Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton’s Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design as Part of the Product Development Programme
2.2. Cell Cultures
2.3. Cell Proliferation Assay
2.4. Flow Cytometry
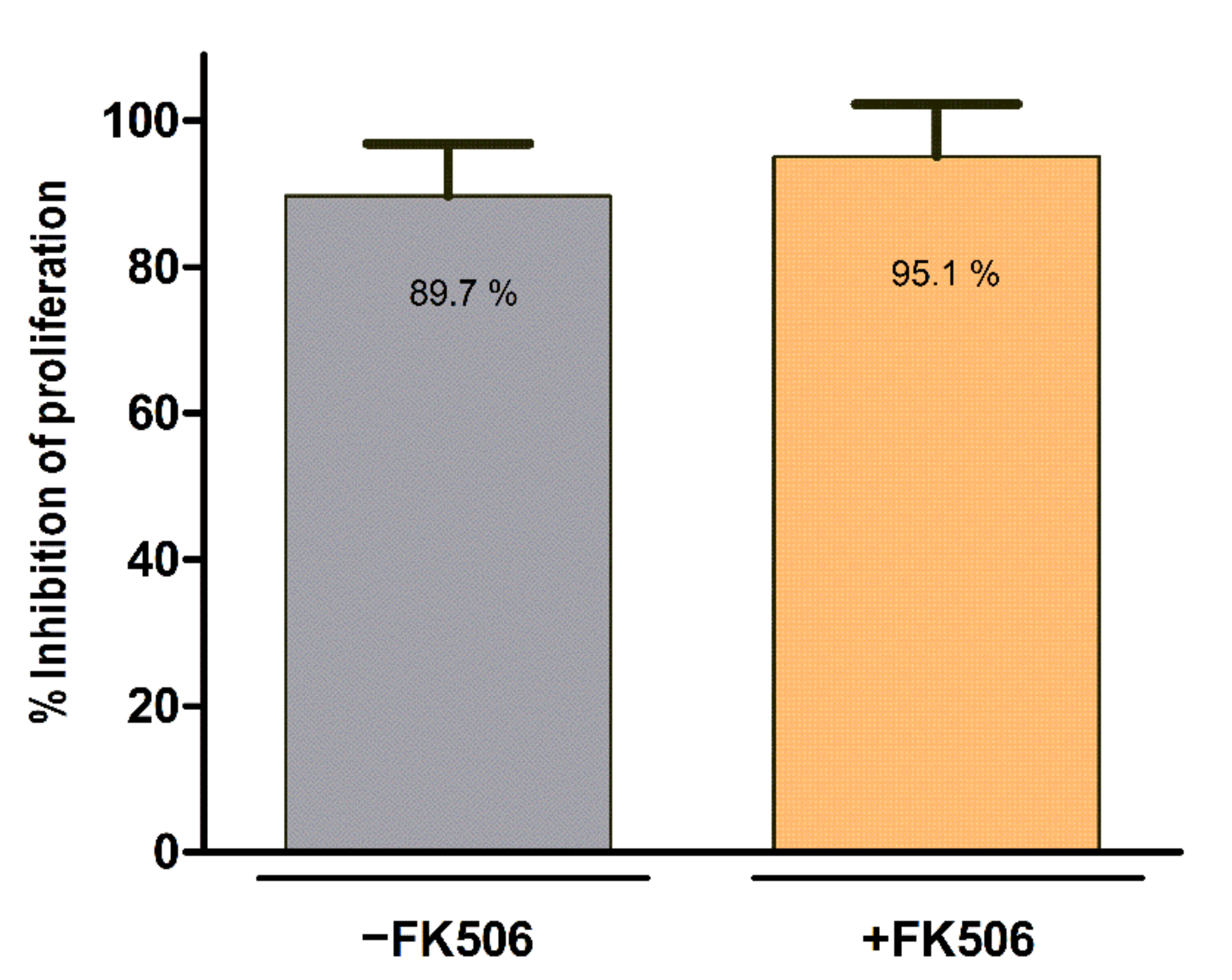
2.5. Lymphocyte Proliferation Assay
2.6. Subchronic In Vivo Toxicity and Biodistribution Study
2.7. Spinal Cord Injury Contusion Model
2.7.1. Cell Administration
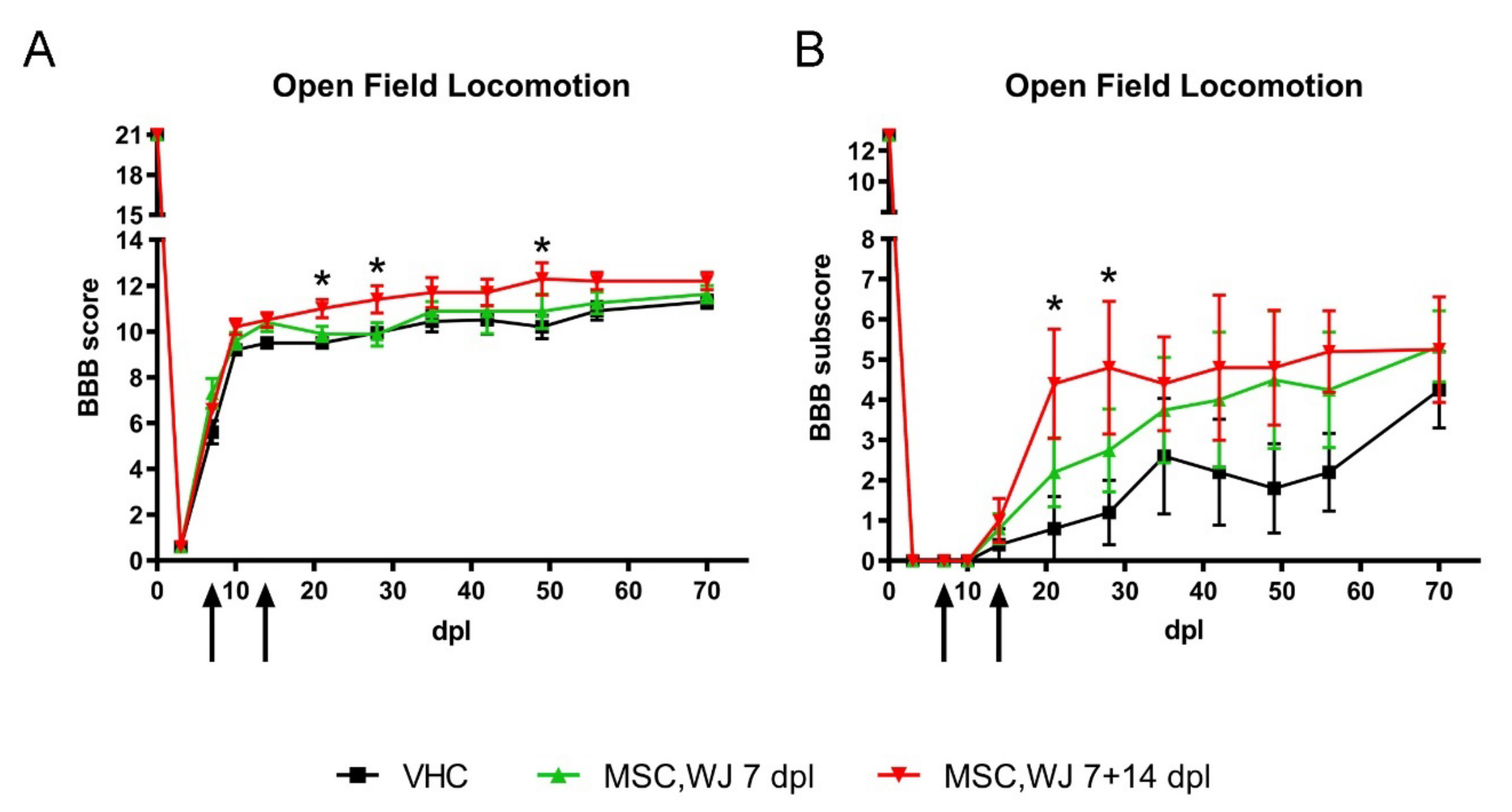
2.7.2. Functional Assessment
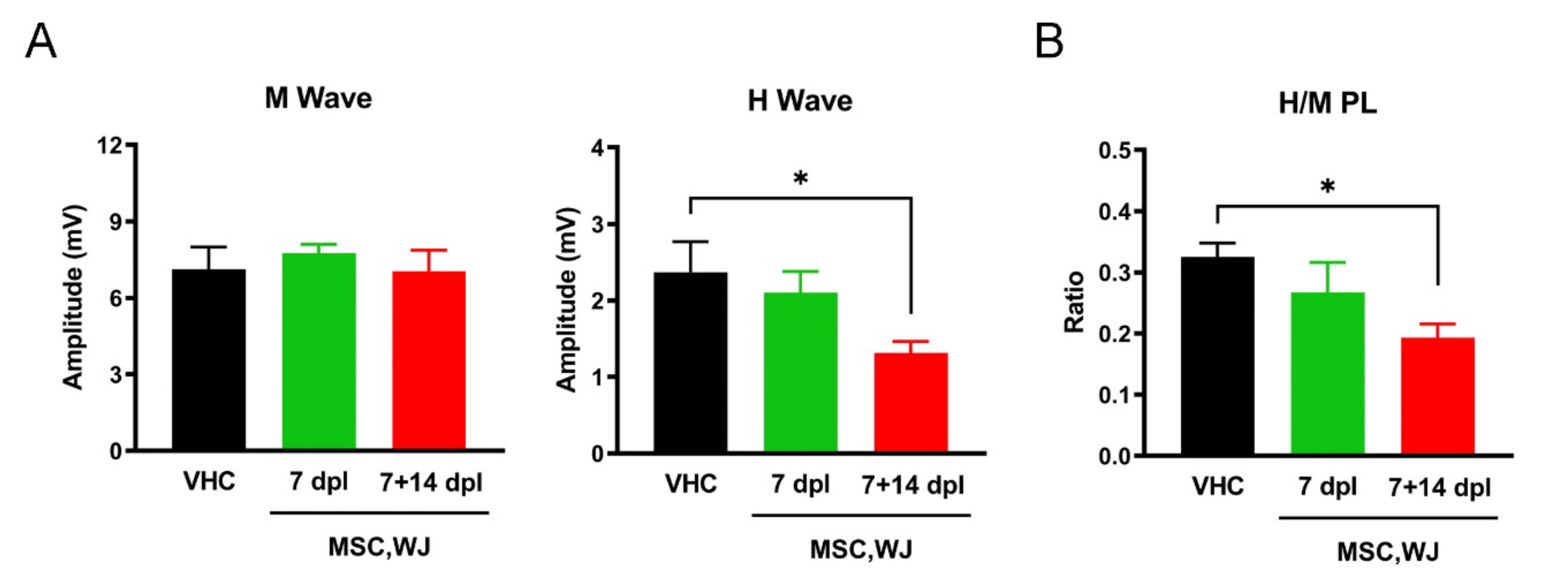
2.7.3. Electrophysiological Tests
2.7.4. Histology
2.8. Statistical Analysis
3. Results
3.1. Characteristics of MSC,WJ
3.2. In Vivo Studies
3.2.1. Toxicity of MSC,WJ Intrathecal Administration
3.2.2. Effects of MSC,WJ on Functional Recovery after SCI
3.3. Histological Results
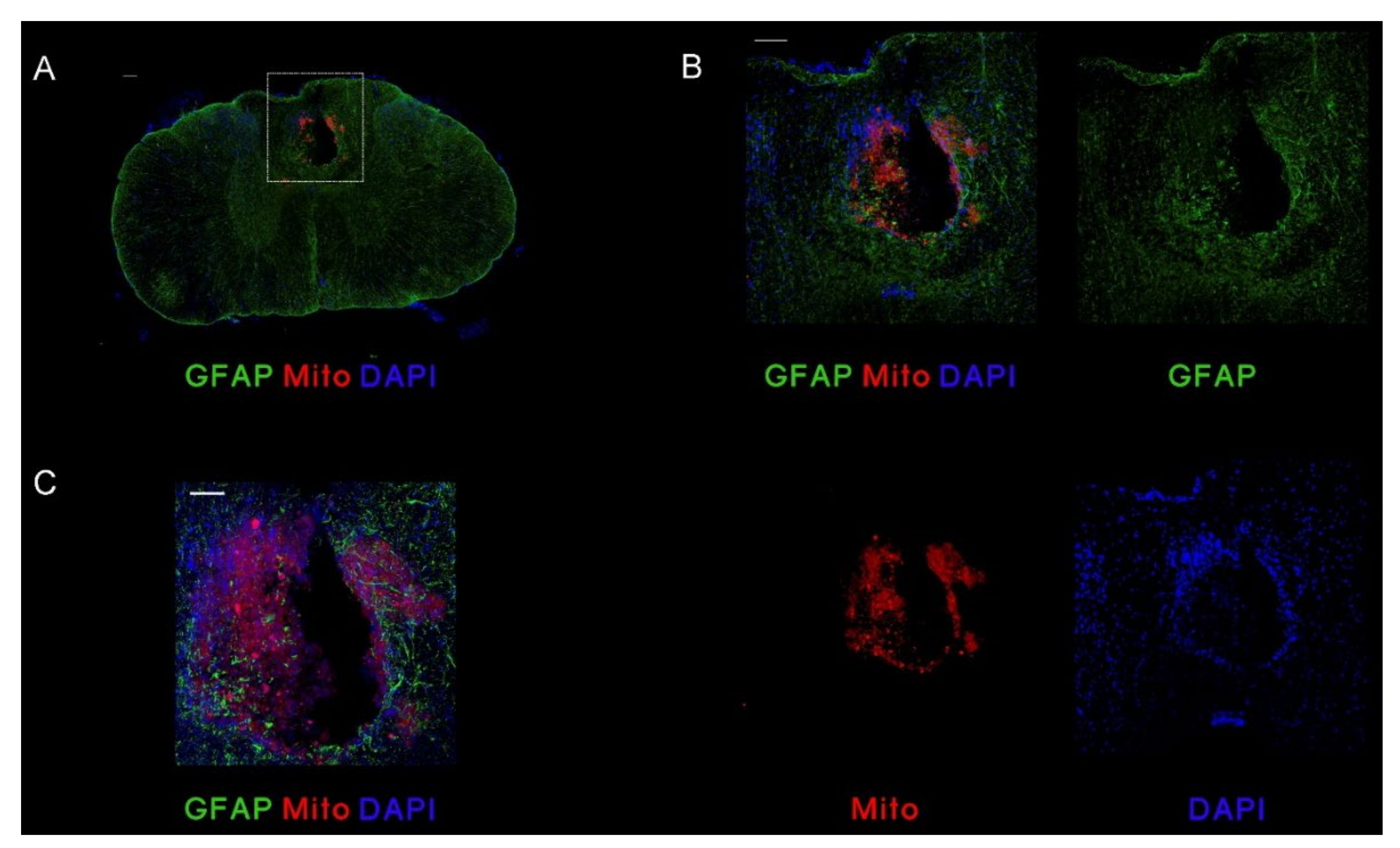
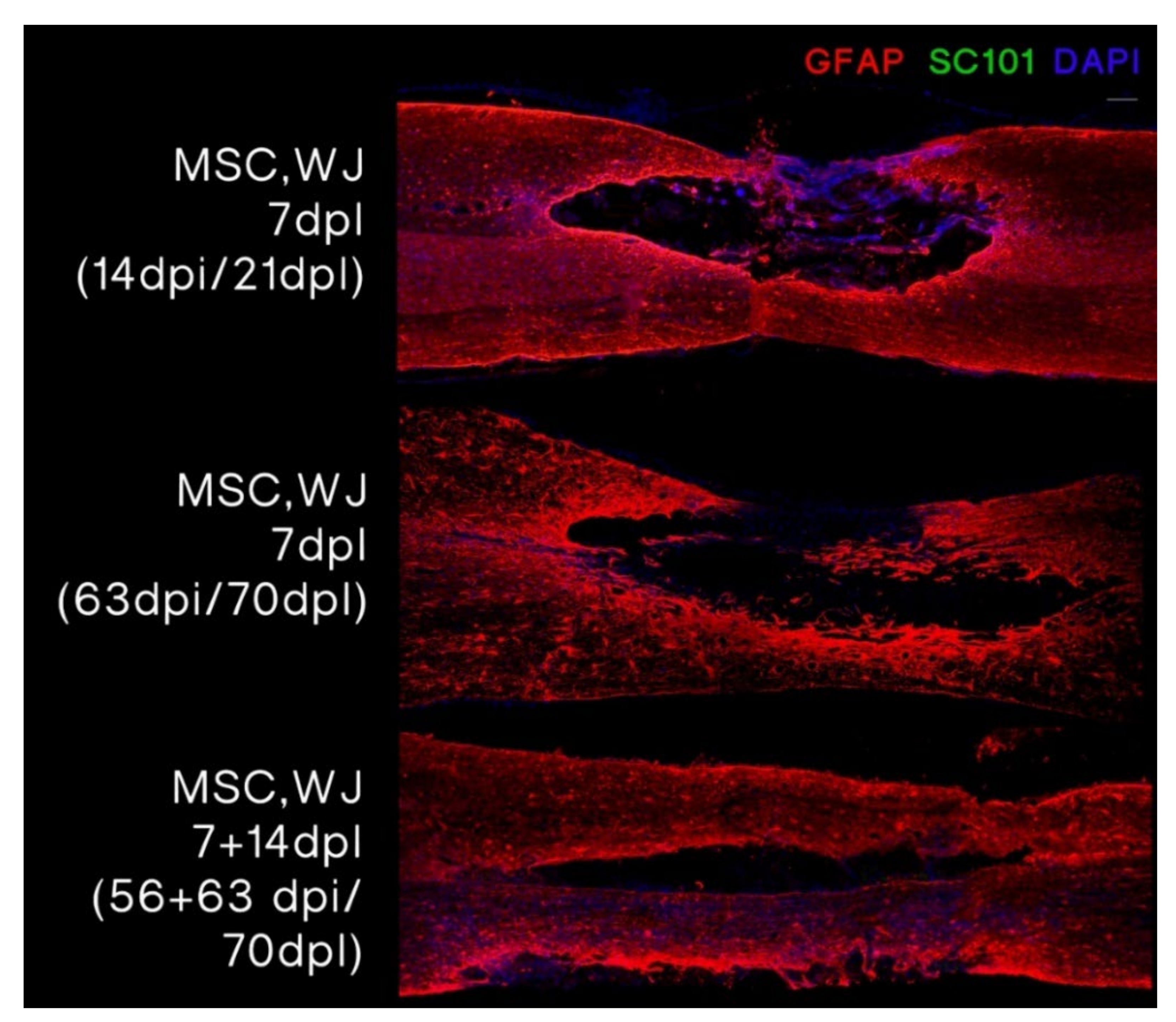
3.3.1. MSC,WJ Localization after Intrathecal Administration
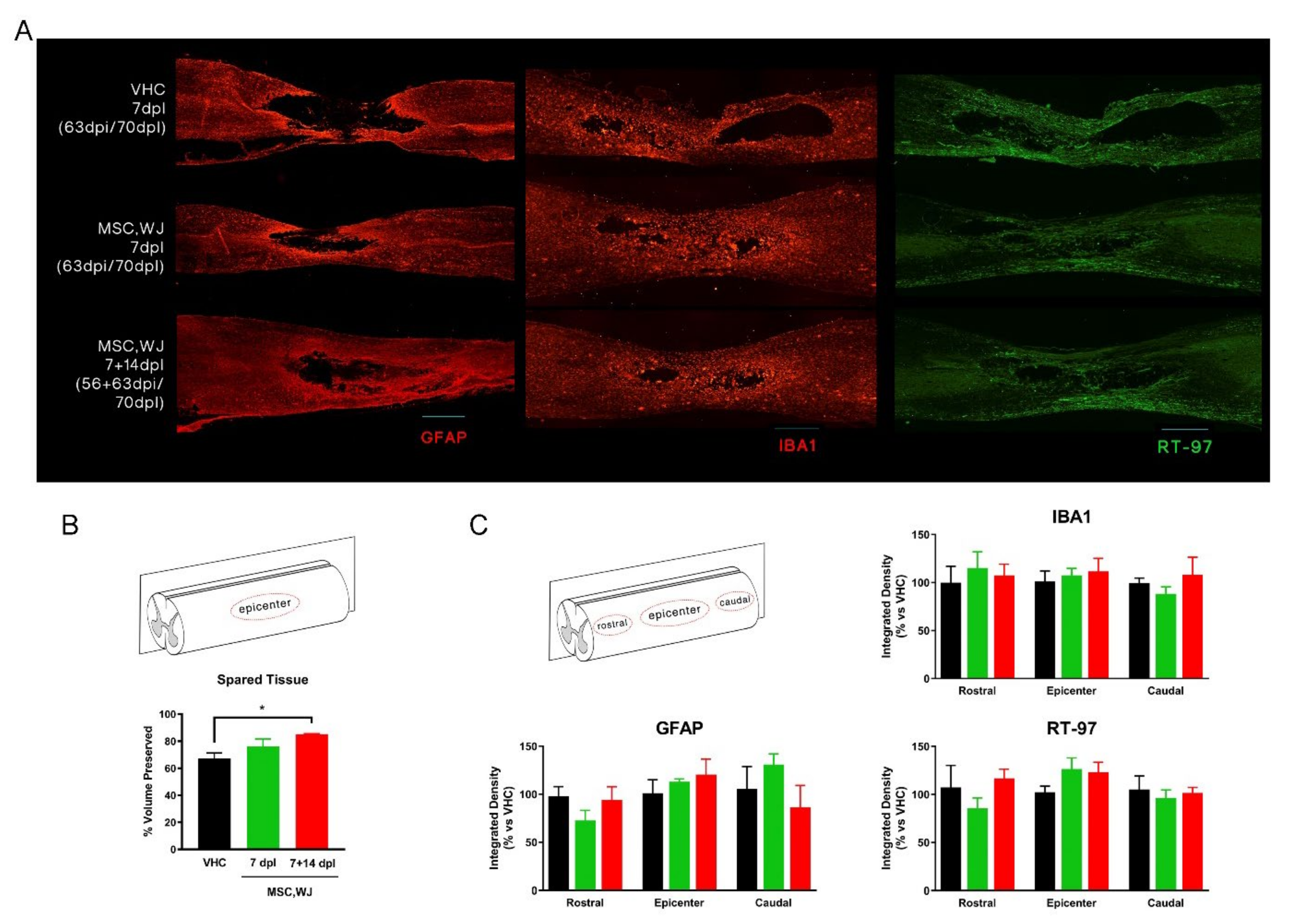
3.3.2. Spared Cord Tissue after SCI and Inflammatory Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BALT | Bronchus-Associated Lymphoid Tissue |
| BBB | Basso, Beattie, Bresnahan |
| CFSE | carboxy–fluorescein diacetate succinimidyl ester |
| CMAP | compound muscle action potential |
| CPD | Cumulative Population Doublings |
| CQA | Critical Quality Attributes |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| Dpi | days post-injection |
| dpl | days post-lesion |
| GM | gastrocnemius medialis |
| GMP | Good Manufacturing Practice |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| hSer | human serum |
| IMPD | Investigational Medicinal Product Dossier |
| MEP | Motor evoked potential |
| MNC | motor nerve conduction |
| MSC | multipotent Mesenchymal Stromal cells |
