Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmid and Bacterial Transformation
2.3. Preparation of Physiactisome
2.4. Treatment with Physiactisome
2.5. Isolation of Total RNA
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction
2.7. Transmission Electron Microscopy in 3D Cultures
2.8. Isolation of C2C12 EVs
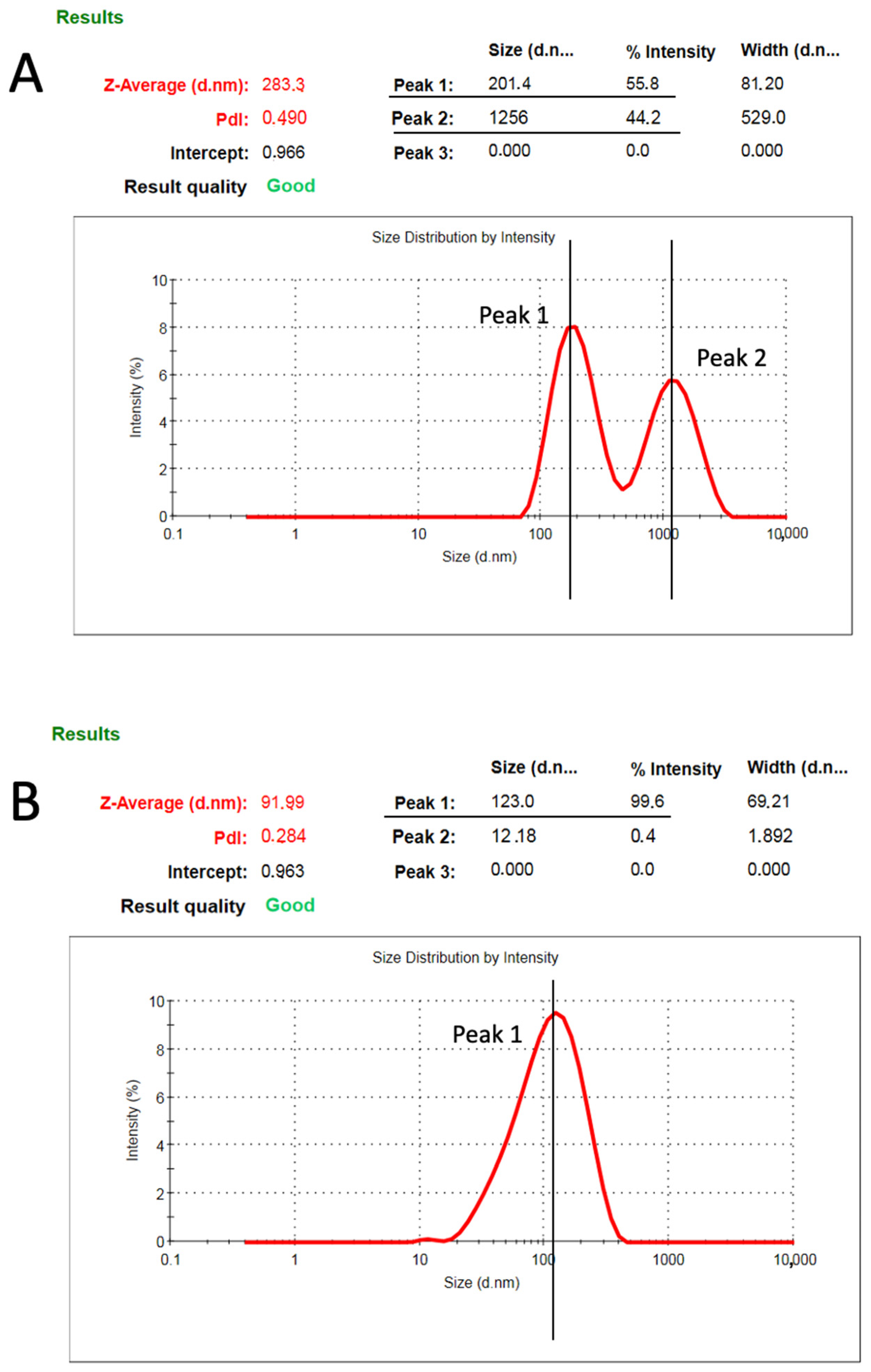
2.9. Size Distribution of C2C12 EVs
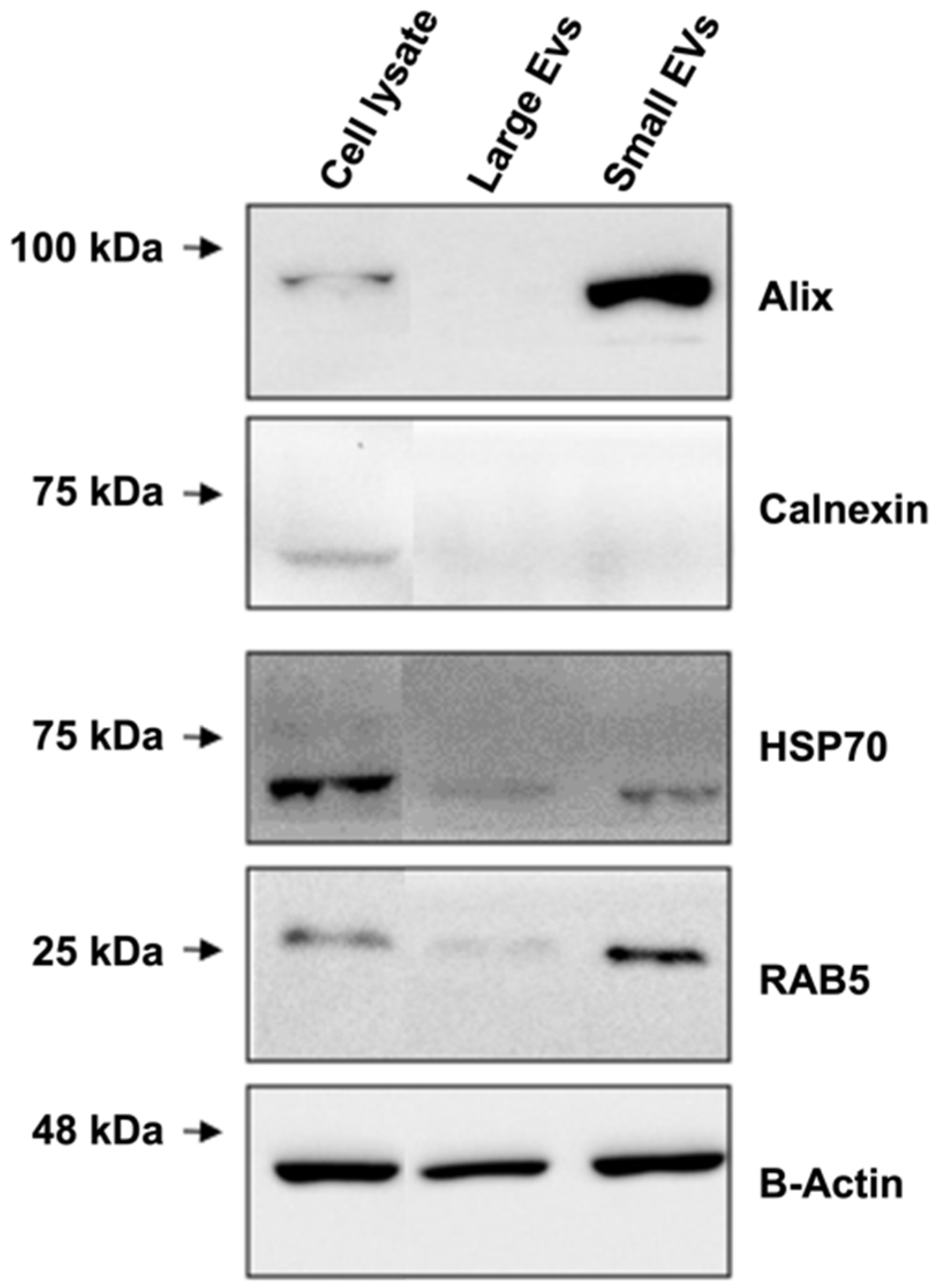
2.10. Western Blotting
2.11. Recombinant Hsp60
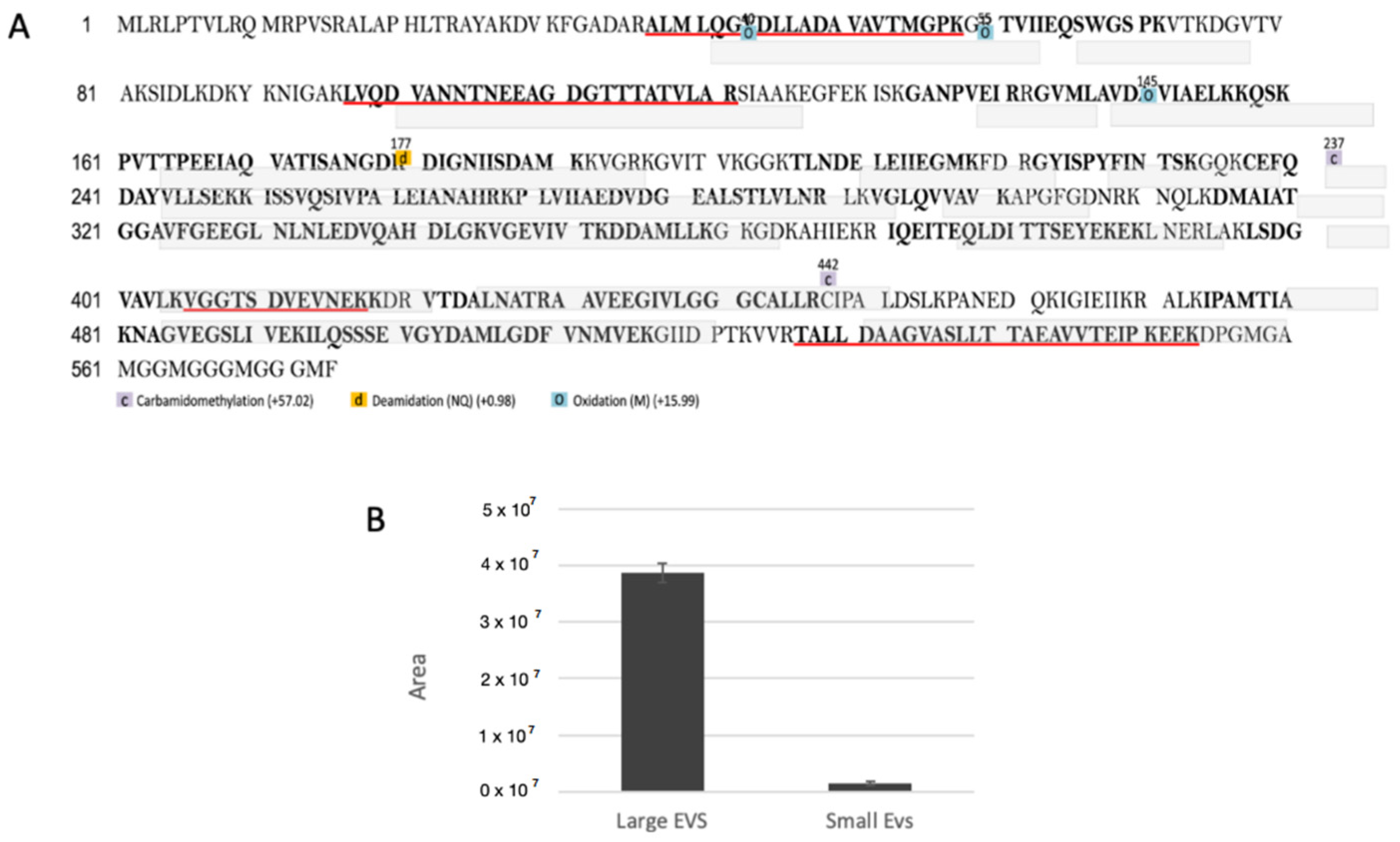
2.12. Proteomic Analysis of Small and Large EVs
2.13. Liquid Chromatography and Tandem Mass Spectrometry
2.14. Database Searches, Protein Identification, and Label-Free Quantification Analysis
2.15. Interaction Analysis
2.16. Statistical Analysis
3. Results
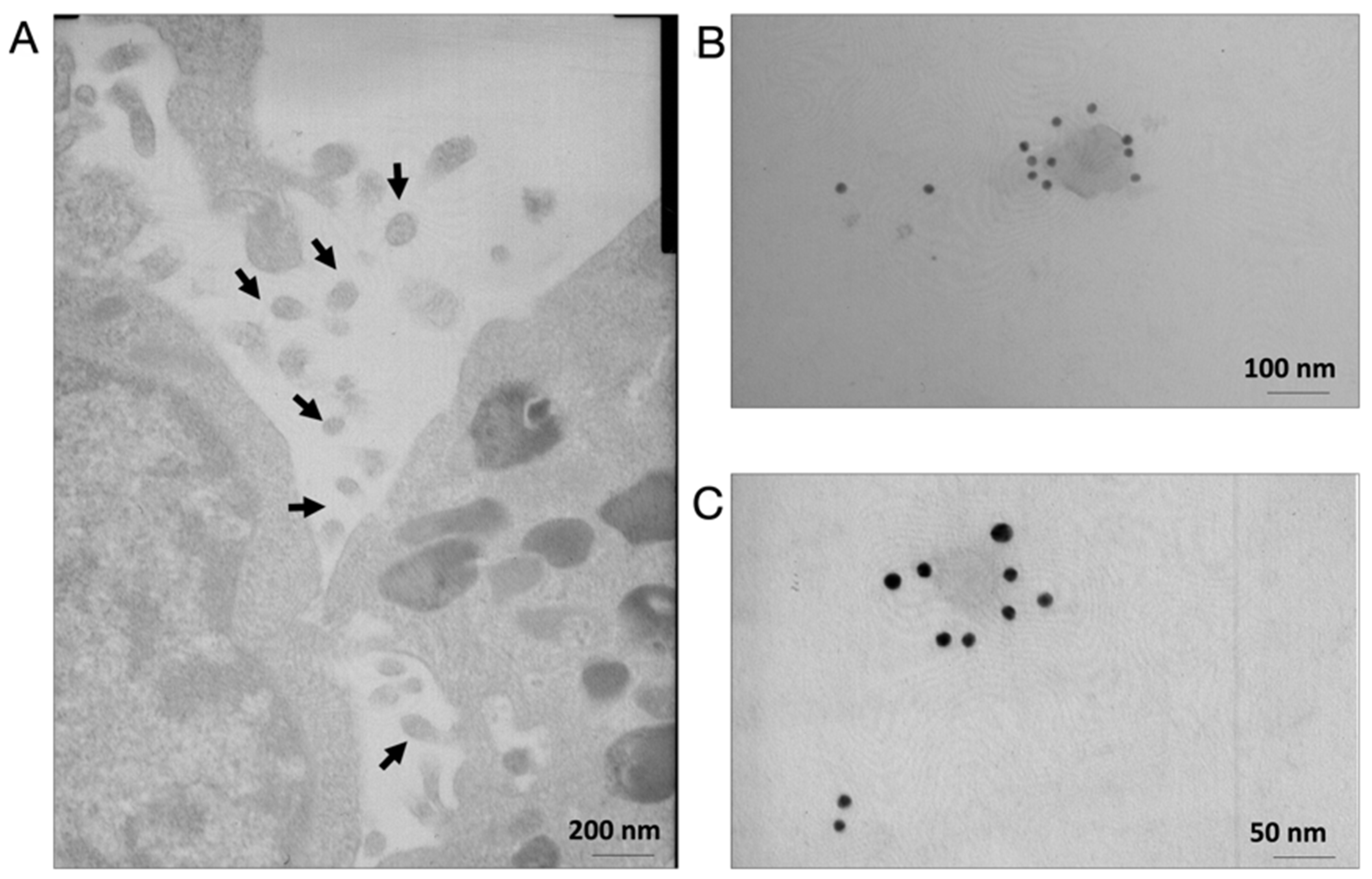
3.1. C2C12 Cell Lines Secrete Nanovesicles
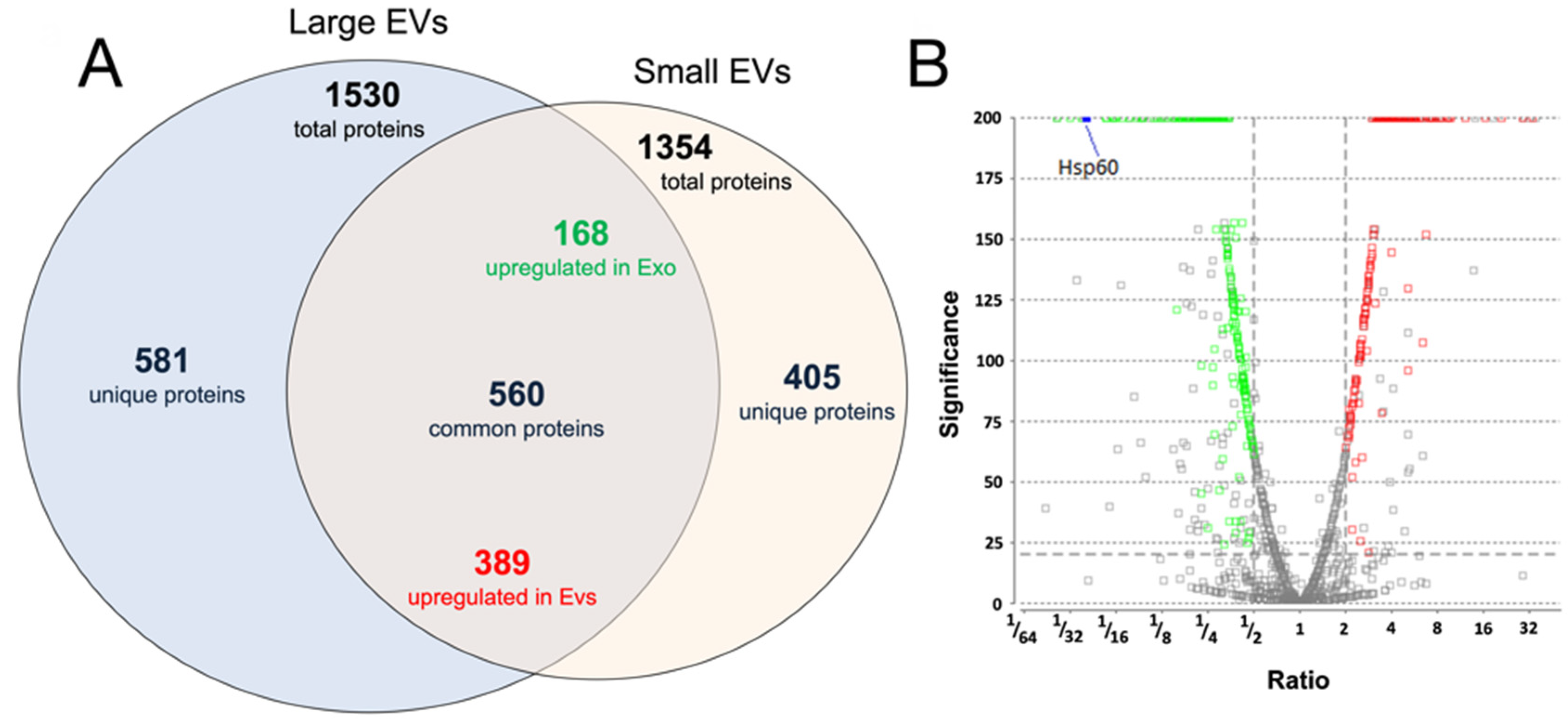
3.2. Proteomic Analysis of Small and Large EVs
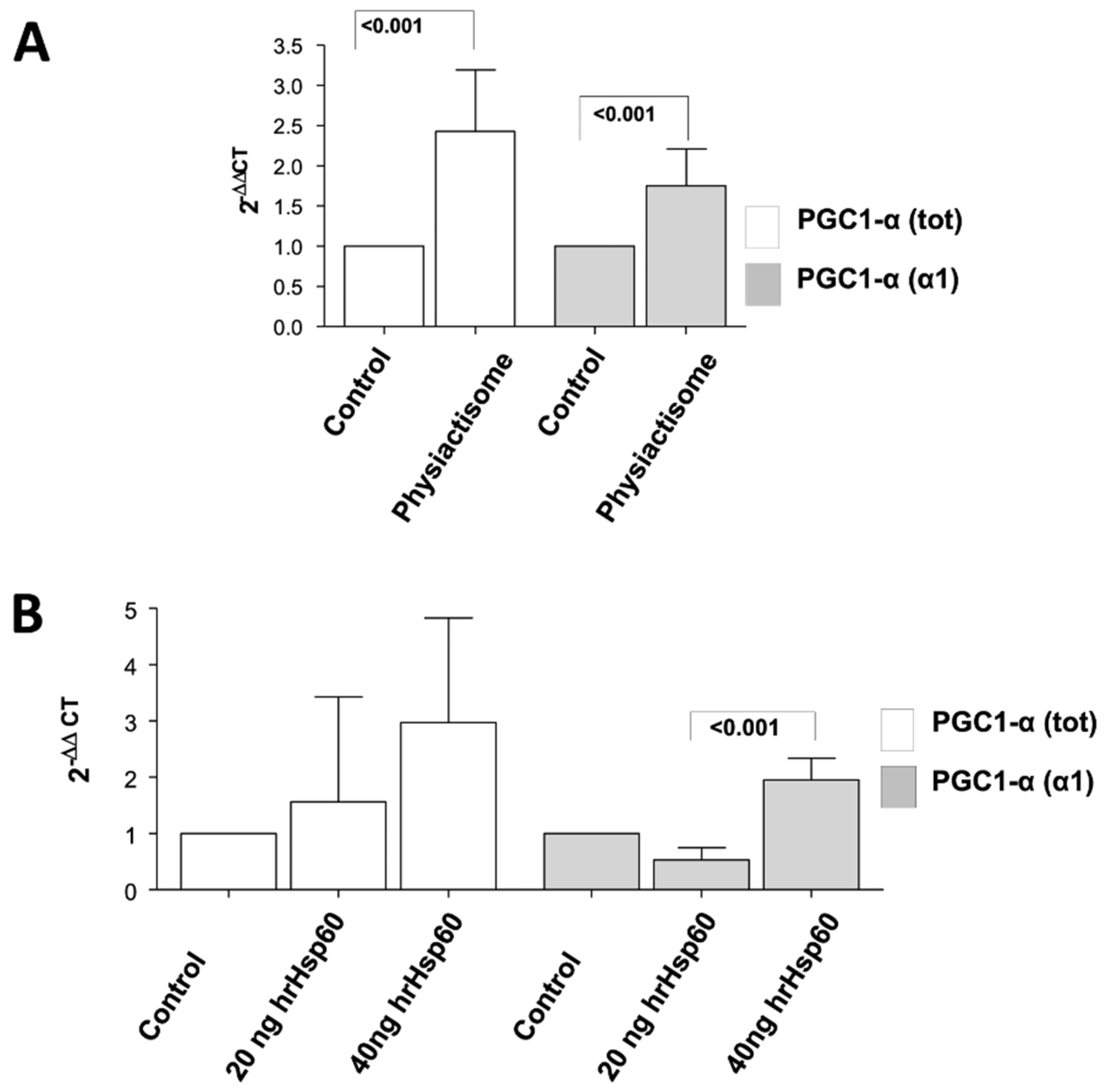
3.3. Development and Characterisation of Physiactisome
3.4. Effect of Physiactisome on the Expression of PGC-1α Isoform 1
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Bokshan, S.L.; Marcaccio, S.E.; DePasse, J.M.; Daniels, A.H. Diagnostic Criteria and Clinical Outcomes in Sarcopenia Research: A Literature Review. J. Clin. Med. 