Epigenetics, Enhancer Function and 3D Chromatin Organization in Reprogramming to Pluripotency
Abstract
1. Introduction
2. Epigenetics of Reprogramming
2.1. Pioneer Factors in Reprogramming
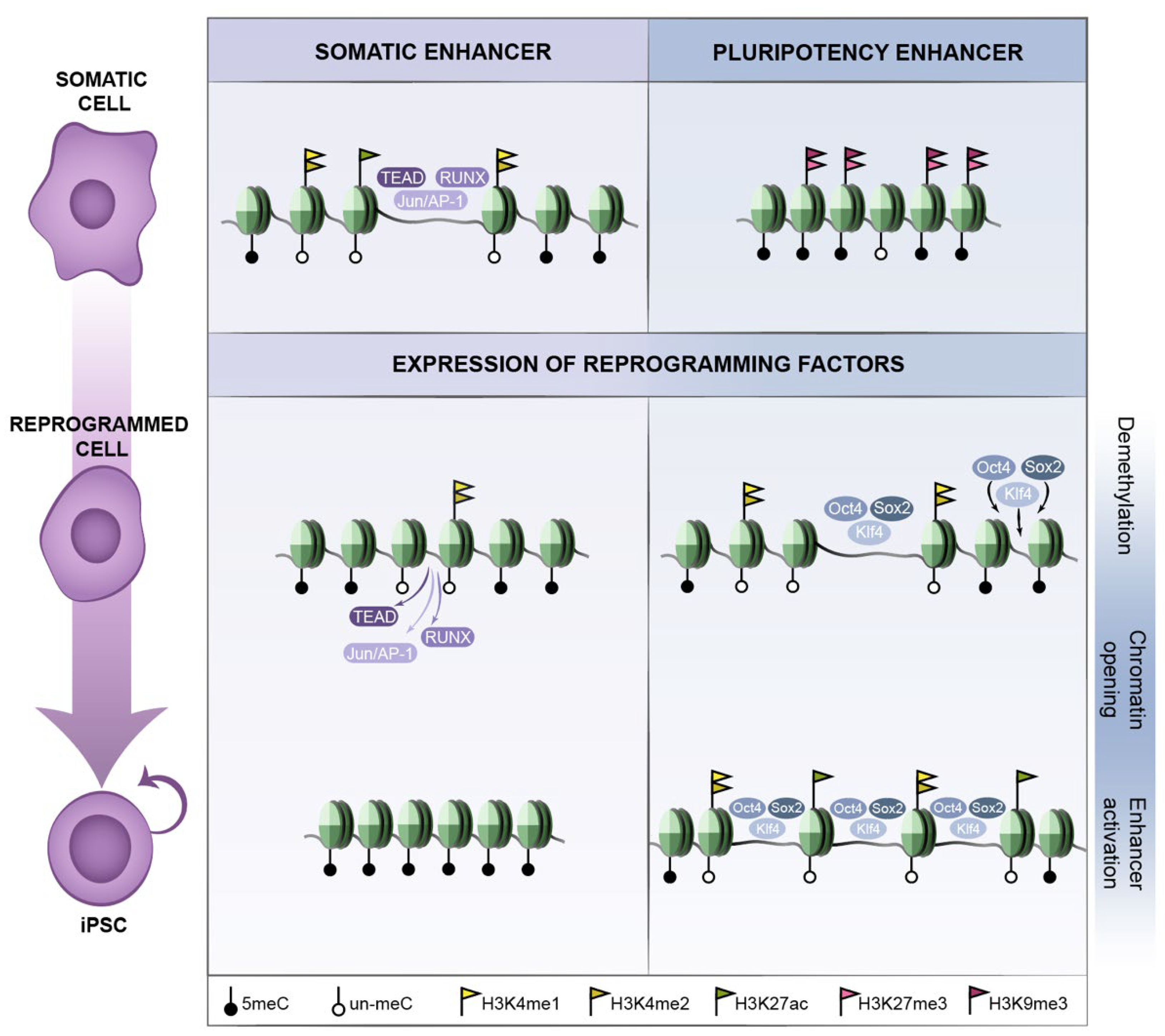
2.2. Early Reprogramming Changes in Enhancers and Promoters
2.3. Epigenetic Barriers to Reprogramming
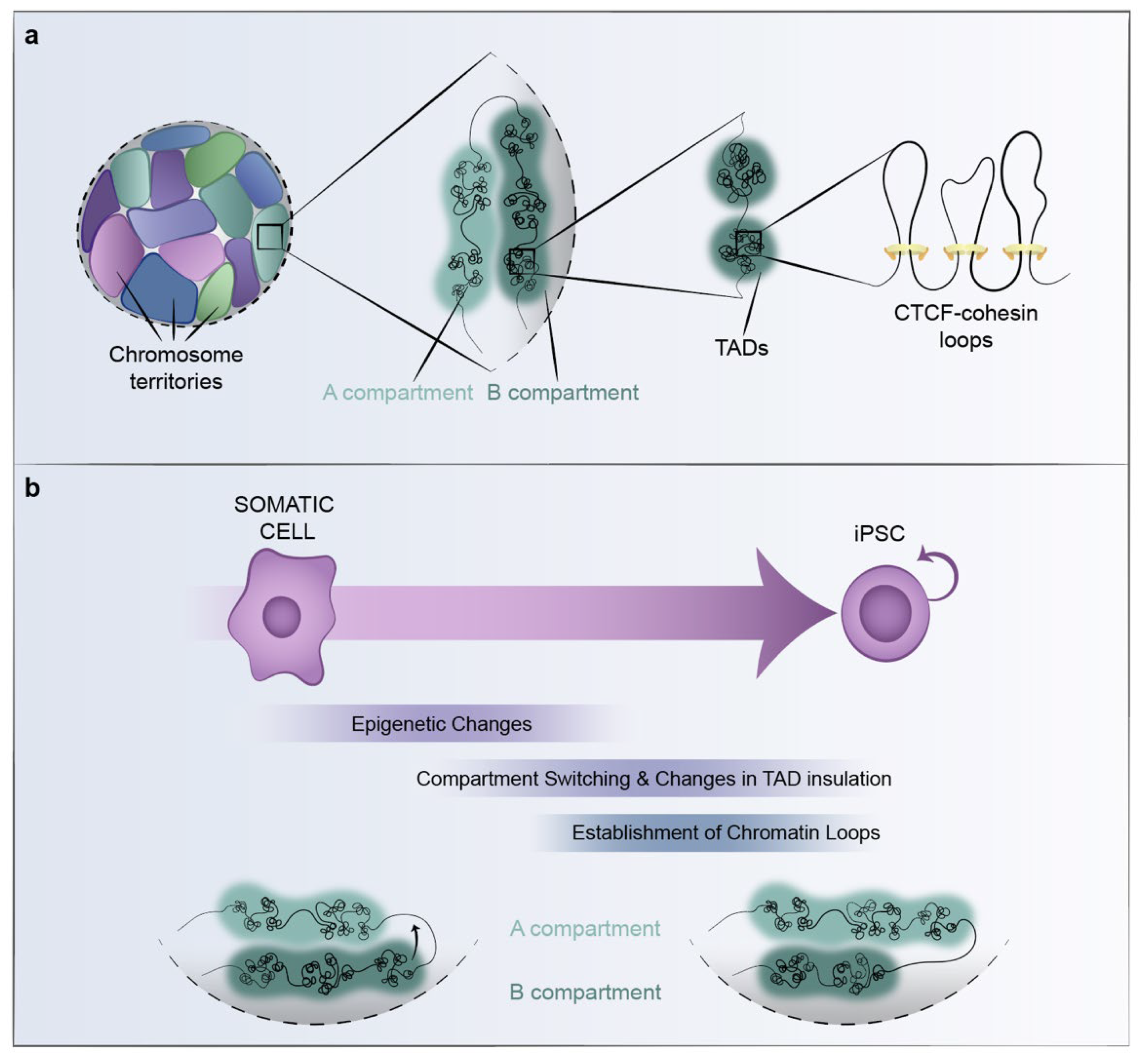
3. 3D-Chromatin Organization in Pluripotency
3.1. Spatial Organization of the Genome and Gene Regulation
3.2. 3D-Chromatin Reorganization during Reprogramming to Pluripotency
3.3. Transcription Factors as Drivers of 3D Chromatin Organization
3.4. Chromatin Accessibility during Reprogramming
4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stadhouders, R.; Filion, G.J.; Graf, T. Transcription Factors and 3D Genome Conformation in Cell-Fate Decisions. Nature 2019, 569, 345–354. [Google Scholar] [CrossRef]
- Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; Ziller, M.J.; et al. Integrative Analysis of 111 Reference Human Epigenomes. Nature 2015, 518, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, Y.; Liu, L.; Yu, K.; Zhang, L.; Wang, H.; He, X.; Wang, J.; Lu, C.; Wu, L.N.; et al. Dual Regulatory Switch through Interactions of Tcf7l2/Tcf4 with Stage-Specific Partners Propels Oligodendroglial Maturation. Nat. Commun. 2016, 7, 10883. [Google Scholar] [CrossRef]
- Bauer, D.C.; Buske, F.A.; Bailey, T.L. Dual-Functioning Transcription Factors in the Developmental Gene Network of Drosophila Melanogaster. BMC Bioinform. 2010, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Lehming, N.; Thanos, D.; Brickman, J.M.; Ma, J.; Maniatis, T.; Ptashne, M. An HMG-like Protein That Can Switch a Transcriptional Activator to a Repressor. Nature 1994, 371, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S.; Mango, S.E. Pioneer Transcription Factors, Chromatin Dynamics, and Cell Fate Control. Curr. Opin. Genet. Dev. 2016, 37, 76–81. [Google Scholar] [CrossRef]
- Remeseiro, S.; Hörnblad, A.; Spitz, F. Gene Regulation during Development in the Light of Topologically Associating Domains. Wiley interdisciplinary reviews. Dev. Biol. 2016, 5, 169–185. [Google Scholar] [CrossRef]
- Gurdon, J.B.; Elsdale, T.R.; Fischberg, M. Sexually Mature Individuals of Xenopus Laevis from the Transplantation of Single Somatic Nuclei. Nature 1958, 182, 64–65. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Li, D.; Liu, Y.; Guo, J.; Li, C.; Yao, Y.; Wang, Y.; Zhao, G.; Wang, X.; et al. Induction of Pluripotent Stem Cells from Mouse Embryonic Fibroblasts by Jdp2-Jhdm1b-Mkk6-Glis1-Nanog-Essrb-Sall4. Cell Rep. 2019, 27, 3473–3485.e5. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct Cell Reprogramming: Approaches, Mechanisms and Progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.S.; Mortazavi, A.; Myers, R.M.; Wold, B. Genome-Wide Mapping of in Vivo Protein-DNA Interactions. Science 2007, 316, 1497–1502. [Google Scholar] [CrossRef]
- Skene, P.J.; Henikoff, S. A Simple Method for Generating High-Resolution Maps of Genome-Wide Protein Binding. eLife 2015, 4, e09225. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Giresi, P.G.; Zaba, L.C.; Chang, H.Y.; Greenleaf, W.J. Transposition of Native Chromatin for Fast and Sensitive Epigenomic Profiling of Open Chromatin, DNA-Binding Proteins and Nucleosome Position. Nat. Methods 2013, 10, 1213–1218. [Google Scholar] [CrossRef]
- Boyle, A.P.; Davis, S.; Shulha, H.P.; Meltzer, P.; Margulies, E.H.; Weng, Z.; Furey, T.S.; Crawford, G.E. High-Resolution Mapping and Characterization of Open Chromatin across the Genome. Cell 2008, 132, 311–322. [Google Scholar] [CrossRef]
- Dostie, J.; Richmond, T.A.; Arnaout, R.A.; Selzer, R.R.; Lee, W.L.; Honan, T.A.; Rubio, E.D.; Krumm, A.; Lamb, J.; Nusbaum, C.; et al. Chromosome Conformation Capture Carbon Copy (5C): A Massively Parallel Solution for Mapping Interactions between Genomic Elements. Genome Res. 2006, 16, 1299–1309. [Google Scholar] [CrossRef]
- Zhao, Z.; Tavoosidana, G.; Sjölinder, M.; Göndör, A.; Mariano, P.; Wang, S.; Kanduri, C.; Lezcano, M.; Sandhu, K.S.; Singh, U.; et al. Circular Chromosome Conformation Capture (4C) Uncovers Extensive Networks of Epigenetically Regulated Intra- and Interchromosomal Interactions. Nat. Genet. 2006, 38, 1341–1347. [Google Scholar] [CrossRef]
- Simonis, M.; Klous, P.; Splinter, E.; Moshkin, Y.; Willemsen, R.; de Wit, E.; van Steensel, B.; de Laat, W. Nuclear Organization of Active and Inactive Chromatin Domains Uncovered by Chromosome Conformation Capture–on-Chip (4C). Nat. Genet. 2006, 38, 1348–1354. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive Mapping of Long-Range Interactions Reveals Folding Principles of the Human Genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef]