| NAD | nucleic acid detection |
| NC | nucleated cells |
| NDS | normal donkey serum |
| NIH | National Institutes of Health |
| OECD | Organisation for Economic Co-operation and Development |
| PBS | phosphate buffered saline |
| PD | pharmacodynamics |
| PFA | paraformaldehyde |
| PK | pharmacokinetics |
| PL | plantar interossei |
| PMA | Phorbol 12-myristate 13-acetate |
| SCI | Spinal Cord Injury |
| SEM | standard error of the mean |
| TA | tibialis anterior |
| Tox | Toxicology |
| WJ | Wharton’s jelly |
References
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Wolfe, D.L.; Team SCIRESR. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-H.; Ha, K.-Y.; Kim, S.-I. Spinal Cord Injury and Related Clinical Trials. Clin. Orthop. Surg. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Rasmusson, I.; Ringdén, O.; Sundberg, B.; Le Blanc, K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp. Cell Res. 2005, 305, 33–41. [Google Scholar] [CrossRef]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Oliver-Vila, I.; Ramírez-Moncayo, C.; Grau-Vorster, M.; Marín-Gallén, S.; Caminal, M.; Vives, J. Optimisation of a potency assay for the assessment of immunomodulative potential of clinical grade multipotent mesenchymal stromal cells. Cytotechnology 2018, 70, 31–44. [Google Scholar] [CrossRef]
- GGrau-Vorster, M.; Rodríguez, L.; del Mazo-Barbara, A.; Mirabel, C.; Blanco, M.; Codinach, M.; Gómez, S.G.; Querol, S.; García-López, J.; Vives, J. Compliance with Good Manufacturing Practice in the Assessment of Immunomodulation Potential of Clinical Grade Multipotent Mesenchymal Stromal Cells Derived from Wharton’s Jelly. Cells 2019, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Uccelli, A.; Laroni, A.; Freedman, M.S. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011, 10, 649–656. [Google Scholar] [CrossRef]