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H., Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Testa, G.; Liguori, I.; Papillo, M.; Flocco, V.; Panicara, V.; Galizia, G.; Della-Morte, D.; Gargiulo, G.; Cacciatore, F.; et al. Sarcopenia and Heart Failure. Nutrients 2020, 12, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.P.; Chen, Y.; Li, A.S.; Reid, M.B. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am. J. Physiol. Cell Physiol. 2003, 285, C806–C812. [Google Scholar] [CrossRef] [Green Version]
- Khal, J.; Wyke, S.M.; Russell, S.T.; Hine, A.V.; Tisdale, M.J. Expression of the ubiquitin-proteasome pathway and muscle loss in experimental cancer cachexia. Br. J. Cancer 2005, 93, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Penna, F.; Costelli, P. New developments in investigational hdac inhibitors for the potential multimodal treatment of cachexia. Expert Opin. Investig. Drugs 2019, 28, 179–189. [Google Scholar] [CrossRef]
- Sirago, G.; Conte, E.; Fracasso, F.; Cormio, A.; Fehrentz, J.A.; Martinez, J.; Musicco, C.; Camerino, G.M.; Fonzino, A.; Rizzi, L.; et al. Growth hormone secretagogues hexarelin and JMV2894 protect skeletal muscle from mitochondrial damages in a rat model of cisplatin-induced cachexia. Sci. Rep. 2017, 7, 13017. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Kim, J.W.; Lee, S.J.; Bae, G.U. Ginsenoside Rg3 protects glucocorticoid-induced muscle atrophy in vitro through improving mitochondrial biogenesis and myotube growth. Mol. Med. Rep. 2022, 25, 94. [Google Scholar] [CrossRef]
