Continuous Percoll Gradient Centrifugation of Erythrocytes—Explanation of Cellular Bands and Compromised Age Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Collection, RBC Preparation, and Solutions
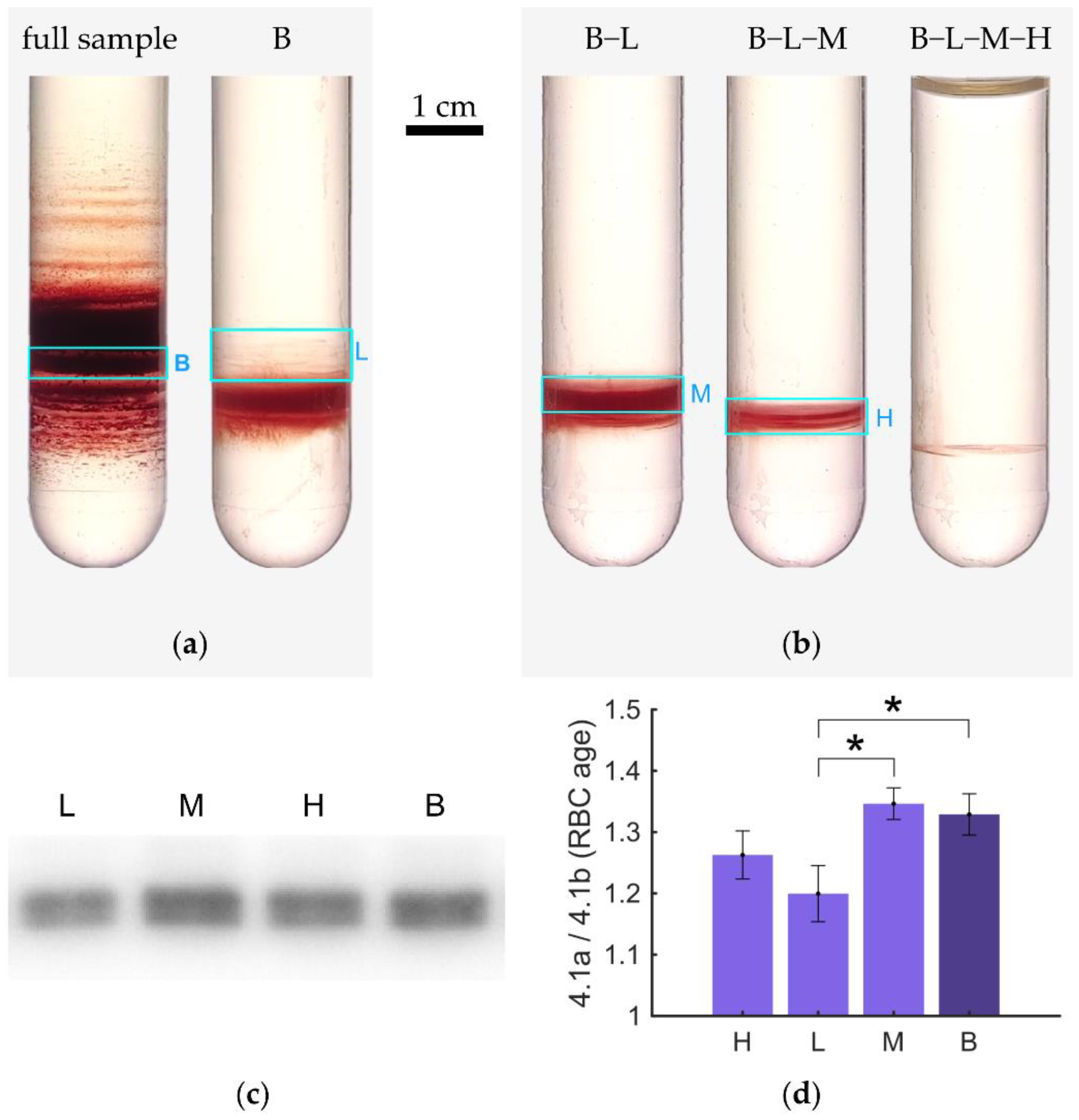