- Oliveri, R.S.; Bello, S.; Biering-Sørensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodríguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2021, 23, 146–156. [Google Scholar] [CrossRef]
- Gastelurrutia, P.; Prat-Vidal, C.; Vives, J.; Coll, R.; Bayes-Genis, A.; Gálvez-Montón, C. Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience. Front. Cardiovasc. Med. 2021, 8, 604434. [Google Scholar] [CrossRef]
- Vives, J.; Blanco, M.; Caminal, M.; Coca, M.I.; Codinach, M.; Coll, R.; Doral, M.; Lloret, M.; Oliver-Vila, I.; Ortega, I.; et al. Development of an advanced cell therapy product indicated for the treatment of gonarthrosis. BMC Proc. 2015, 9, O9. [Google Scholar] [CrossRef] [Green Version]
- Vives, J.; Carmona, G.; Vives, J.; Carmona, G. Guide to Cell Therapy GxP, 1st ed.; Academic Press (Elsevier): Amsterdam, The Netherlands, 2015; p. 266. [Google Scholar]
- Oliver-Vila, I.; Coca, M.I.; Grau-Vorster, M.; Pujals-Fonts, N.; Caminal, M.; Casamayor-Genescà, A. Evaluation of a cell-banking strategy for the production of clinical grade mesenchymal stromal cells from Wharton’s jelly. Cytotherapy 2016, 18, 25–35. [Google Scholar] [CrossRef]
- García-Muñoz, E.; Vives, J. Towards the standardization of methods of tissue processing for the isolation of mesenchymal stromal cells for clinical use. Cytotechnology 2021, 73, 513–522. [Google Scholar] [CrossRef]
- Vivas, D.; Grau-Vorster, M.; Oliver-Vila, I.; García-López, J.; Vives, J. Evaluation of a cell-based osteogenic formulation compliant with good manufacturing practice for use in tissue engineering. Mol. Biol. Rep. 2020, 47, 5145–5154. [Google Scholar] [CrossRef]