- Currow, D.; Temel, J.S.; Abernethy, A.; Milanowski, J.; Friend, J.; Fearon, K.C. ROMANA 3: A phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann. Oncol. 2017, 28, 1949–1956. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Taaffe, D.R.; Kim, J.S.; Luo, H.; Yang, L.; Fairman, C.M.; Qiao, Y.; Newton, R.; Galvão, D.A. Protective effects of physical activity in colon cancer and underlying mechanisms: A review of epidemiological and biological evidence. Crit. Rev. Oncol. Hematol. 2022, 170, 103578. [Google Scholar] [CrossRef]
- Lenk, K.; Erbs, S.; Höllriegel, R.; Beck, E.; Linke, A.; Gielen, S.; Winkler, S.M.; Sandri, M.; Hambrecht, R.; Schuler, G.; et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur. J. Prev. Cardiol. 2012, 19, 404–411. [Google Scholar] [CrossRef]
- Smart, N.A.; Steele, M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: A systematic review. Congest. Heart Fail. 2011, 17, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Daou, H.N. Exercise as an anti-inflammatory therapy for cancer cachexia: A focus on interleukin-6 regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R296–R310. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujie, S.; Uchida, M.; Iemitsu, M. Resistance exercise—Induced increase in muscle 5α-dihydrotestosterone contributes to the activation of muscle Akt/mTOR/p70S6K- and Akt/AS160/GLUT4-signaling pathways in type 2 diabetic rats. FASEB J. 2020, 34, 11047–11057. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Yan, Z.; Spaulding, H.R. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020, 32, 101508. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.C.; Ferreira, D.M.; Ruas, J.L. Intercellular: Local and systemic actions of skeletal muscle PGC-1s. Trends Endocrinol. Metab. 2015, 26, 305–314. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; Marino Gammazza, A.; Macaluso, F.; Paladino, L.; Scalia, F.; Spinoso, G.; Dimauro, I.; Caporossi, D.; Cappello, F.; Di Felice, V.; et al. Sex-based differences after a single bout of exercise on PGC1α isoforms in skeletal muscle: A pilot study. FASEB. J. 2021, 35, e21328. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Marino Gammazza, A.; Macaluso, F.; Di Felice, V.; Cappello, F.; Barone, R. Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology. Cells 2018, 7, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, J.P.; Ross, C.R.; Kilgore, J.L.; Musch, T.I. Induction of mitochondrial stress proteins following treadmill running. Med. Sci. Sports Exerc. 2000, 32, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Maclaren, D.P.M.; Cable, N.T.; Campbell, I.T.; Evans, L.; Kayani, A.C.; McArdle, A.; Drust, B. Trained men display increased basal heat shock protein content of skeletal muscle. Med. Sci. Sports Exerc. 2008, 40, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Takahashi, M.; Connor, M.K.; Freyssenet, D. Assembly of the Cellular Powerhouse: Current Issues in Muscle Mitochondrial Biogenesis. Exerc. Sport Sci. Rev. 2000, 28, 68–73. [Google Scholar] [PubMed]
- Campanella, C.; Bucchieri, F.; Ardizzone, N.M.; Marino Gammazza, A.; Montalbano, A.; Ribbene, A.; Di Felice, V.; Bellafiore, M.; David, S.; Rappa, F.; et al. Upon oxidative stress, the antiapoptotic Hsp60/procaspase-3 complex persists in mucoepidermoid carcinoma cells. Eur. J. Histochem. 2008, 52, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, R.; Macaluso, F.; Sangiorgi, C.; Campanella, C.; Marino Gammazza, A.; Moresi, V.; Coletti, D.; Conway de Macario, E.; Macario, A.J.I.; Cappello, F.; et al. Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression. Sci. Rep. 2016, 6, 19781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romancino, D.P.; Paterniti, G.; Campos, Y.; De Luca, A.; Di Felice, V.; d’Azzo, A.; Bongiovanni, A. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013, 587, 1379–1384. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, J.; Sun, M.; Li, J.; Wang, H.D. Toward the next-generation phyto-nanomedicines: Cell-derived nanovesicles (CDNs) for natural product delivery. Biomed. Pharm. 