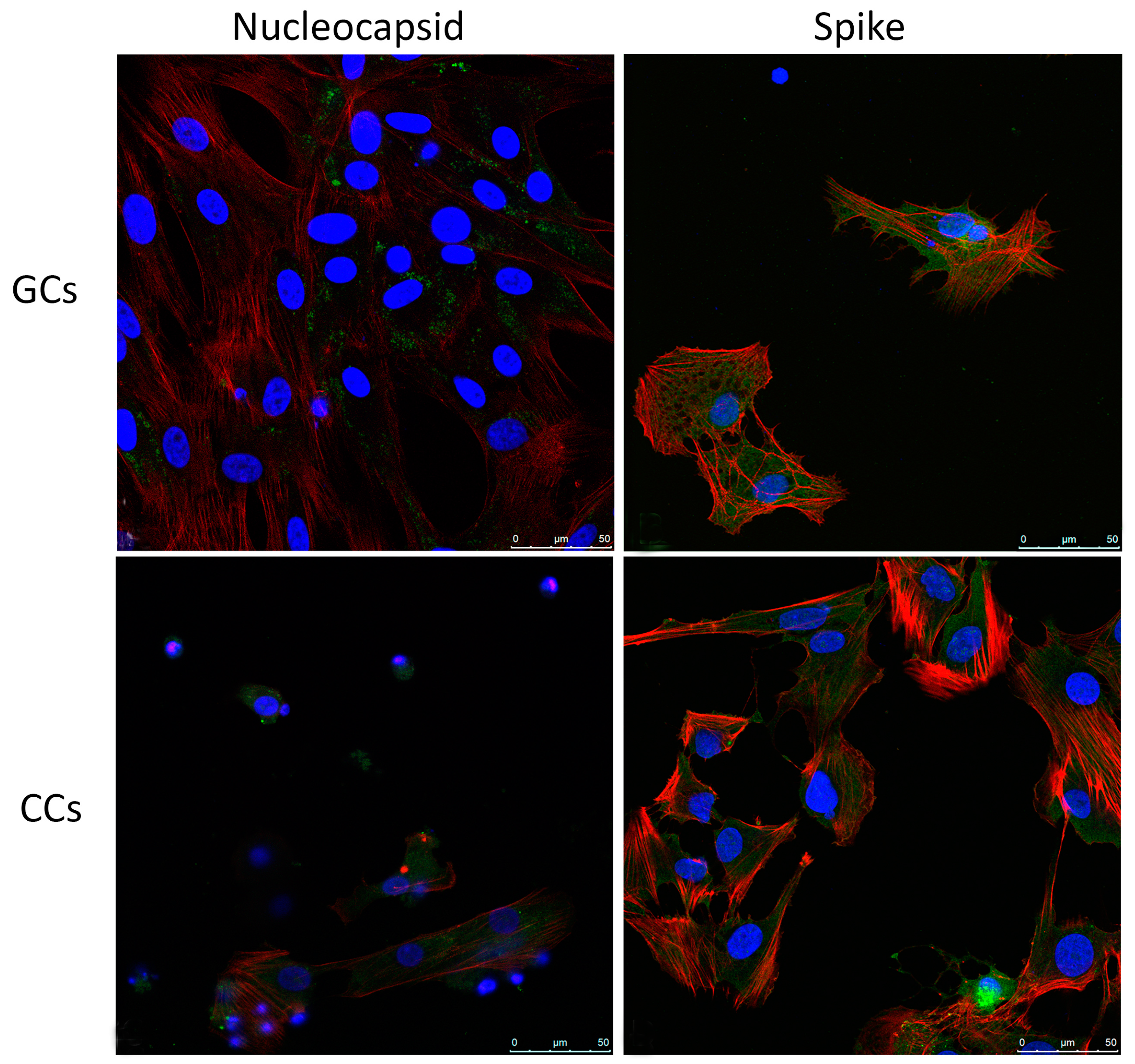
3.2. SARS-CoV-2 Infects Human Granulosa and Cumulus Cells
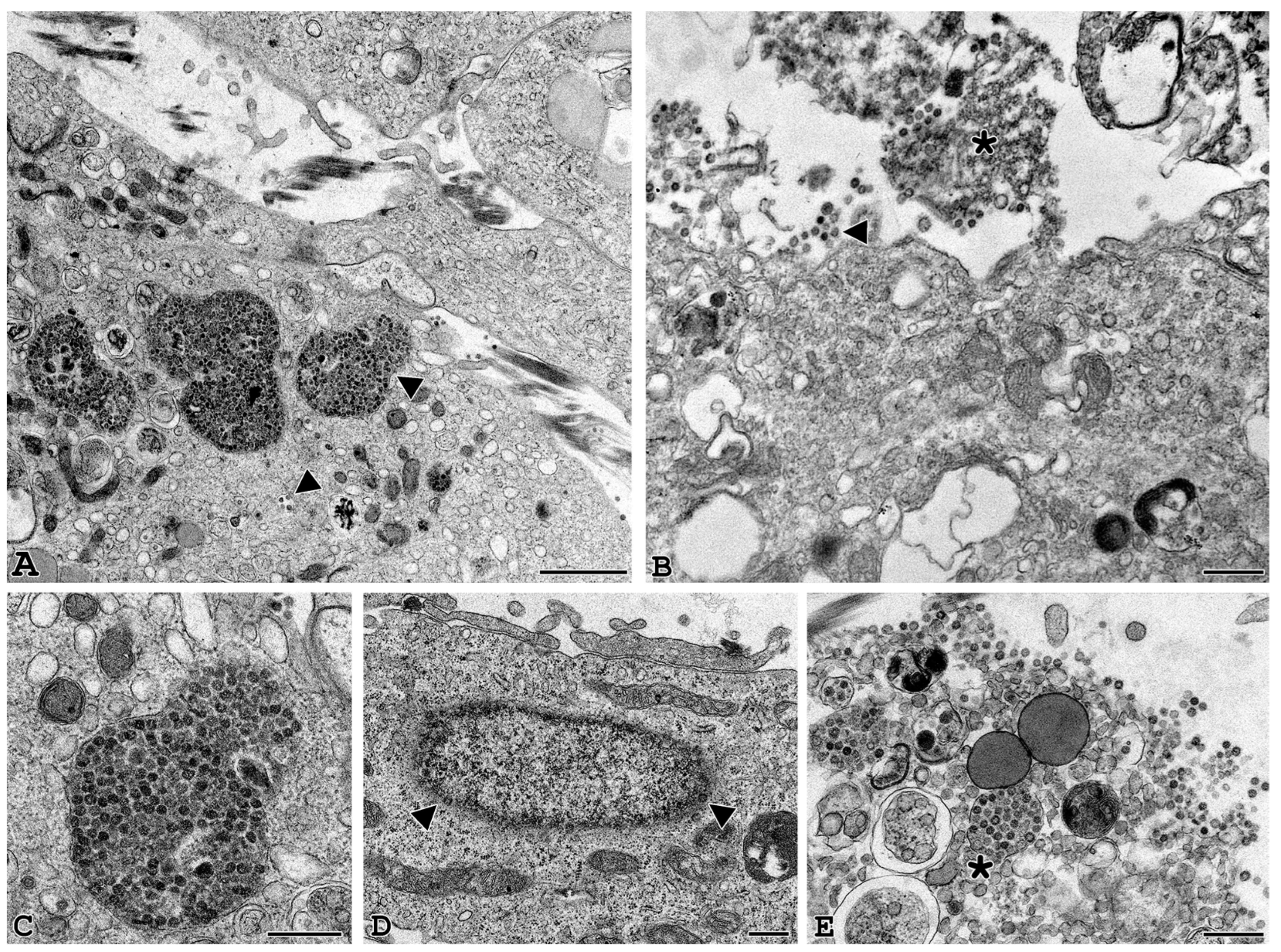
3.3. Electron Microscopy Identification of SARS-CoV-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 9 December 2021).
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Lukassen, S.; Chua, R.L.; Trefzer, T.; Kahn, N.C.; Schneider, M.A.; Muley, T.; Winter, H.; Meister, M.; Veith, C.; Boots, A.W.; et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020, 39, e105114. [Google Scholar] [CrossRef]
- Orooji, Y.; Sohrabi, H.; Hemmat, N.; Oroojalian, F.; Baradaran, B.; Mokhtarzadeh, A.; Mohaghegh, M.; Karimi-Maleh, H. An overview on SARS-CoV-2 (COVID-19) and other human coronaviruses and their detection capability via amplification assay, chemical sensing, biosensing, immunosensing, and clinical assays. Nano-Micro Lett. 2020, 13, 18. [Google Scholar] [CrossRef]
- Shilts, J.; Crozier, T.W.M.; Greenwood, E.J.D.; Lehner, P.J.; Wright, G.J. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci. Rep. 2021, 11, 413. [Google Scholar] [CrossRef]
- Tu, Y.-F.; Chien, C.-S.; Yarmishyn, A.A.; Lin, Y.-Y.; Luo, Y.-H.; Lin, Y.-T.; Lai, W.-Y.; Yang, D.-M.; Chou, S.-J.; Yang, Y.-P.; et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Run-Qian, L.; Hao-Ran, W.; Hao-Ran, C.; Ya-Bin, L.; Yang, G.; Fei, C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020, 26, 367–373. [Google Scholar] [CrossRef]
- Pereira, V.M.; Reis, F.M.; Santos, R.A.S.; Cassali, G.D.; Santos, S.H.S.; Honorato-Sampaio, K.; dos Reis, A.M. Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod. Sci. 2009, 16, 1165–1174. [Google Scholar] [CrossRef]
- Chang, H.-M.; Qiao, J.; Leung, P.C.K. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum. Reprod. Update 2016, 23, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, L.K.; Rodrigues, P.; Limback, D. Structural aspects of oocyte maturation. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 176–182. ISBN 978-0-12-815145-7. [Google Scholar]
- Luddi, A.; Gori, M.; Marrocco, C.; Capaldo, A.; Pavone, V.; Bianchi, L.; Boschi, L.; Morgante, G.; Piomboni, P.; de Leo, V. Matrix metalloproteinases and their inhibitors in human cumulus and granulosa cells as biomarkers for oocyte quality estimation. Fertil. Steril. 2018, 109, 930–939.e3. [Google Scholar] [CrossRef] [Green Version]