- Codinach, M.; Blanco, M.; Ortega, I.; Lloret, M.; Reales, L.; Coca, M.I.; Torrents, S.; Doral, M.; Oliver-Vila, I.; Requena-Montero, M.; et al. Design and validation of a consistent and reproducible manufacture process for the production of clinical-grade bone marrow–derived multipotent mesenchymal stromal cells. Cytotherapy 2016, 18, 1197–1208. [Google Scholar] [CrossRef]
- Reyes, B.; Coca, M.I.; Codinach, M.; López-Lucas, M.D.; del Mazo-Barbara, A.; Caminal, M.; Oliver-Vila, I.; Cabañas, V.; Lope-Piedrafita, S.; García-López, J.; et al. Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies. Cytotherapy 2017, 19, 1060–1069. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Forés, J.; Herrando-Grabulosa, M.; Valls, R.; Leiva-Rodríguez, T.; Galea, E.; González-Pérez, F.; Navarro, X.; Petegnief, V.; Bosch, A.; et al. Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence. Sci. Rep. 2018, 8, 1879. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.; Beattie, M.S.; Bresnahan, J.C. Graded Histological and Locomotor Outcomes after Spinal Cord Contusion Using the NYU Weight-Drop Device versus Transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.M. Behavioral Testing After Spinal Cord Injury: Congruities, Complexities, and Controversies. J. Neurotrauma 2004, 21, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Avendano, C.; Roda, J.; Carceller, F.; Díez-Tejedor, E. Morphometric study of focal cerebral ischemia in rats: A stereological evaluation. Brain Res. 1995, 673, 83–92. [Google Scholar] [CrossRef]
- RRedondo-Castro, E.; Navarro, X.; García-Alías, G. Longitudinal Evaluation of Residual Cortical and Subcortical Motor Evoked Potentials in Spinal Cord Injured Rats. J. Neurotrauma 2016, 33, 907–916. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Vives, J.; Mirabel, C. Multipotent Mesenchymal Stromal Cells From Bone Marrow for Current and Potential Clinical Applications. In Reis RL, editor. Encyclopedia of Tissue Engineering and Regenerative Medicine; Oxford: Oxford, UK, 2019; pp. 503–512. [Google Scholar]