2022, 145, 112416. [Google Scholar] [CrossRef]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2021, 151, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Botzler, C.; Li, G.; Issels, R.D.; Multhoff, G. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones 1998, 3, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Saletti, R.; Reina, S.; Pittalà, M.G.G.; Magrì, A.; Cunsolo, V.; Foti, S.; De Pinto, V. Post-translational modifications of VDAC1 and VDAC2 cysteines from rat liver mitochondria. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 806–816. [Google Scholar] [CrossRef]
- Saletti, R.; Reina, S.; Pittalà, M.G.; Belfiore, R.; Cunsolo, V.; Messina, A.; De Pinto, V.; Foti, S. High resolution mass spectrometry characterization of the oxidation pattern of methionine and cysteine residues in rat liver mitochondria voltage-dependent anion selective channel 3 (VDAC3). Biochim. Biophys. Acta Biomembr. 2017, 1859, 301–311. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef] [Green Version]
- Guescini, M.; Maggio, S.; Ceccaroli, P.; Battistelli, M.; Annibalini, G.; Piccoli, G.; Sestili, P.; Stocchi, V. Extracellular Vesicles Released by Oxidatively Injured or Intact C2C12 Myotubes Promote Distinct Responses Converging toward Myogenesis. Int. J. Mol. Sci. 2017, 18, 2488. [Google Scholar] [CrossRef] [Green Version]