2.2. Percoll Density Preparation, Centrifugation, and Sub-Band Extraction
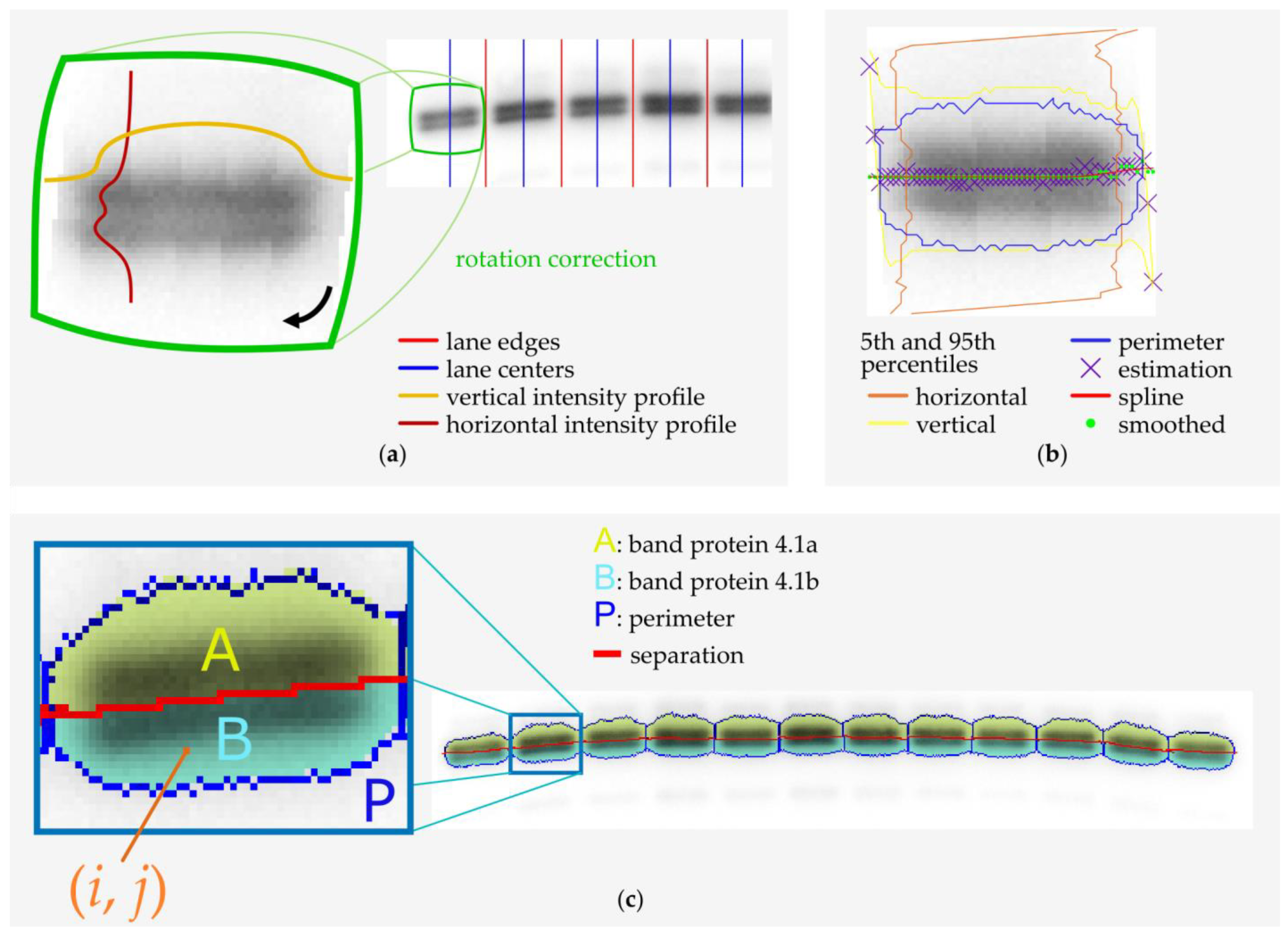
2.3. Microscopy and Image Analysis
2.4. Band 4.1 Protein Deamidation Detection by Western Blot