- Reis, M.; Ogonek, J.; Qesari, M.; Borges, N.M.; Nicholson, L.; Preußner, L.; Dickinson, A.M.; Wang, X.-N.; Weissinger, E.M.; Richter, A. Recent Developments in Cellular Immunotherapy for HSCT-Associated Complications. Front. Immunol. 2016, 7, 500. [Google Scholar] [CrossRef] [Green Version]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Canadian Critical Care Trials Group. Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Mei, S.H.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.; et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. eClinicalMedicine 2020, 19, 100249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramallo, M.; Carreras-Sánchez, I.; López-Fernández, A.; Vélez, R.; Aguirre, M.; Feldman, S.; Vives, J. Advances in translational orthopaedic research with species-specific multipotent mesenchymal stromal cells derived from the umbilical cord. Histol. Histopathol. 2020, 36, 18249. [Google Scholar]
- García-Fernández, C.; López-Fernández, A.; Borrós, S.; Lecina, M.; Vives, J. Strategies for large-scale expansion of clinical-grade human multipotent mesenchymal stromal cells. Biochem. Eng. J. 2020, 159, 107601. [Google Scholar] [CrossRef]
- Prat-Vidal, C.; Rodríguez-Gómez, L.; Aylagas, M.; Nieto-Nicolau, N.; Gastelurrutia, P.; Agustí, E.; Gálvez-Montón, C.; Jorba, I.; Teis, A.; Monguió-Tortajada, M.; et al. First-in-human PeriCord cardiac bioimplant: Scalability and GMP manufacturing of an allogeneic engineered tissue graft. eBioMedicine 2020, 54, 102729. [Google Scholar] [CrossRef]