- Saludas, L.; Garbayo, E.; Ruiz-Villalba, A.; Hernández, S.; Vader, P.; Prósper, F.; Blanco-Prieto, M.J. Isolation methods of large and small extracellular vesicles derived from cardiovascular progenitors: A comparative study. Eur. J. Pharm. Biopharm. 2022, 170, 187–196. [Google Scholar] [CrossRef]
- Yuan, W.; Song, C. The Emerging Role of Rab5 in Membrane Receptor Trafficking and Signaling Pathways. Biochem. Res. Int. 2020, 2020, 4186308. [Google Scholar] [CrossRef] [Green Version]
- Yerlikaya, A.; Kanbur, E.; Stanley, B.A.; Tümer, E. The Ubiquitin-Proteasome Pathway and Epigenetic Modifications in Cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2021, 21, 20–32. [Google Scholar] [CrossRef]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [Green Version]
- Simon, T.; Kumaran, A.; Veselu, D.F.; Giamas, G. Three Method-Combination Protocol for Improving Purity of Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 3071. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Mitochondrial dysregulation and muscle disuse atrophy. F1000Research 2019, 8, 1621. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.T.; Patel, B.M. Mitochondrial Metabolism in Cancer Cachexia: Novel Drug Target. Curr. Drug Metab. 2019, 20, 1141–1153. [Google Scholar] [CrossRef] [PubMed]

| Primer | Target Sequence | Forward | Reverse |
|---|---|---|---|
| PGC1 tot | [31] | 5′-TGATGTGAATGACTTGGATACAGACA-3′ | 5′-GCTCATTGTTGTACTGGTTGGATATG-3′ |
| PGC1 α1 | [31] | 5′-GGACATGTGCAGCCAAGACTCT-3′ | 5′-CACTTCAATCCACCCAGAAAGCT-3′ |