2.5. Statistical Analysis
3. Results
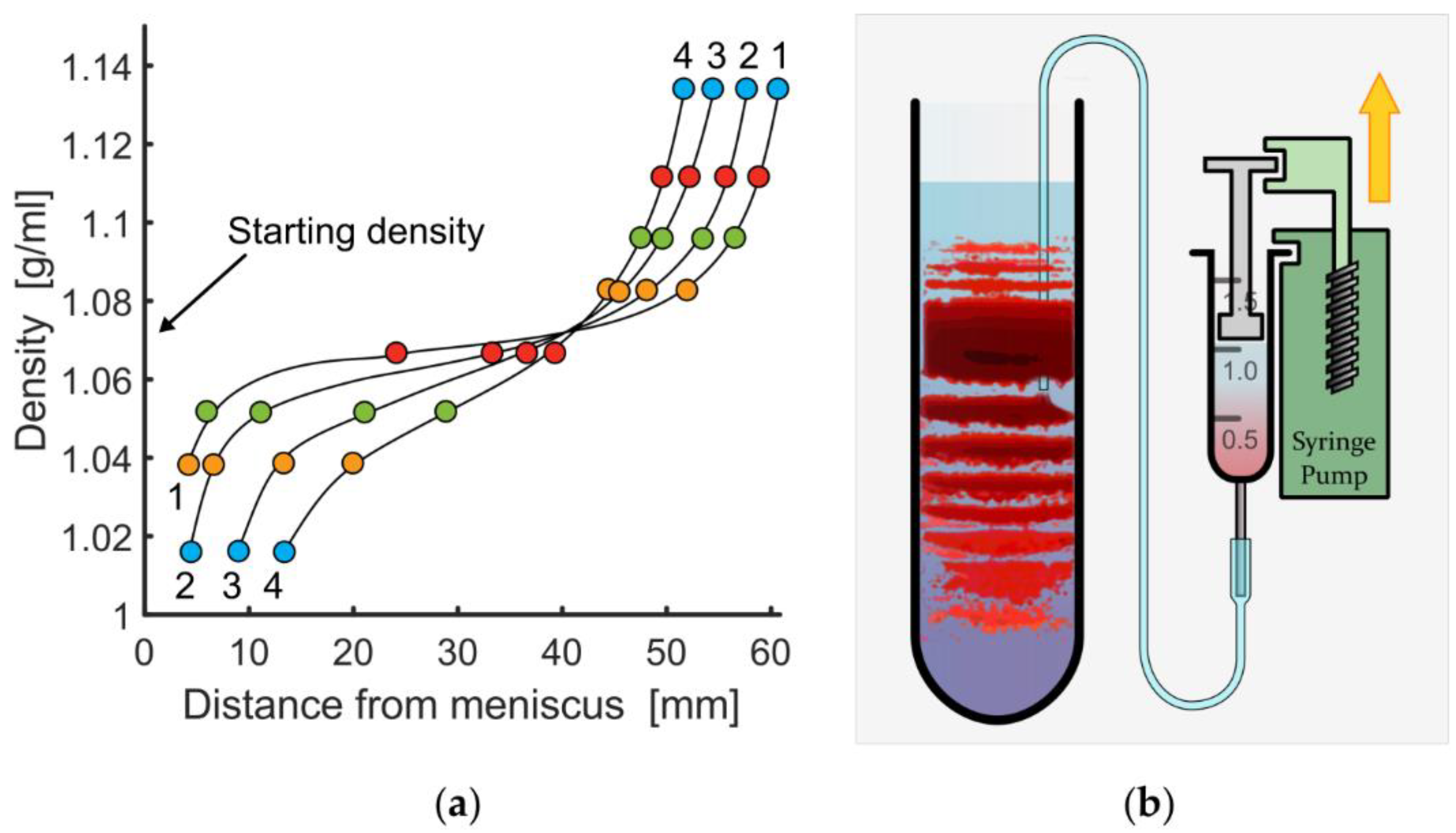
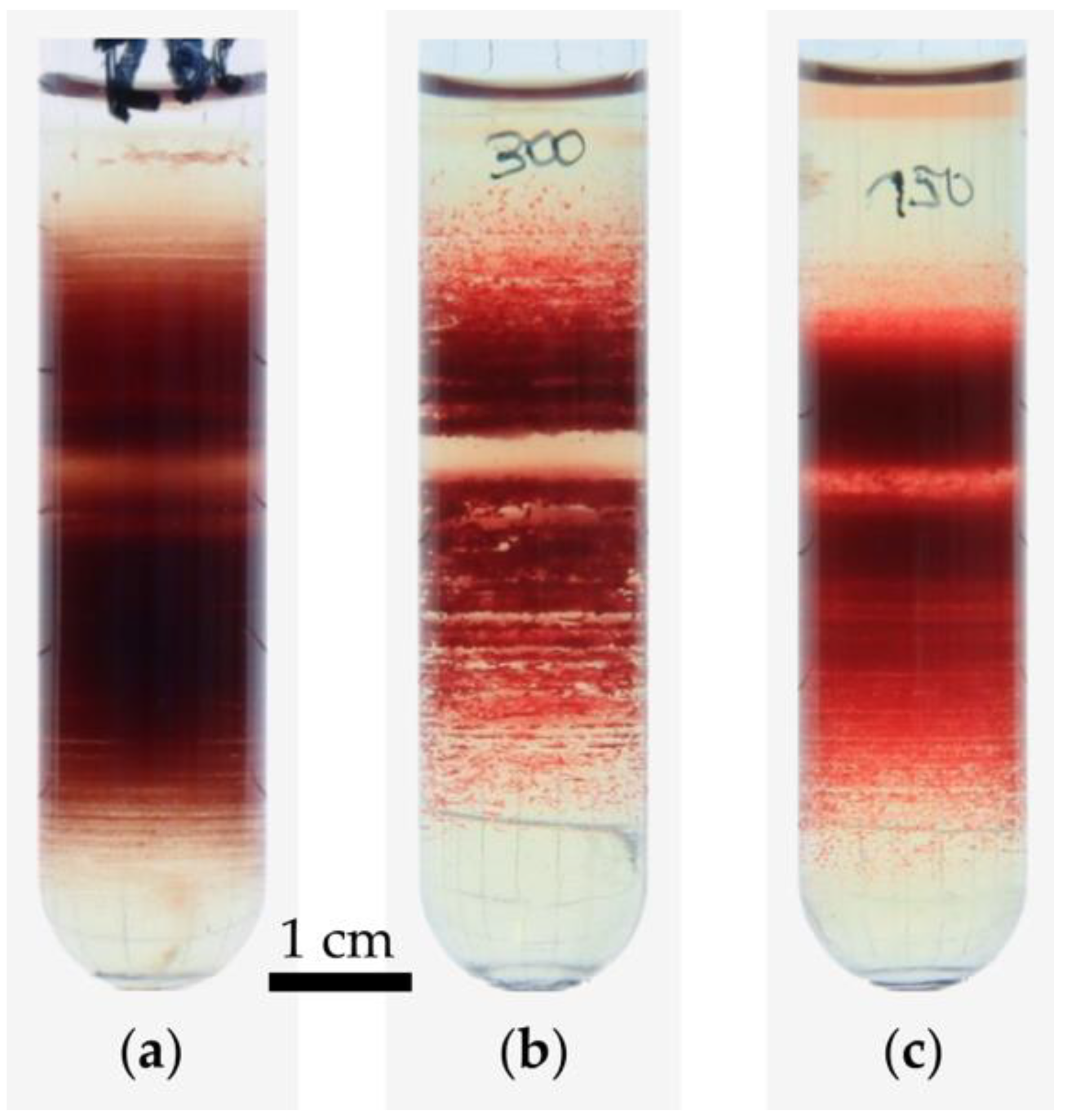
3.1. Variation of Basic Experimental Parameters
3.2. Recentrifugation of Particular Bands
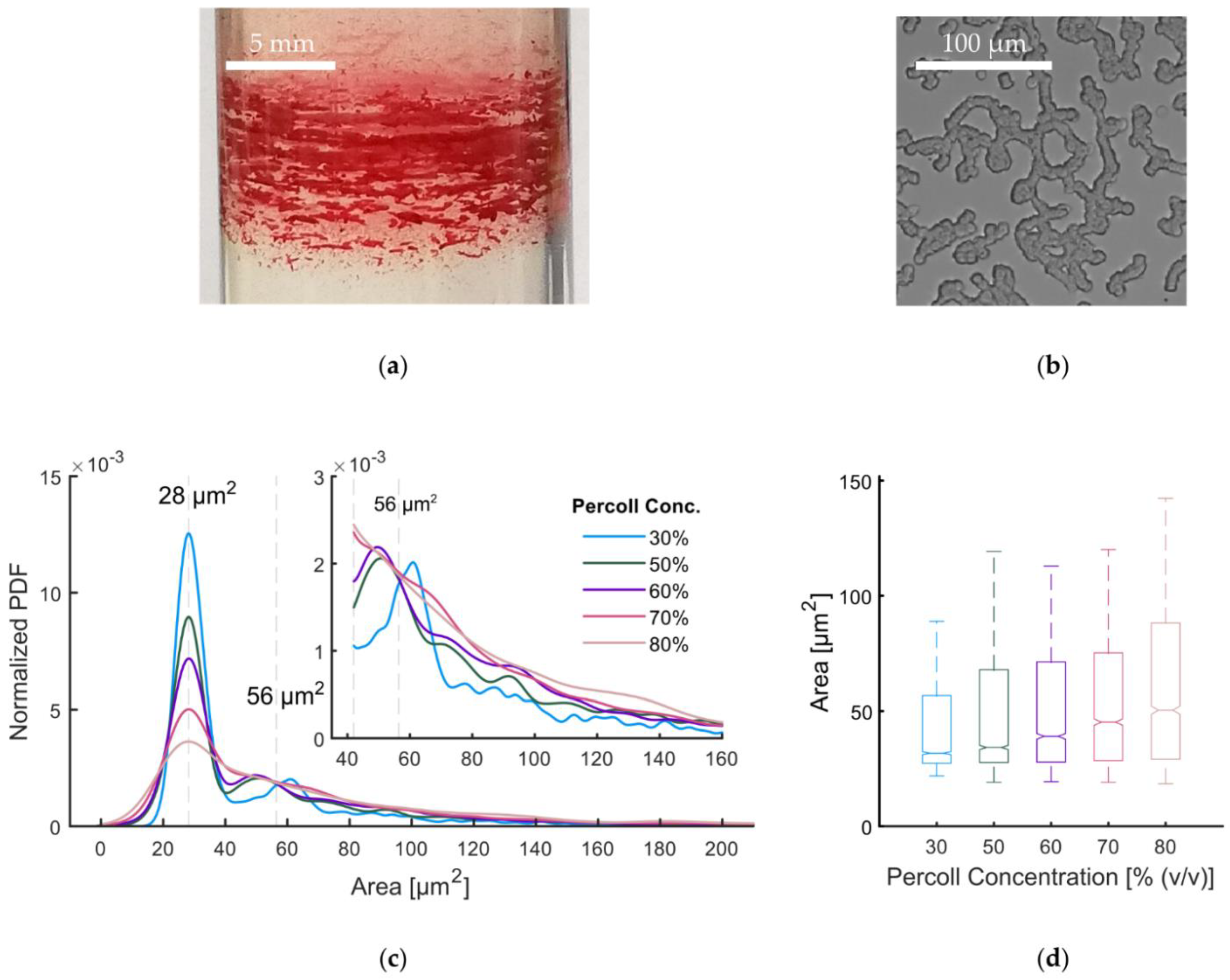