- Dixon, J.R.; Jung, I.; Selvaraj, S.; Shen, Y.; Antosiewicz-Bourget, J.E.; Lee, A.Y.; Ye, Z.; Kim, A.; Rajagopal, N.; Xie, W.; et al. Chromatin Architecture Reorganization during Stem Cell Differentiation. Nature 2015, 518, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Mumbach, M.R.; Rubin, A.J.; Flynn, R.A.; Dai, C.; Khavari, P.A.; Greenleaf, W.J.; Chang, H.Y. HiChIP: Efficient and Sensitive Analysis of Protein-Directed Genome Architecture. Nat. Methods 2016, 13, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Yu, M.; Li, G.; Chee, S.; Liu, T.; Schmitt, A.D.; Ren, B. Mapping of Long-Range Chromatin Interactions by Proximity Ligation-Assisted ChIP-Seq. Cell Res. 2016, 26, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Xu, L.; Wang, E. Quantifying the Waddington Landscape and Biological Paths for Development and Differentiation. Proc. Natl. Acad. Sci. USA 2011, 108, 8257–8262. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J. Quantifying Cell Fate Decisions for Differentiation and Reprogramming of a Human Stem Cell Network: Landscape and Biological Paths. PLoS Comput. Biol. 2013, 9, e1003165. [Google Scholar] [CrossRef]
- Rajapakse, I.; Groudine, M.; Mesbahi, M. Dynamics and Control of State-Dependent Networks for Probing Genomic Organization. Proc. Natl. Acad. Sci. USA 2011, 108, 17257–17262. [Google Scholar] [CrossRef]
- Cahan, P.; Li, H.; Morris, S.A.; Lummertz da Rocha, E.; Daley, G.Q.; Collins, J.J. CellNet: Network Biology Applied to Stem Cell Engineering. Cell 2014, 158, 903–915. [Google Scholar] [CrossRef]
- D’Alessio, A.C.; Fan, Z.P.; Wert, K.J.; Baranov, P.; Cohen, M.A.; Saini, J.S.; Cohick, E.; Charniga, C.; Dadon, D.; Hannett, N.M.; et al. A Systematic Approach to Identify Candidate Transcription Factors That Control Cell Identity. Stem Cell Rep. 2015, 5, 763–775. [Google Scholar] [CrossRef]
- Consortium, T.F.; Rackham, O.J.L.; Firas, J.; Fang, H.; Oates, M.E.; Holmes, M.L.; Knaupp, A.S.; Suzuki, H.; Nefzger, C.M.; Daub, C.O.; et al. A Predictive Computational Framework for Direct Reprogramming between Human Cell Types. Nat. Genet. 2016, 48, 331–335. [Google Scholar] [CrossRef]
- Vecchio, D.D.; Abdallah, H.; Qian, Y.; Collins, J.J. A Blueprint for a Synthetic Genetic Feedback Controller to Reprogram Cell Fate. Cell Syst. 2017, 4, 109–120.e11. [Google Scholar] [CrossRef]
- Rajagopal, N.; Srinivasan, S.; Kooshesh, K.; Guo, Y.; Edwards, M.D.; Banerjee, B.; Syed, T.; Emons, B.J.M.; Gifford, D.K.; Sherwood, R.I. High-Throughput Mapping of Regulatory DNA. Nat. Biotechnol. 2016, 34, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Canver, M.C.; Smith, E.C.; Sher, F.; Pinello, L.; Sanjana, N.E.; Shalem, O.; Chen, D.D.; Schupp, P.G.; Vinjamur, D.S.; Garcia, S.P.; et al. BCL11A Enhancer Dissection by Cas9-Mediated in Situ Saturating Mutagenesis. Nature 2015, 527, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Fang, R.; Li, B.; Meng, Z.; Yu, J.; Qiu, Y.; Lin, K.C.; Huang, H.; Liu, T.; Marina, R.J.; et al. A Tiling-Deletion-Based Genetic Screen for Cis-Regulatory Element Identification in Mammalian Cells. Nat. Methods 2017, 14, 629–635. [Google Scholar] [CrossRef]
- Fulco, C.P.; Nasser, J.; Jones, T.R.; Munson, G.; Bergman, D.T.; Subramanian, V.; Grossman, S.R.; Anyoha, R.; Doughty, B.R.; Patwardhan, T.A.; et al. Activity-by-Contact Model of Enhancer– Promoter Regulation from Thousands of CRISPR Perturbations. Nat. Genet. 2019, 51, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, N.E.; Wright, J.; Zheng, K.; Shalem, O.; Fontanillas, P.; Joung, J.; Cheng, C.; Regev, A.; Zhang, F. High-Resolution Interrogation of Functional Elements in the Noncoding Genome. Science 2016, 353, 1545–1549. [Google Scholar] [CrossRef]
- Vierstra, J.; Reik, A.; Chang, K.-H.; Stehling-Sun, S.; Zhou, Y.; Hinkley, S.J.; Paschon, D.E.; Zhang, L.; Psatha, N.; Bendana, Y.R.; et al. Functional Footprinting of Regulatory DNA. Nat. Methods 2015, 12, 927–930. [Google Scholar] [CrossRef]
- Korkmaz, G.; Lopes, R.; Ugalde, A.P.; Nevedomskaya, E.; Han, R.; Myacheva, K.; Zwart, W.; Elkon, R.; Agami, R. Functional Genetic Screens for Enhancer Elements in the Human Genome Using CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 192–198. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Yuan, P.; Fang, F.; Huss, M.; Vega, V.B.; Wong, E.; Orlov, Y.L.; Zhang, W.; Jiang, J.; et al. Integration of External Signaling Pathways with the Core Transcriptional Network in Embryonic Stem Cells. Cell 2008, 133, 1106–1117. [Google Scholar] [CrossRef]
- Kim, J.; Chu, J.; Shen, X.; Wang, J.; Orkin, S.H. An Extended Transcriptional Network for Pluripotency of Embryonic Stem Cells. Cell 2008, 132, 1049–1061. [Google Scholar] [CrossRef]