| GADPH | MGI:MGI:95640 | 5′-CAAGGACACTGAGCAAGAGA-3′ | 5′-GCCCCTCCTGTTATTATGGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Felice, V.; Barone, R.; Trovato, E.; D’Amico, D.; Macaluso, F.; Campanella, C.; Marino Gammazza, A.; Muccilli, V.; Cunsolo, V.; Cancemi, P.; et al. Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia. Cells 2022, 11, 1406. https://doi.org/10.3390/cells11091406
Di Felice V, Barone R, Trovato E, D’Amico D, Macaluso F, Campanella C, Marino Gammazza A, Muccilli V, Cunsolo V, Cancemi P, et al. Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia. Cells. 2022; 11(9):1406. https://doi.org/10.3390/cells11091406
Chicago/Turabian StyleDi Felice, Valentina, Rosario Barone, Eleonora Trovato, Daniela D’Amico, Filippo Macaluso, Claudia Campanella, Antonella Marino Gammazza, Vera Muccilli, Vincenzo Cunsolo, Patrizia Cancemi, and et al. 2022. "Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia" Cells 11, no. 9: 1406. https://doi.org/10.3390/cells11091406
APA StyleDi Felice, V., Barone, R., Trovato, E., D’Amico, D., Macaluso, F., Campanella, C., Marino Gammazza, A., Muccilli, V., Cunsolo, V., Cancemi, P., Multhoff, G., Coletti, D., Adamo, S., Farina, F., & Cappello, F. (2022). Physiactisome: A New Nanovesicle Drug Containing Heat Shock Protein 60 for Treating Muscle Wasting and Cachexia. Cells, 11(9), 1406. https://doi.org/10.3390/cells11091406