- Garcia-Lopez, J.; Delgadillo, J.; Vilarrodona, A.; Querol, S.; Ovejo, J.; Coll, R.; Millan, A.; Madrigal, A.; Soria, G.; Vidal, F.; et al. SARS-CoV-2/COVID-19 pandemic: First wave, impact, response and lessons learnt in a fully integrated Regional Blood and Tissue Bank. A narrative report. Blood Transfus. 2021, 19, 158–167. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sanchez-Diaz, M.; Quiñones-Vico, M.; de la Torre, R.S.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Barberini, D.J.; Aleman, M.; Aristizábal, F.; Spriet, M.; Clark, K.C.; Walker, N.J.; Galuppo, L.D.; Amorim, R.M.; Woolard, K.D.; Borjesson, D.L. Safety and tracking of intrathecal allogeneic mesenchymal stem cell transplantation in healthy and diseased horses. Stem Cell Res. Ther. 2018, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Cheriyan, J.; Ryan, D.J.; Weinreb, J.H.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T. Spinal cord injury models: A review. Spinal Cord 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Mothe, A.J.; Bozkurt, G.; Catapano, J.; Zabojova, J.; Wang, X.; Keating, A.; Tator, C.H. Intrathecal transplantation of stem cells by lumbar puncture for thoracic spinal cord injury in the rat. Spinal Cord 2011, 49, 967–973. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, T.-T.; Tian, Z.-M.; Gao, H.; Wen, H.-Q.; Pang, M.; He, W.-J.; Wang, N.-X.; Chen, Y.-Y.; Wang, Y.; et al. Subarachnoid transplantation of human umbilical cord mesenchymal stem cell in rodent model with subacute incomplete spinal cord injury: Preclinical safety and efficacy study. Exp. Cell Res. 2020, 395, 112184. [Google Scholar] [CrossRef]

| GxP | Type | Description | Ref. |
|---|---|---|---|
| GMP | Production process and Product characterisation | Validation of a bioprocess design for the derivation and scale up of human MSC,WJ for clinical use | [9,16] |