- Valerio, D.; Luddi, A.; De Leo, V.; Labella, D.; Longobardi, S.; Piomboni, P. SA1/SA2 cohesion proteins and SIRT1-NAD+ deacetylase modulate telomere homeostasis in cumulus cells and are eligible biomarkers of ovarian aging. Hum. Reprod. 2018, 33, 887–894. [Google Scholar] [CrossRef]
- Lai, A.; Bergna, A.; Menzo, S.; Zehender, G.; Caucci, S.; Ghisetti, V.; Rizzo, F.; Maggi, F.; Cerutti, F.; Giurato, G.; et al. Circulating SARS-CoV-2 variants in Italy, October 2020–March 2021. Virol. J. 2021, 18, 168. [Google Scholar] [CrossRef]
- Vicenti, I.; Martina, M.G.; Boccuto, A.; De Angelis, M.; Giavarini, G.; Dragoni, F.; Marchi, S.; Trombetta, C.M.; Crespan, E.; Maga, G.; et al. System-oriented optimization of multi-target 2,6-diaminopurine derivatives: Easily accessible broad-spectrum antivirals active against flaviviruses, influenza virus and SARS-CoV-2. Eur. J. Med. Chem. 2021, 224, 113683. [Google Scholar] [CrossRef]
- Lai, A. Decreased neutralization of the Eta SARS-CoV-2 variant by sera of previously infected and uninfected vaccinated individuals. J. Infect. 2021, 84, 94–118. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent end points. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Governini, L.; Marrocco, C.; Semplici, B.; Pavone, V.; Belmonte, G.; Luisi, S.; Petraglia, F.; Luddi, A.; Piomboni, P. Extracellular matrix remodeling and inflammatory pathway in human endometrium: Insights from uterine leiomyomas. Fertil. Steril. 2021, 116, 1404–1414. [Google Scholar] [CrossRef]
- Neuman, B.W.; Adair, B.D.; Yoshioka, C.; Quispe, J.D.; Orca, G.; Kuhn, P.; Milligan, R.A.; Yeager, M.; Buchmeier, M.J. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006, 80, 7918–7928. [Google Scholar] [CrossRef] [Green Version]
- Stanley, K.E.; Thomas, E.; Leaver, M.; Wells, D. Coronavirus disease-19 and fertility: Viral host entry protein expression in male and female reproductive tissues. Fertil. Steril. 2020, 114, 33–43. [Google Scholar] [CrossRef]
- Entezami, F.; Samama, M.; Dejucq-Rainsford, N.; Bujan, L. SARS-CoV-2 and human reproduction: An open question. EClinicalMedicine 2020, 25, 100473. [Google Scholar] [CrossRef]