3.3. Percoll-Induced Cell Aggregation
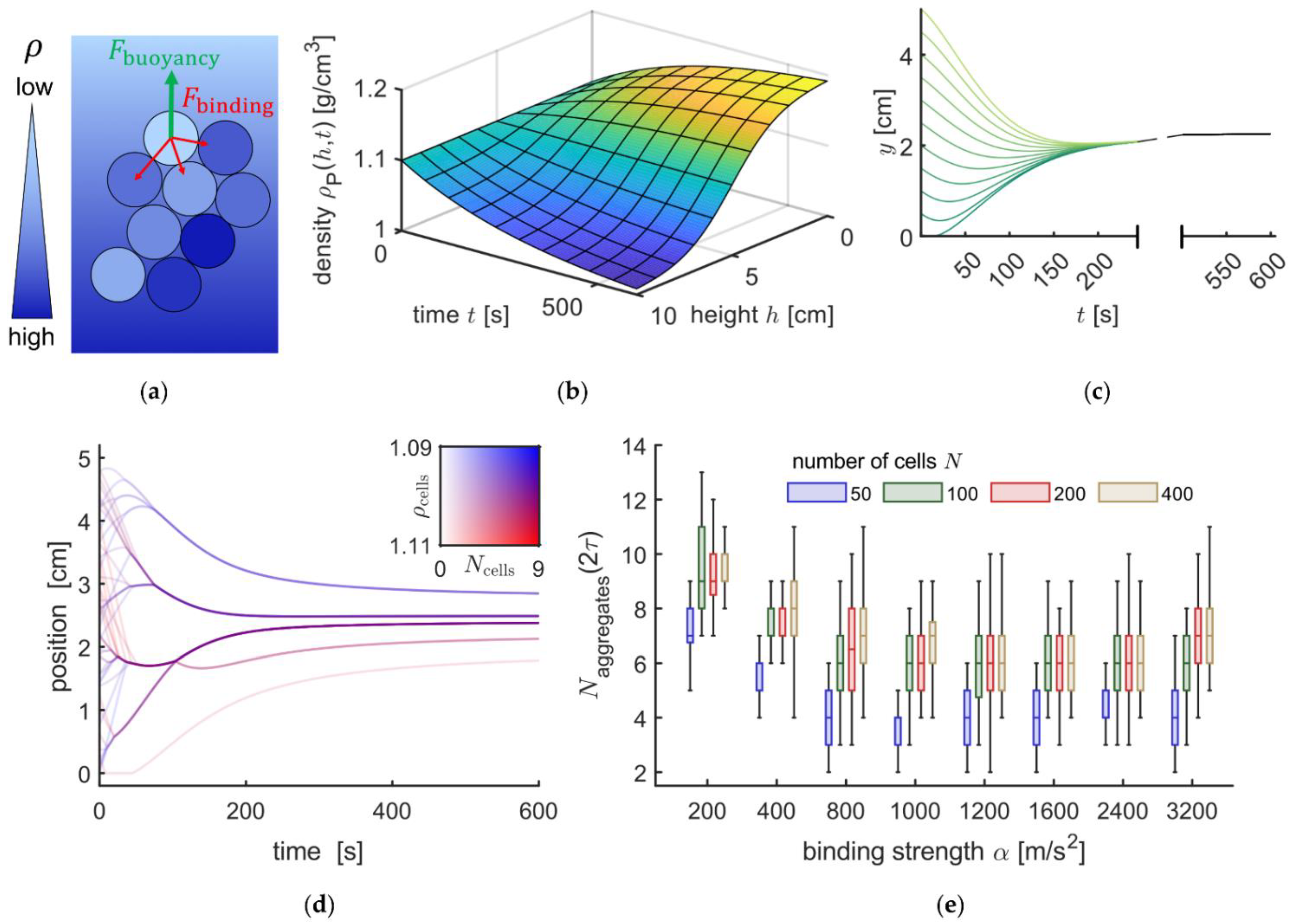
3.4. Mathematical Modelling of the Sedimentation Process
3.5. Experimental Conditions Suppressing RBC Aggregate Formation
3.6. RBC Age Determination in Hypotonic Solutions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
- The displacement force (buoyancy) that includes acceleration of cell volume and liquid volume must be equal to the sum of binding forces for each cell.