| GLP | Safety in vitro | Assessment of telomerase activity, proto-oncogene expression, G-banding karyotype and senescence of clinical grade human MSC,WJ | [16] |
| GLP | Safety in vivo | Biodistribution and toxicity assessment after administration of clinical grade human MSC,WJ in immunodeficient mice by intrathecal and tail vein injection | [16] |
| n/a | Product characterisation | Evaluation of potential interaction of FK506 and MSC,WJ in terms of viability, identity, proliferation and immunopotency | This work |
| GLP | PK/Tox in vivo | Subchronic toxicity assessment and biodistribution of human MSC,WJ after intrathecal administration in immunodeficient rats | This work |
| n/a | PD/PK in vivo | Evaluation of the effect and persistence of intrathecal administration of clinical grade human MSC,WJ in chronic traumatic model of SCI in rat | This work |
| GCP | Phase I/IIa | Randomized double-blind, crossover, placebo-controlled phase I/IIa clinical trial in 10 patients (7 males, 3 females, age range: 25–47 years) with chronic complete SCI (AIS-A) at dorsal level (T2-T11) (1 dose 1 × 107 MSC,WJ). | [12] EudraCT No. 2015-005786-23; Clinicaltrials.gov Id. NCT03003364 |
| GCP | Phase I/IIa | Randomized double-blind, placebo-controlled phase I/II clinical trial in patients with chronic (1 to 5 years) incomplete cervical lesion (2 doses 1 × 106 MSC,WJ/Kg) | EudraCT No. 2021-000346-18; Clinicaltrials.gov Id. NCT05054803 |
| Acceptance Criteria | Values (%) | |||
|---|---|---|---|---|
| Viability | ≥70% | 97.2 | ||
| Identity | Positive (≥95%) for CD 105, CD 90, CD 73 | CD105 99.8 | CD90 99.9 | CD73 99.8 |
| Negative (≤5 %) for CD 45, CD 31, HLA-DR | CD45 0.0 | CD31 0.0 | HLA-DR 0.0 | |
| Acceptance Criteria | Values (%) (−FK506/+FK506) | |||
|---|---|---|---|---|
| Viability | ≥70% | 95.7/93.9 | ||