- Visel, A.; Blow, M.J.; Li, Z.; Zhang, T.; Akiyama, J.A.; Holt, A.; Plajzer-Frick, I.; Shoukry, M.; Wright, C.; Chen, F.; et al. ChIP-Seq Accurately Predicts Tissue-Specific Activity of Enhancers. Nature 2009, 457, 854–858. [Google Scholar] [CrossRef]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of Induced Pluripotent Stem Cells without Myc from Mouse and Human Fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wernig, M.; Meissner, A.; Foreman, R.; Brambrink, T.; Ku, M.; Hochedlinger, K.; Bernstein, B.E.; Jaenisch, R. In Vitro Reprogramming of Fibroblasts into a Pluripotent ES-Cell-like State. Nature 2007, 448, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zviran, A.; Mor, N.; Rais, Y.; Gingold, H.; Peles, S.; Chomsky, E.; Viukov, S.; Buenrostro, J.D.; Scognamiglio, R.; Weinberger, L.; et al. Deterministic Somatic Cell Reprogramming Involves Continuous Transcriptional Changes Governed by Myc and Epigenetic-Driven Modules. Cell Stem Cell 2019, 24, 328–341.e9. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Hu, G.; Wei, G.; Cui, K.; Yamane, A.; Resch, W.; Wang, R.; Green, D.R.; Tessarollo, L.; Casellas, R.; et al. C-Myc Is a Universal Amplifier of Expressed Genes in Lymphocytes and Embryonic Stem Cells. Cell 2012, 151, 68–79. [Google Scholar] [CrossRef]
- Soufi, A.; Donahue, G.; Zaret, K.S. Facilitators and Impediments of the Pluripotency Reprogramming Factors’ Initial Engagement with the Genome. Cell 2012, 151, 994–1004. [Google Scholar] [CrossRef]
- Sridharan, R.; Tchieu, J.; Mason, M.J.; Yachechko, R.; Kuoy, E.; Horvath, S.; Zhou, Q.; Plath, K. Role of the Murine Reprogramming Factors in the Induction of Pluripotency. Cell 2009, 136, 364–377. [Google Scholar] [CrossRef]
- Zaret, K.S.; Carroll, J.S. Pioneer Transcription Factors: Establishing Competence for Gene Expression. Gene Dev. 2011, 25, 2227–2241. [Google Scholar] [CrossRef]
- Beato, M.; Eisfeld, K. Transcription Factor Access to Chromatin. Nucleic Acids Res. 1997, 25, 3559–3563. [Google Scholar] [CrossRef]
- Roberts, G.A.; Ozkan, B.; Gachulincová, I.; O’Dwyer, M.R.; Hall-Ponsele, E.; Saxena, M.; Robinson, P.J.; Soufi, A. Dissecting OCT4 Defines the Role of Nucleosome Binding in Pluripotency. Nat. Cell Biol. 2021, 23, 834–845. [Google Scholar] [CrossRef]
- Teif, V.B.; Vainshtein, Y.; Caudron-Herger, M.; Mallm, J.-P.; Marth, C.; Höfer, T.; Rippe, K. Genome-Wide Nucleosome Positioning during Embryonic Stem Cell Development. Nat. Struct. Mol. Biol. 2012, 19, 1185–1192. [Google Scholar] [CrossRef]
- You, J.S.; Kelly, T.K.; Carvalho, D.D.D.; Taberlay, P.C.; Liang, G.; Jones, P.A. OCT4 Establishes and Maintains Nucleosome-Depleted Regions That Provide Additional Layers of Epigenetic Regulation of Its Target Genes. Proc. Natl. Acad. Sci. USA 2011, 108, 14497–14502. [Google Scholar] [CrossRef]
- Soufi, A.; Garcia, M.F.; Jaroszewicz, A.; Osman, N.; Pellegrini, M.; Zaret, K.S. Pioneer Transcription Factors Target Partial DNA Motifs on Nucleosomes to Initiate Reprogramming. Cell 2015, 161, 555–568. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Yang, X.; Zhou, C.; Guo, J.; Wu, C.; Qin, Y.; Guo, L.; He, J.; Yu, S.; et al. Chromatin Accessibility Dynamics during IPSC Reprogramming. Cell Stem Cell 2017, 21, 819–833.e6. [Google Scholar] [CrossRef]
- Malik, V.; Glaser, L.V.; Zimmer, D.; Velychko, S.; Weng, M.; Holzner, M.; Arend, M.; Chen, Y.; Srivastava, Y.; Veerapandian, V.; et al. Pluripotency Reprogramming by Competent and Incompetent POU Factors Uncovers Temporal Dependency for Oct4 and Sox2. Nat. Commun. 2019, 10, 3477. [Google Scholar] [CrossRef]
- Chronis, C.; Fiziev, P.; Papp, B.; Butz, S.; Bonora, G.; Sabri, S.; Ernst, J.; Plath, K. Cooperative Binding of Transcription Factors Orchestrates Reprogramming. Cell 2017, 168, 442–459.e20. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, X.; Shi, J.; Yao, M.; Lin, J.; Li, J.; Liu, H.; Li, H.; Shi, G.; Wang, Z.; et al. Dynamically Reorganized Chromatin Is the Key for the Reprogramming of Somatic Cells to Pluripotent Cells. Sci. Rep. 2015, 5, 17691. [Google Scholar] [CrossRef]
- Koche, R.P.; Smith, Z.D.; Adli, M.; Gu, H.; Ku, M.; Gnirke, A.; Bernstein, B.E.; Meissner, A. Reprogramming Factor Expression Initiates Widespread Targeted Chromatin Remodelling. Cell Stem Cell 2011, 8, 96–105. [Google Scholar] [CrossRef]
- Stadtfeld, M.; Hochedlinger, K. Induced Pluripotency: History, Mechanisms, and Applications. Gene Dev. 2010, 24, 2239–2263. [Google Scholar] [CrossRef]
- Ernst, J.; Kheradpour, P.; Mikkelsen, T.S.; Shoresh, N.; Ward, L.D.; Epstein, C.B.; Zhang, X.; Wang, L.; Issner, R.; Coyne, M.; et al. Mapping and Analysis of Chromatin State Dynamics in Nine Human Cell Types. Nature 2011, 473, 43–49. [Google Scholar] [CrossRef]
- Rada-Iglesias, A.; Bajpai, R.; Swigut, T.; Brugmann, S.A.; Flynn, R.A.; Wysocka, J. A Unique Chromatin Signature Uncovers Early Developmental Enhancers in Humans. Nature 2011, 470, 279–283. [Google Scholar] [CrossRef]