- The average of all cell densities in an aggregate equals the suspension density at the average position of those cells.
References
- Fahraeus, R. The Suspension Stability of the Blood. Physiol. Rev. 1929, 9, 241–274. [Google Scholar] [CrossRef]
- Mateyko, G.M.; Kopac, M.J. Part II: Isopyknotic Cushioning during High-Speed Centrifugation. Ann. N. Y. Acad. Sci. 1963, 105, 219–285. [Google Scholar] [CrossRef]
- Pertoft, H.; Laurent, T.C. Isopycnic Separation of Cells and Cell Organelles by Centrifugation in Modified Colloidal Silica Gradients. In Methods of Cell Separation; Catsimpoolas, N., Ed.; Springer: Boston, MA, USA, 1977; pp. 25–65. [Google Scholar]
- Pertoft, H.; Laurent, T.C.; Låås, T.; Kågedal, L. Density Gradients Prepared from Colloidal Silica Particles Coated by Polyvinylpyrrolidone (Percoll). Anal. Biochem. 1978, 88, 271–282. [Google Scholar] [CrossRef]
- Healthcare, G.E. Cell Separation Media—Methodology and Applications; GE Healthcare Bio-Sciences AB: Uppsala, Sweden, 1985. [Google Scholar]
- Bessis, M. Corpuscles, Atlas of Red Blood Cell Shapes; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar] [CrossRef]
- Simionato, G.; Hinkelmann, K.; Chachanidze, R.; Bianchi, P.; Fermo, E.; van Wijk, R.; Leonetti, M.; Wagner, C.; Kaestner, L.; Quint, S. Red Blood Cell Phenotyping from 3D Confocal Images Using Artificial Neural Networks. PloS Comput. Biol. 2021, 17, e1008934. [Google Scholar] [CrossRef]
- Mohandas, N.; Evans, E. Mechanical Properties of the Red Cell Membrane in Relation to Molecular Structure and Genetic Defects. Annu. Rev. Biophys. Biomol. 1994, 23, 787–818. [Google Scholar] [CrossRef]
- Quint, S.; Christ, A.F.; Guckenberger, A.; Himbert, S.; Kaestner, L.; Gekle, S.; Wagner, C. 3D Tomography of Cells in Micro-Channels. Appl. Phys. Lett. 2017, 111, 103701. [Google Scholar] [CrossRef] [Green Version]
- Kihm, A.; Quint, S.; Laschke, M.W.; Menger, M.D.; John, T.; Kaestner, L.; Wagner, C. Lingering Dynamics in Microvascular Blood Flow. Biophys. J. 2021, 120, 432–439. [Google Scholar] [CrossRef]
- Beutler, E. Biochemistry of the Erythrocyte. Experientia 1969, 25, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Smith, B.L.; Hartel-Schenk, S. Biochemistry of the Erythrocyte Rh Polypeptides: A Review. Yale J. Biol. Med. 1990, 63, 461–467. [Google Scholar] [PubMed]
- Wang, J.; Hertz, L.; Ruppenthal, S.; Nemer, W.E.; Connes, P.; Goede, J.S.; Bogdanova, A.; Birnbaumer, L.; Kaestner, L. Lysophosphatidic Acid-Activated Calcium Signaling Is Elevated in Red Cells from Sickle Cell Disease Patients. Cells 2021, 10, 456. [Google Scholar] [CrossRef]
- Bessis, M. Living Blood Cells and Their Ultrastructure; Springer: Berlin/Heidelberg, Germany, 1973; ISBN 3-540-05981-4. [Google Scholar]
- Bernhardt, I.; Ellory, J.C. Red Cell Membrane Transport in Health and Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; ISBN 9783540442271. [Google Scholar]
- Kaestner, L. Cation Channels in Erythrocytes—Historical and Future Perspective. Open Biol. J. 2011, 4, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Kaestner, L. Channelizing the Red Blood Cell: Molecular Biology Competes with Patch-Clamp. Front. Mol. Biosci. 2015, 2, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.L.Y.; Bouyer, G.; Cueff, A.; Egée, S.; Glogowska, E.; Ollivaux, C. Ion Channels in Human Red Blood Cell Membrane: Actors or Relics? Blood Cells Mol. Dis. 2011, 46, 261–265. [Google Scholar] [CrossRef]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.-T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. Mrs Bull. 2010, 35, 382–388. [Google Scholar] [CrossRef]
- Kaestner, L.; Bogdanova, A.; Egee, S. Calcium Channels and Calcium-Regulated Channels in Human Red Blood Cells. Adv. Exp. Med. Biol. 2020, 1131, 625–648. [Google Scholar] [CrossRef]
- Danielczok, J.G.; Terriac, E.; Hertz, L.; Petkova-Kirova, P.; Lautenschläger, F.; Laschke, M.W.; Kaestner, L. Red Blood Cell Passage of Small Capillaries Is Associated with Transient Ca2+-Mediated Adaptations. Front. Physiol. 2017, 8, 979. [Google Scholar] [CrossRef] [Green Version]
- Egee, S.; Kaestner, L. The Transient Receptor Potential Vanilloid Type 2 (TRPV2) Channel—A New Druggable Ca2+ Pathway in Red Cells, Implications for Red Cell Ion Homeostasis. Front. Physiol. 2021, 12, 677573. [Google Scholar] [CrossRef]