| Identity | Positive (≥95%) for CD 105, CD 90, CD 73 | CD105 99.5/99.8 | CD90 99.9/99.8 | CD73 99.5/99.5 |
| Negative (≤5 %) for CD 45, CD 31, HLA-DR | CD45 0.0/0.0 | CD31 0.0/0.0 | HLA-DR 0.0/0.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vives, J.; Hernández, J.; Mirabel, C.; Puigdomenech-Poch, M.; Romeo-Guitart, D.; Marmolejo-Martínez-Artesero, S.; Cabrera-Pérez, R.; Jaramillo, J.; Kumru, H.; García-López, J.; et al. Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton’s Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance. Cells 2022, 11, 2153. https://doi.org/10.3390/cells11142153
Vives J, Hernández J, Mirabel C, Puigdomenech-Poch M, Romeo-Guitart D, Marmolejo-Martínez-Artesero S, Cabrera-Pérez R, Jaramillo J, Kumru H, García-López J, et al. Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton’s Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance. Cells. 2022; 11(14):2153. https://doi.org/10.3390/cells11142153
Chicago/Turabian StyleVives, Joaquim, Joaquim Hernández, Clémentine Mirabel, Maria Puigdomenech-Poch, David Romeo-Guitart, Sara Marmolejo-Martínez-Artesero, Raquel Cabrera-Pérez, Jessica Jaramillo, Hatice Kumru, Joan García-López, and et al. 2022. "Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton’s Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance" Cells 11, no. 14: 2153. https://doi.org/10.3390/cells11142153
APA StyleVives, J., Hernández, J., Mirabel, C., Puigdomenech-Poch, M., Romeo-Guitart, D., Marmolejo-Martínez-Artesero, S., Cabrera-Pérez, R., Jaramillo, J., Kumru, H., García-López, J., Vidal-Samsó, J., Navarro, X., & Coll-Bonet, R. (2022). Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton’s Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance. Cells, 11(14), 2153. https://doi.org/10.3390/cells11142153