- Calo, E.; Wysocka, J. Modification of Enhancer Chromatin: What, How, and Why? Mol. Cell 2013, 49, 825–837. [Google Scholar] [CrossRef]
- Zentner, G.E.; Tesar, P.J.; Scacheri, P.C. Epigenetic Signatures Distinguish Multiple Classes of Enhancers with Distinct Cellular Functions. Genome Res. 2011, 21, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac Separates Active from Poised Enhancers and Predicts Developmental State. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Molina, S.; Respuela, P.; Tebartz, C.; Kolovos, P.; Nikolic, M.; Fueyo, R.; van Ijcken, W.F.J.; Grosveld, F.; Frommolt, P.; Bazzi, H.; et al. PRC2 Facilitates the Regulatory Topology Required for Poised Enhancer Function during Pluripotent Stem Cell Differentiation. Cell Stem Cell 2017, 20, 689–705.e9. [Google Scholar] [CrossRef]
- Crispatzu, G.; Rehimi, R.; Pachano, T.; Bleckwehl, T.; Cruz-Molina, S.; Xiao, C.; Mahabir, E.; Bazzi, H.; Rada-Iglesias, A. The Chromatin, Topological and Regulatory Properties of Pluripotency-Associated Poised Enhancers Are Conserved in Vivo. Nat. Commun. 2021, 12, 4344. [Google Scholar] [CrossRef] [PubMed]
- Whyte, W.A.; Orlando, D.A.; Hnisz, D.; Abraham, B.J.; Lin, C.Y.; Kagey, M.H.; Rahl, P.B.; Lee, T.I.; Young, R.A. Master Transcription Factors and Mediator Establish Super-Enhancers at Key Cell Identity Genes. Cell 2013, 153, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Bonn, S.; Zinzen, R.P.; Girardot, C.; Gustafson, E.H.; Perez-Gonzalez, A.; Delhomme, N.; Ghavi-Helm, Y.; Wilczyński, B.; Riddell, A.; Furlong, E.E.M. Tissue-Specific Analysis of Chromatin State Identifies Temporal Signatures of Enhancer Activity during Embryonic Development. Nat. Genet. 2012, 44, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Won, K.-J.; Xu, Z.; Zhang, X.; Whitaker, J.W.; Shoemaker, R.; Ren, B.; Xu, Y.; Wang, W. Global Identification of Transcriptional Regulators of Pluripotency and Differentiation in Embryonic Stem Cells. Nucleic Acids Res. 2012, 40, 8199–8209. [Google Scholar] [CrossRef][Green Version]
- Mikkelsen, T.S.; Hanna, J.; Zhang, X.; Ku, M.; Wernig, M.; Schorderet, P.; Bernstein, B.E.; Jaenisch, R.; Lander, E.S.; Meissner, A. Dissecting Direct Reprogramming through Integrative Genomic Analysis. Nature 2008, 454, 49–55. [Google Scholar] [CrossRef]
- Sardina, J.L.; Collombet, S.; Tian, T.V.; Gómez, A.; Stefano, B.D.; Berenguer, C.; Brumbaugh, J.; Stadhouders, R.; Segura-Morales, C.; Gut, M.; et al. Transcription Factors Drive Tet2-Mediated Enhancer Demethylation to Reprogram Cell Fate. Cell Stem Cell 2018, 23, 727–741.e9. [Google Scholar] [CrossRef]
- Adachi, K.; Kopp, W.; Wu, G.; Heising, S.; Greber, B.; Stehling, M.; Araúzo-Bravo, M.J.; Boerno, S.T.; Timmermann, B.; Vingron, M.; et al. Esrrb Unlocks Silenced Enhancers for Reprogramming to Naive Pluripotency. Cell Stem Cell 2018, 23, 266–275.e6. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.A.; Cetinbas, M.; Clement, K.; Walsh, R.M.; Cheloufi, S.; Gu, H.; Langkabel, J.; Kamiya, A.; Schorle, H.; Meissner, A.; et al. Prospective Isolation of Poised IPSC Intermediates Reveals Principles of Cellular Reprogramming. Cell Stem Cell 2018, 23, 289–305.e5. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Jaenisch, R. De Novo DNA Methylation by Dnmt3a and Dnmt3b Is Dispensable for Nuclear Reprogramming of Somatic Cells to a Pluripotent State. Gene Dev. 2011, 25, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Adli, M.; Zou, J.Y.; Verstappen, G.; Coyne, M.; Zhang, X.; Durham, T.; Miri, M.; Deshpande, V.; De Jager, P.L.; et al. Genome-Wide Chromatin State Transitions Associated with Developmental and Environmental Cues. Cell 2013, 152, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Samavarchi-Tehrani, P.; Golipour, A.; David, L.; Sung, H.; Beyer, T.A.; Datti, A.; Woltjen, K.; Nagy, A.; Wrana, J.L. Functional Genomics Reveals a BMP-Driven Mesenchymal-to-Epithelial Transition in the Initiation of Somatic Cell Reprogramming. Cell Stem Cell 2010, 7, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, J.; Ni, S.; Zhou, T.; Qing, X.; Li, H.; He, W.; Chen, J.; Li, F.; Zhuang, Q.; et al. A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts. Cell Stem Cell 2010, 7, 51–63. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.R.; et al. Epigenetic Memory in Induced Pluripotent Stem Cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef]
- Polo, J.M.; Liu, S.; Figueroa, M.E.; Kulalert, W.; Eminli, S.; Tan, K.Y.; Apostolou, E.; Stadtfeld, M.; Li, Y.; Shioda, T.; et al. Cell Type of Origin Influences the Molecular and Functional Properties of Mouse Induced Pluripotent Stem Cells. Nat. Biotechnol. 2010, 28, 848–855. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef]