- Piccinini, G.; Minetti, G.; Balduini, C.; Brovelli, A. Oxidation State of Glutathione and Membrane Proteins in Human Red Cells of Different Age. Mech. Ageing Dev. 1995, 78, 15–26. [Google Scholar] [CrossRef]
- Piomelli, S.; Seaman, C. Mechanism of Red Blood Cell Aging: Relationship of Cell Density and Cell Age. Am. J. Hematol. 1993, 42, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Kaestner, L.; Bogdanova, A.Y.; Minetti, G.; Rudloff, S.; Lundby, C.; Makhro, A.; Seiler, E.; Cromvoirt, A.; Fenk, S.; et al. Absence of Neocytolysis in Humans Returning from a 3-week High-altitude Sojourn. Acta Physiol. 2021, 232, e13647. [Google Scholar] [CrossRef] [PubMed]
- Vettore, L.; Matteis, M.C.D.; Zampini, P. A New Density Gradient System for the Separation of Human Red Blood Cells. Am. J. Hematol. 1980, 8, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.H.; Werre, J.M.; Schipper, L.; Roerdinkholder-Stoelwinder, B.; Huls, T.; Willekens, F.L.A.; Wichers, G.; Halie, M.R. Determinants of Red Blood Cell Deformability in Relation to Cell Age. Eur. J. Haematol. 2009, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Minetti, G. The Potential of Erythrocytes as Cellular Aging Models. Cell Death Differ. 2017, 24, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U.; Stammler, P.; Fasler, S.; Ingold, M.; Fehr, J. Density Separation of Human Red Blood Cells on Self Forming Percoll Gradients: Correlation with Cell Age. Biochim. Biophys. Acta 1992, 1116, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Wagner-Britz, L.; Bogdanova, A.; Ruppenthal, S.; Wiesen, K.; Kaiser, E.; Tian, Q.; Krause, E.; Bernhardt, I.; Lipp, P.; et al. Morphologically Homogeneous Red Blood Cells Present a Heterogeneous Response to Hormonal Stimulation. PLoS ONE 2013, 8, e67697. [Google Scholar] [CrossRef] [Green Version]
- Hertz, L.; Ruppenthal, S.; Simionato, G.; Quint, S.; Kihm, A.; Abay, A.; Petkova-Kirova, P.; Boehm, U.; Weissgerber, P.; Wagner, C.; et al. The Evolution of Erythrocytes Becoming Red in Respect to Fluorescence. Front. Physiol. 2019, 10, 753. [Google Scholar] [CrossRef] [Green Version]
- Mueller, T.J.; Jackson, C.W.; Dockter, M.E.; Morrison, M. Membrane Skeletal Alterations during in Vivo Mouse Red Cell Aging. Increase in the Band 4.1a:4.1b Ratio. J. Clin. Investig. 1987, 79, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Inaba, M.; Gupta, K.; Kuwabara, M.; Takahashi, T.; Benz, E.J.; Maede, Y. Deamidation of Human Erythrocyte Protein 4.1: Possible Role in Aging. Blood 1992, 79, 3355–3361. [Google Scholar] [CrossRef] [Green Version]
- Robinson, N.E.; Robinson, A.B. Molecular Clocks: Deamidation of Asparaginyl and Glutaminyl Residues in Peptides and Proteins; Althouse Press: Cave Junction, OR, USA, 2004; ISBN 978-1590872505. [Google Scholar]
- Vacha, J. Red Cell Life-Span. In Red Blood Cells of Domestic Mammals; Agar, N.S., Board, P.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 67–132. [Google Scholar]
- Makhro, A.; Kaestner, L.; Bogdanova, A. NMDA Receptor Activity in Circulating Red Blood Cells: Methods of Detection. Methods Mol. Biol. Clifton N. J. 2017, 1677, 265–282. [Google Scholar] [CrossRef]
- Huisjes, R.; Makhro, A.; Llaudet-Planas, E.; Hertz, L.; Petkova-Kirova, P.; Verhagen, L.P.; Pignatelli, S.; Rab, M.A.; Schiffelers, R.M.; Seiler, E.; et al. Density, Heterogeneity and Deformability of Red Cells as Markers of Clinical Severity in Hereditary Spherocytosis. Haematologica 2019, 105, 188151. [Google Scholar] [CrossRef]
- Makhro, A.; Huisjes, R.; Verhagen, L.P.; del Mañú-Pereira, M.; Llaudet-Planas, E.; Petkova-Kirova, P.; Wang, J.; Eichler, H.; Bogdanova, A.; van Wijk, R.; et al. Red Cell Properties after Different Modes of Blood Transportation. Front. Physiol. 2016, 7, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadafi, A.; Makhro, A.; Livshits, L.; Navab, N.; Bogdanova, A.; Albarqouni, S.; Marr, C. Sickle Cell Disease Severity Prediction from Percoll Gradient Images Using Graph Convolutional Networks. 2021. Available online: https://arxiv.org/abs/2109.05372v1 (accessed on 4 October 2021).