- Rajput, S.K.; Logsdon, D.M.; Kile, B.; Engelhorn, H.J.; Goheen, B.; Khan, S.; Swain, J.; McCormick, S.; Schoolcraft, W.B.; Yuan, Y.; et al. Human eggs, zygotes, and embryos express the receptor angiotensin 1-converting enzyme 2 and transmembrane serine protease 2 protein necessary for severe acute respiratory syndrome coronavirus 2 infection. F S Sci. 2021, 2, 33–42. [Google Scholar] [CrossRef]
- Essahib, W.; Verheyen, G.; Tournaye, H.; Van de Velde, H. SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J. Assist. Reprod. Genet. 2020, 37, 2657–2660. [Google Scholar] [CrossRef]
- Beumer, J.; Geurts, M.H.; Lamers, M.M.; Puschhof, J.; Zhang, J.; van der Vaart, J.; Mykytyn, A.Z.; Breugem, T.I.; Riesebosch, S.; Schipper, D.; et al. A CRISPR/Cas9 genetically engineered organoid biobank reveals essential host factors for coronaviruses. Nat. Commun. 2021, 12, 5498. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ni, H.; Ma, X.-H.; Xu, L.-B.; Kadomatsu, K.; Muramatsu, T.; Yang, Z.-M. Basigin expression and regulation in mouse ovary during the sexual maturation and development of corpus luteum. Mol. Reprod. Dev. 2004, 68, 135–141. [Google Scholar] [CrossRef]
- García, V.; Kohen, P.; Maldonado, C.; Sierralta, W.; Muñoz, A.; Villarroel, C.; Strauss, J.F.; Devoto, L. Transient expression of progesterone receptor and cathepsin-l in human granulosa cells during the periovulatory period. Fertil. Steril. 2012, 97, 707–713.e1. [Google Scholar] [CrossRef]
- Murgolo, N.; Therien, A.G.; Howell, B.; Klein, D.; Koeplinger, K.; Lieberman, L.A.; Adam, G.C.; Flynn, J.; McKenna, P.; Swaminathan, G.; et al. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLoS Pathog. 2021, 17, e1009225. [Google Scholar] [CrossRef]
- Laue, M.; Kauter, A.; Hoffmann, T.; Möller, L.; Michel, J.; Nitsche, A. Morphometry of SARS-CoV and SARS-CoV-2 particles in ultrathin plastic sections of infected vero cell cultures. Sci. Rep. 2021, 11, 3515. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Ren, X.; Hu, J.; Li, Z.; Long, R.; Xi, Q.; Zhu, L.; Jin, L. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: A retrospective cohort study. EClinicalMedicine 2021, 38, 101013. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Wang, T.R.; Willis, M.D.; Yland, J.; Crowe, H.M.; Geller, R.J.; Willis, S.K.; Perkins, R.B.; et al. A Prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am. J. Epidemiol. 2022, kwac011. [Google Scholar] [CrossRef]