- Mikkelsen, T.S.; Ku, M.; Jaffe, D.B.; Issac, B.; Lieberman, E.; Giannoukos, G.; Alvarez, P.; Brockman, W.; Kim, T.-K.; Koche, R.P.; et al. Genome-Wide Maps of Chromatin State in Pluripotent and Lineage-Committed Cells. Nature 2007, 448, 553–560. [Google Scholar] [CrossRef]
- Wang, A.; Yue, F.; Li, Y.; Xie, R.; Harper, T.; Patel, N.A.; Muth, K.; Palmer, J.; Qiu, Y.; Wang, J.; et al. Epigenetic Priming of Enhancers Predicts Developmental Competence of HESC-Derived Endodermal Lineage Intermediates. Cell Stem Cell 2015, 16, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Mirny, L. The 3D Genome as Moderator of Chromosomal Communication. Cell 2016, 164, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Cavalli, G. Organization and Function of the 3D Genome. Nat. Rev. Genet. 2016, 17, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Fam, S.; Barrionuevo, F.; Dohrmann, U.; Günther, T.; Schüle, R.; Kemler, R.; Mallo, M.; Kanzler, B.; Scherer, G. Long-Range Upstream and Downstream Enhancers Control Distinct Subsets of the Complex Spatiotemporal Sox9 Expression Pattern. Dev. Biol. 2006, 291, 382–397. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.J.; Buckle, A.; van Heyningen, V.; Kleinjan, D.A. DNaseI Hypersensitivity and Ultraconservation Reveal Novel, Interdependent Long-Range Enhancers at the Complex Pax6 Cis-Regulatory Region. PLoS ONE 2011, 6, e28616. [Google Scholar] [CrossRef] [PubMed]
- Will, A.J.; Cova, G.; Osterwalder, M.; Chan, W.-L.; Wittler, L.; Brieske, N.; Heinrich, V.; de Villartay, J.-P.; Vingron, M.; Klopocki, E.; et al. Composition and Dosage of a Multipartite Enhancer Cluster Control Developmental Expression of Ihh (Indian Hedgehog). Nat. Genet. 2017, 49, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Hörnblad, A.; Bastide, S.; Langenfeld, K.; Langa, F.; Spitz, F. Dissection of the Fgf8 Regulatory Landscape by in Vivo CRISPR-Editing Reveals Extensive Intra- and Inter-Enhancer Redundancy. Nat. Commun. 2021, 12, 439. [Google Scholar] [CrossRef]
- Osterwalder, M.; Barozzi, I.; Tissières, V.; Fukuda-Yuzawa, Y.; Mannion, B.J.; Afzal, S.Y.; Lee, E.A.; Zhu, Y.; Plajzer-Frick, I.; Pickle, C.S.; et al. Enhancer Redundancy Provides Phenotypic Robustness in Mammalian Development. Nature 2018, 554, 239–243. [Google Scholar] [CrossRef]
- Barolo, S. Shadow Enhancers: Frequently Asked Questions about Distributed Cis-Regulatory Information and Enhancer Redundancy. BioEssays 2011, 34, 135–141. [Google Scholar] [CrossRef]
- Guelen, L.; Pagie, L.; Brasset, E.; Meuleman, W.; Faza, M.B.; Talhout, W.; Eussen, B.H.; de Klein, A.; Wessels, L.; de Laat, W.; et al. Domain Organization of Human Chromosomes Revealed by Mapping of Nuclear Lamina Interactions. Nature 2008, 453, 948–951. [Google Scholar] [CrossRef]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological Domains in Mammalian Genomes Identified by Analysis of Chromatin Interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Nora, E.P.; Lajoie, B.R.; Schulz, E.G.; Giorgetti, L.; Okamoto, I.; Servant, N.; Piolot, T.; van Berkum, N.L.; Meisig, J.; Sedat, J.; et al. Spatial Partitioning of the Regulatory Landscape of the X-Inactivation Centre. Nature 2012, 485, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Lupiáñez, D.G.; Kraft, K.; Heinrich, V.; Krawitz, P.; Brancati, F.; Klopocki, E.; Horn, D.; Kayserili, H.; Opitz, J.M.; Laxova, R.; et al. Disruptions of Topological Chromatin Domains Cause Pathogenic Rewiring of Gene-Enhancer Interactions. Cell 2015, 161, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Dowen, J.M.; Fan, Z.P.; Hnisz, D.; Ren, G.; Abraham, B.J.; Zhang, L.N.; Weintraub, A.S.; Schuijers, J.; Lee, T.I.; Zhao, K.; et al. Control of Cell Identity Genes Occurs in Insulated Neighborhoods in Mammalian Chromosomes. Cell 2014, 159, 374–387. [Google Scholar] [CrossRef]
- Symmons, O.; Uslu, V.V.; Tsujimura, T.; Ruf, S.; Nassari, S.; Schwarzer, W.; Ettwiller, L.; Spitz, F. Functional and Topological Characteristics of Mammalian Regulatory Domains. Genome Res. 2014, 24, 390–400. [Google Scholar] [CrossRef]
- Sun, F.; Chronis, C.; Kronenberg, M.; Chen, X.-F.; Su, T.; Lay, F.D.; Plath, K.; Kurdistani, S.K.; Carey, M.F. Promoter-Enhancer Communication Occurs Primarily within Insulated Neighborhoods. Mol. Cell 2018. [CrossRef]
- Crane, E.; Bian, Q.; McCord, R.P.; Lajoie, B.R.; Wheeler, B.S.; Ralston, E.J.; Uzawa, S.; Dekker, J.; Meyer, B.J. Condensin-Driven Remodelling of X Chromosome Topology during Dosage Compensation. Nature 2015, 523, 240–244. [Google Scholar] [CrossRef]
- Peñalosa-Ruiz, G.; Bright, A.R.; Mulder, K.W.; Veenstra, G.J.C. The Interplay of Chromatin and Transcription Factors during Cell Fate Transitions in Development and Reprogramming. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2019, 1862, 194407. [Google Scholar] [CrossRef]
- Sanborn, A.L.; Rao, S.S.P.; Huang, S.-C.; Durand, N.C.; Huntley, M.H.; Jewett, A.I.; Bochkov, I.D.; Chinnappan, D.; Cutkosky, A.; Li, J.; et al. Chromatin Extrusion Explains Key Features of Loop and Domain Formation in Wild-Type and Engineered Genomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6456–E6465. [Google Scholar] [CrossRef]
- Fudenberg, G.; Imakaev, M.; Lu, C.; Goloborodko, A.; Abdennur, N.; Mirny, L.A. Formation of Chromosomal Domains by Loop Extrusion. Cell Rep. 2016, 15, 2038–2049. [Google Scholar] [CrossRef]
- Beagan, J.A.; Phillips-Cremins, J.E. On the Existence and Functionality of Topologically Associating Domains. Nat. Genet. 2020, 52, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.S.; Cattoglio, C.; Slobodyanyuk, E.; Hansen, A.S.; Rando, O.J.; Tjian, R.; Darzacq, X. Resolving the 3D Landscape of Transcription-Linked Mammalian Chromatin Folding. Mol. Cell 2020, 78, 539–553.e8. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.P.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Vian, L.; Pękowska, A.; Rao, S.S.P.; Kieffer-Kwon, K.-R.; Jung, S.; Baranello, L.; Huang, S.-C.; Khattabi, L.E.; Dose, M.; Pruett, N.; et al. The Energetics and Physiological Impact of Cohesin Extrusion. Cell 2018, 173, 1165–1178.e20. [Google Scholar] [CrossRef]
- Cuadrado, A.; Losada, A. Specialized Functions of Cohesins STAG1 and STAG2 in 3D Genome Architecture. Curr. Opin. Genet. Dev. 2020, 61, 9–16. [Google Scholar] [CrossRef]
- Cuadrado, A.; Giménez-Llorente, D.; Kojic, A.; Rodríguez-Corsino, M.; Cuartero, Y.; Martín-Serrano, G.; Gómez-López, G.; Marti-Renom, M.A.; Losada, A. Specific Contributions of Cohesin-SA1 and Cohesin-SA2 to TADs and Polycomb Domains in Embryonic Stem Cells. Cell Rep. 2019, 27, 3500–3510.e4. [Google Scholar] [CrossRef]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A Phase Separation Model for Transcriptional Control. Cell 2017, 169, 13–23. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855.e16. [Google Scholar] [CrossRef]
- Chong, S.; Dugast-Darzacq, C.; Liu, Z.; Dong, P.; Dailey, G.M.; Cattoglio, C.; Heckert, A.; Banala, S.; Lavis, L.; Darzacq, X.; et al. Imaging Dynamic and Selective Low-Complexity Domain Interactions That Control Gene Transcription. Science 2018, 361, eaar2555. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator Condensation at Super-Enhancers Links Phase Separation and Gene Control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Cho, W.-K.; Spille, J.-H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA Polymerase II Clusters Associate in Transcription-Dependent Condensates. Science 2018, 361, eaar4199. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Hassan, A.B.; Errington, R.J.; Cook, P.R. Visualization of Focal Sites of Transcription within Human Nuclei. EMBO J 1993, 12, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, J.; Zhou, Y.; Fasolino, M.; Goldman, N.; Schwartz, G.W.; Mumbach, M.R.; Nguyen, S.C.; Rome, K.S.; Sela, Y.; Zapataro, Z.; et al. Oncogenic Notch Promotes Long-Range Regulatory Interactions within Hyperconnected 3D Cliques. Mol. Cell 2019, 73, 1174–1190.e12. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.S.; Li, C.H.; Zamudio, A.V.; Sigova, A.A.; Hannett, N.M.; Day, D.S.; Abraham, B.J.; Cohen, M.A.; Nabet, B.; Buckley, D.L.; et al. YY1 Is a Structural Regulator of Enhancer-Promoter Loops. Cell 2017, 171, 1573–1588.e28. [Google Scholar] [CrossRef]
- Ji, X.; Dadon, D.B.; Powell, B.E.; Fan, Z.P.; Borges-Rivera, D.; Shachar, S.; Weintraub, A.S.; Hnisz, D.; Pegoraro, G.; Lee, T.I.; et al. 3D Chromosome Regulatory Landscape of Human Pluripotent Cells. Cell Stem Cell 2016, 18, 262–275. [Google Scholar] [CrossRef]
- Li, G.; Ruan, X.; Auerbach, R.K.; Sandhu, K.S.; Zheng, M.; Wang, P.; Poh, H.M.; Goh, Y.; Lim, J.; Zhang, J.; et al. Extensive Promoter-Centered Chromatin Interactions Provide a Topological Basis for Transcription Regulation. Cell 2012, 148, 84–98. [Google Scholar] [CrossRef]
- Pelham-Webb, B.; Murphy, D.; Apostolou, E. Dynamic 3D Chromatin Reorganization during Establishment and Maintenance of Pluripotency. Stem Cell Rep. 2020, 15, 1176–1195. [Google Scholar] [CrossRef]
- Krijger, P.H.L.; Di Stefano, B.; de Wit, E.; Limone, F.; van Oevelen, C.; de Laat, W.; Graf, T. Cell-of-Origin-Specific 3D Genome Structure Acquired during Somatic Cell Reprogramming. Cell Stem Cell 2016, 18, 597–610. [Google Scholar] [CrossRef]
- Apostolou, E.; Ferrari, F.; Walsh, R.M.; Bar-Nur, O.; Stadtfeld, M.; Cheloufi, S.; Stuart, H.T.; Polo, J.M.; Ohsumi, T.K.; Borowsky, M.L.; et al. Genome-Wide Chromatin Interactions of the Nanog Locus in Pluripotency, Differentiation, and Reprogramming. Cell Stem Cell 2013, 12, 699–712. [Google Scholar] [CrossRef]