- Carp-Veliscu, A.; Mehedintu, C.; Frincu, F.; Bratila, E.; Rasu, S.; Iordache, I.; Bordea, A.; Braga, M. The effects of SARS-CoV-2 infection on female fertility: A review of the literature. Int. J. Environ. Res. Public Health 2022, 19, 984. [Google Scholar] [CrossRef] [PubMed]
- Boudry, L.; Essahib, W.; Mateizel, I.; Velde, H.V.D.; Geyter, D.D.; Pierard, D.; Uvin, V.; Tournaye, H.; Brucker, M.D. Undetectable viral RNA in follicular fluid (FF), cumulus cells (CC) and endometrial tissue in SARS-CoV–2 positive patients. Hum. Reprod. 2021, 36 (Suppl. S1), 274. [Google Scholar] [CrossRef]
- Barragan, M.; Guillén, J.J.; Martin-Palomino, N.; Rodriguez, A.; Vassena, R. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum. Reprod. 2021, 36, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Herrero, Y.; Pascuali, N.; Velázquez, C.; Oubiña, G.; Hauk, V.; de Zúñiga, I.; Peña, M.G.; Martínez, G.; Lavolpe, M.; Veiga, F.; et al. SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2022, 1868, 166295. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luongo, F.P.; Dragoni, F.; Boccuto, A.; Paccagnini, E.; Gentile, M.; Canosi, T.; Morgante, G.; Luddi, A.; Zazzi, M.; Vicenti, I.; et al. SARS-CoV-2 Infection of Human Ovarian Cells: A Potential Negative Impact on Female Fertility. Cells 2022, 11, 1431. https://doi.org/10.3390/cells11091431
Luongo FP, Dragoni F, Boccuto A, Paccagnini E, Gentile M, Canosi T, Morgante G, Luddi A, Zazzi M, Vicenti I, et al. SARS-CoV-2 Infection of Human Ovarian Cells: A Potential Negative Impact on Female Fertility. Cells. 2022; 11(9):1431. https://doi.org/10.3390/cells11091431
Chicago/Turabian StyleLuongo, Francesca P., Filippo Dragoni, Adele Boccuto, Eugenio Paccagnini, Mariangela Gentile, Tamara Canosi, Giuseppe Morgante, Alice Luddi, Maurizio Zazzi, Ilaria Vicenti, and et al. 2022. "SARS-CoV-2 Infection of Human Ovarian Cells: A Potential Negative Impact on Female Fertility" Cells 11, no. 9: 1431. https://doi.org/10.3390/cells11091431
APA StyleLuongo, F. P., Dragoni, F., Boccuto, A., Paccagnini, E., Gentile, M., Canosi, T., Morgante, G., Luddi, A., Zazzi, M., Vicenti, I., & Piomboni, P. (2022). SARS-CoV-2 Infection of Human Ovarian Cells: A Potential Negative Impact on Female Fertility. Cells, 11(9), 1431. https://doi.org/10.3390/cells11091431