- Beagan, J.A.; Gilgenast, T.G.; Kim, J.; Plona, Z.; Norton, H.K.; Hu, G.; Hsu, S.C.; Shields, E.J.; Lyu, X.; Apostolou, E.; et al. Local Genome Topology Can Exhibit an Incompletely Rewired 3D-Folding State during Somatic Cell Reprogramming. Cell Stem Cell 2016, 18, 611–624. [Google Scholar] [CrossRef]
- Stadhouders, R.; Vidal, E.; Serra, F.; Stefano, B.D.; Dily, F.L.; Quilez, J.; Gomez, A.; Collombet, S.; Berenguer, C.; Cuartero, Y.; et al. Transcription Factors Orchestrate Dynamic Interplay between Genome Topology and Gene Regulation during Cell Reprogramming. Nat. Genet. 2018, 50, 238–249. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, F.; Kim, S.; Yang, H.; Lyu, J.; An, W.; Wang, K.; Lu, W. Klf4 Organizes Long-Range Chromosomal Interactions with the Oct4 Locus in Reprogramming and Pluripotency. Cell Stem Cell 2013, 13, 36–47. [Google Scholar] [CrossRef]
- Denholtz, M.; Bonora, G.; Chronis, C.; Splinter, E.; de Laat, W.; Ernst, J.; Pellegrini, M.; Plath, K. Long-Range Chromatin Contacts in Embryonic Stem Cells Reveal a Role for Pluripotency Factors and Polycomb Proteins in Genome Organization. Cell Stem Cell 2013, 13, 602–616. [Google Scholar] [CrossRef]
- Mumbach, M.R.; Satpathy, A.T.; Boyle, E.A.; Dai, C.; Gowen, B.G.; Cho, S.W.; Nguyen, M.L.; Rubin, A.J.; Granja, J.M.; Kazane, K.R.; et al. Enhancer Connectome in Primary Human Cells Identifies Target Genes of Disease-Associated DNA Elements. Nat. Genet. 2017, 49, 1602–1612. [Google Scholar] [CrossRef]
- Giammartino, D.C.D.; Kloetgen, A.; Polyzos, A.; Liu, Y.; Kim, D.; Murphy, D.; Abuhashem, A.; Cavaliere, P.; Aronson, B.; Shah, V.; et al. KLF4 Is Involved in the Organization and Regulation of Pluripotency-Associated Three-Dimensional Enhancer Networks. Nat. Cell Biol. 2019, 21, 1179–1190. [Google Scholar] [CrossRef]
- Giammartino, D.C.D.; Polyzos, A.; Apostolou, E. Transcription Factors: Building Hubs in the 3D Space. Cell Cycle 2020, 19, 2395–2410. [Google Scholar] [CrossRef]
- Johanson, T.M.; Lun, A.T.L.; Coughlan, H.D.; Tan, T.; Smyth, G.K.; Nutt, S.L.; Allan, R.S. Transcription-Factor-Mediated Supervision of Global Genome Architecture Maintains B Cell Identity. Nat Immunol. 2018, 19, 1257–1264. [Google Scholar] [CrossRef]
- Dall’Agnese, A.; Caputo, L.; Nicoletti, C.; di Iulio, J.; Schmitt, A.; Gatto, S.; Diao, Y.; Ye, Z.; Forcato, M.; Perera, R.; et al. Transcription Factor-Directed Re-Wiring of Chromatin Architecture for Somatic Cell Nuclear Reprogramming toward Trans-Differentiation. Mol. Cell 2019, 76, 453–472.e8. [Google Scholar] [CrossRef]
- Bertolini, J.A.; Favaro, R.; Zhu, Y.; Pagin, M.; Ngan, C.Y.; Wong, C.H.; Tjong, H.; Vermunt, M.W.; Martynoga, B.; Barone, C.; et al. Mapping the Global Chromatin Connectivity Network for Sox2 Function in Neural Stem Cell Maintenance. Cell Stem Cell 2019, 24, 462–476.e6. [Google Scholar] [CrossRef]
- Magli, A.; Baik, J.; Pota, P.; Cordero, C.O.; Kwak, I.-Y.; Garry, D.J.; Love, P.E.; Dynlacht, B.D.; Perlingeiro, R.C.R. Pax3 Cooperates with Ldb1 to Direct Local Chromosome Architecture during Myogenic Lineage Specification. Nat. Commun. 2019, 10, 2316. [Google Scholar] [CrossRef]
- Nitzsche, A.; Paszkowski-Rogacz, M.; Matarese, F.; Janssen-Megens, E.M.; Hubner, N.C.; Schulz, H.; de Vries, I.; Ding, L.; Huebner, N.; Mann, M.; et al. RAD21 Cooperates with Pluripotency Transcription Factors in the Maintenance of Embryonic Stem Cell Identity. PLoS ONE 2011, 6, e19470. [Google Scholar] [CrossRef]
- Monahan, K.; Horta, A.; Lomvardas, S. Lhx2/Ldb1-Mediated Trans Interactions Regulate Olfactory Receptor Choice. Nature 2019, 565, 448–453. [Google Scholar] [CrossRef]
- Kagey, M.H.; Newman, J.J.; Bilodeau, S.; Zhan, Y.; Orlando, D.A.; van Berkum, N.L.; Ebmeier, C.C.; Goossens, J.; Rahl, P.B.; Levine, S.S.; et al. Mediator and Cohesin Connect Gene Expression and Chromatin Architecture. Nature 2010, 467, 430–435. [Google Scholar] [CrossRef]
- Barrington, C.; Georgopoulou, D.; Pezic, D.; Varsally, W.; Herrero, J.; Hadjur, S. Enhancer Accessibility and CTCF Occupancy Underlie Asymmetric TAD Architecture and Cell Type Specific Genome Topology. Nat. Commun. 2019, 10, 2908. [Google Scholar] [CrossRef]
- Nora, E.P.; Goloborodko, A.; Valton, A.-L.; Gibcus, J.H.; Uebersohn, A.; Abdennur, N.; Dekker, J.; Mirny, L.A.; Bruneau, B.G. Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization. Cell 2017, 169, 930–944.e22. [Google Scholar] [CrossRef]
- Wutz, G.; Várnai, C.; Nagasaka, K.; Cisneros, D.A.; Stocsits, R.R.; Tang, W.; Schoenfelder, S.; Jessberger, G.; Muhar, M.; Hossain, M.J.; et al. Topologically Associating Domains and Chromatin Loops Depend on Cohesin and Are Regulated by CTCF, WAPL, and PDS5 Proteins. EMBO J. 2017, 36, 3573–3599. [Google Scholar] [CrossRef]
- Zhu, F.; Farnung, L.; Kaasinen, E.; Sahu, B.; Yin, Y.; Wei, B.; Dodonova, S.O.; Nitta, K.R.; Morgunova, E.; Taipale, M.; et al. The Interaction Landscape between Transcription Factors and the Nucleosome. Nature 2018, 562, 76–81. [Google Scholar] [CrossRef]
- Friman, E.T.; Deluz, C.; Meireles-Filho, A.C.; Govindan, S.; Gardeux, V.; Deplancke, B.; Suter, D.M. Dynamic Regulation of Chromatin Accessibility by Pluripotency Transcription Factors across the Cell Cycle. Elife 2019, 8, e50087. [Google Scholar] [CrossRef]
- Francesconi, M.; Stefano, B.D.; Berenguer, C.; de Andrés-Aguayo, L.; Plana-Carmona, M.; Mendez-Lago, M.; Guillaumet-Adkins, A.; Rodriguez-Esteban, G.; Gut, M.; Gut, I.G.; et al. Single Cell RNA-Seq Identifies the Origins of Heterogeneity in Efficient Cell Transdifferentiation and Reprogramming. Elife 2019, 8, e41627. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Zhu, J.; Liu, Y.; Zhang, Q.; Yi, Z.; Chen, S.; Jiao, Z.; Xu, X.; Xu, J.; et al. Single-Cell RNA-Seq Reveals Dynamic Early Embryonic-like Programs during Chemical Reprogramming. Cell Stem Cell 2018, 23, 31–45.e7. [Google Scholar] [CrossRef]
- Biddy, B.A.; Kong, W.; Kamimoto, K.; Guo, C.; Waye, S.E.; Sun, T.; Morris, S.A. Single-Cell Mapping of Lineage and Identity in Direct Reprogramming. Nature 2018, 564, 219–224. [Google Scholar] [CrossRef]
- Schiebinger, G.; Shu, J.; Tabaka, M.; Cleary, B.; Subramanian, V.; Solomon, A.; Gould, J.; Liu, S.; Lin, S.; Berube, P.; et al. Optimal-Transport Analysis of Single-Cell Gene Expression Identifies Developmental Trajectories in Reprogramming. Cell 2019, 176, 928–943.e22. [Google Scholar] [CrossRef]
- Guo, L.; Lin, L.; Wang, X.; Gao, M.; Cao, S.; Mai, Y.; Wu, F.; Kuang, J.; Liu, H.; Yang, J.; et al. Resolving Cell Fate Decisions during Somatic Cell Reprogramming by Single-Cell RNA-Seq. Mol. Cell 2019, 73, 815–829.e7. [Google Scholar] [CrossRef]
- Wapinski, O.L.; Vierbuchen, T.; Qu, K.; Lee, Q.Y.; Chanda, S.; Fuentes, D.R.; Giresi, P.G.; Ng, Y.H.; Marro, S.; Neff, N.F.; et al. Hierarchical Mechanisms for Direct Reprogramming of Fibroblasts to Neurons. Cell 2013, 155, 621–635. [Google Scholar] [CrossRef]
- Gomes, A.M.; Kurochkin, I.; Chang, B.; Daniel, M.; Law, K.; Satija, N.; Lachmann, A.; Wang, Z.; Ferreira, L.; Ma’ayan, A.; et al. Cooperative Transcription Factor Induction Mediates Hemogenic Reprogramming. Cell Rep. 2018, 25, 2821–2835.e7. [Google Scholar] [CrossRef]
- Horisawa, K.; Udono, M.; Ueno, K.; Ohkawa, Y.; Nagasaki, M.; Sekiya, S.; Suzuki, A. The Dynamics of Transcriptional Activation by Hepatic Reprogramming Factors. Mol. Cell 2020, 79, 660–676.e8. [Google Scholar] [CrossRef]
- Rosa, F.F.; Pires, C.F.; Kurochkin, I.; Halitzki, E.; Zahan, T.; Arh, N.; Zimmermannová, O.; Ferreira, A.G.; Li, H.; Karlsson, S.; et al. Single-Cell Transcriptional Profiling Informs Efficient Reprogramming of Human Somatic Cells to Cross-Presenting Dendritic Cells. Sci. Immunol. 2022, 7, eabg5539. [Google Scholar] [CrossRef]
- Adli, M. The CRISPR Tool Kit for Genome Editing and Beyond. Nat. Commun. 2018, 9, 1911. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next Generation of CRISPR–Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Nakamura, M.; Gao, Y.; Dominguez, A.A.; Qi, L.S. CRISPR Technologies for Precise Epigenome Editing. Nat. Cell Biol. 2021, 23, 11–22. [Google Scholar] [CrossRef]
- Wheat, J.C.; Sella, Y.; Willcockson, M.; Skoultchi, A.I.; Bergman, A.; Singer, R.H.; Steidl, U. Single-Molecule Imaging of Transcription Dynamics in Somatic Stem Cells. Nature 2020, 583, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Heller, I.; Sitters, G.; Broekmans, O.D.; Farge, G.; Menges, C.; Wende, W.; Hell, S.W.; Peterman, E.J.G.; Wuite, G.J.L. STED Nanoscopy Combined with Optical Tweezers Reveals Protein Dynamics on Densely Covered DNA. Nat. Methods 2013, 10, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Tutucci, E.; Vera, M.; Biswas, J.; Garcia, J.; Parker, R.; Singer, R.H. An Improved MS2 System for Accurate Reporting of the MRNA Life Cycle. Nat. Methods 2018, 15, 81–89. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörnblad, A.; Remeseiro, S. Epigenetics, Enhancer Function and 3D Chromatin Organization in Reprogramming to Pluripotency. Cells 2022, 11, 1404. https://doi.org/10.3390/cells11091404
Hörnblad A, Remeseiro S. Epigenetics, Enhancer Function and 3D Chromatin Organization in Reprogramming to Pluripotency. Cells. 2022; 11(9):1404. https://doi.org/10.3390/cells11091404
Chicago/Turabian StyleHörnblad, Andreas, and Silvia Remeseiro. 2022. "Epigenetics, Enhancer Function and 3D Chromatin Organization in Reprogramming to Pluripotency" Cells 11, no. 9: 1404. https://doi.org/10.3390/cells11091404
APA StyleHörnblad, A., & Remeseiro, S. (2022). Epigenetics, Enhancer Function and 3D Chromatin Organization in Reprogramming to Pluripotency. Cells, 11(9), 1404. https://doi.org/10.3390/cells11091404