- Kaestner, L. Artificial Intelligence Meets Hematology. Transfus. Apher. Sci. 2020, 59, 102986. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Kaestner, L. Early Career Scientists’ Guide to the Red Blood Cell—Don’t Panic! Front. Physiol. 2020, 11, 588. [Google Scholar] [CrossRef]
- Abay, A.; Simionato, G.; Chachanidze, R.; Bogdanova, A.; Hertz, L.; Bianchi, P.; van den Akker, E.; von Lindern, M.; Leonetti, M.; Minetti, G.; et al. Glutaraldehyde—A Subtle Tool in the Investigation of Healthy and Pathologic Red Blood Cells. Front. Physiol. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Linkert, M.; Rueden, C.T.; Allan, C.; Burel, J.-M.; Moore, W.; Patterson, A.; Loranger, B.; Moore, J.; Neves, C.; Macdonald, D.; et al. Metadata Matters: Access to Image Data in the Real World. J. Cell Biol. 2010, 189, 777–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, R.; Tukey, J.W.; Larsen, W.A. Variations of Box Plots. Am. Stat. 1978, 32, 12. [Google Scholar] [CrossRef]
- Darras, A.; Peikert, K.; Rabe, A.; Yaya, F.; Simionato, G.; John, T.; Dasanna, A.K.; Buvalyy, S.; Geisel, J.; Hermann, A.; et al. Acanthocyte Sedimentation Rate as a Diagnostic Biomarker for Neuroacanthocytosis Syndromes: Experimental Evidence and Physical Justification. Cells 2021, 10, 788. [Google Scholar] [CrossRef]
- Darras, A.; Dasanna, A.K.; John, T.; Gompper, G.; Kaestner, L.; Fedosov, D.A.; Wagner, C. Erythrocyte Sedimentation: Collapse of a High-Volume-Fraction Soft-Particle Gel. Phys. Rev. Lett. 2022, 128, 088101. [Google Scholar] [CrossRef]
- Dasanna, A.K.; Darras, A.; John, T.; Gompper, G.; Kaestner, L.; Wagner, C.; Fedosov, D.A. Erythrocyte Sedimentation: Effect of Aggregation Energy on Gel Structure during Collapse. Phys. Rev. E 2022, 105, 024610. [Google Scholar] [CrossRef]
- Darras, A.; Breunig, H.G.; John, T.; Zhao, R.; Koch, J.; Kummerow, C.; König, K.; Wagner, C.; Kaestner, L. Imaging Erythrocyte Sedimentation in Whole Blood. Front. Physiol. 2022, 12, 729191. [Google Scholar] [CrossRef]
- Price, C.A. Centrifugation in Density Gradients; Academic Press: Cambridge, MA, USA, 1982; ISBN 0-12-564580-5. [Google Scholar]
- Flormann, D.; Aouane, O.; Kaestner, L.; Ruloff, C.; Misbah, C.; Podgorski, T.; Wagner, C. The Buckling Instability of Aggregating Red Blood Cells. Sci. Rep. 2017, 7, 7928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew, V.L.; Tiffert, T. The Terminal Density Reversal Phenomenon of Aging Human Red Blood Cells. Front. Physiol. 2013, 4, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermolinskiy, P.; Lugovtsov, A.; Yaya, F.; Lee, K.; Kaestner, L.; Wagner, C.; Priezzhev, A. Effect of Red Blood Cell Aging In Vivo on Their Aggregation Properties In Vitro: Measurements with Laser Tweezers. Appl. Sci. 2020, 10, 7581. [Google Scholar] [CrossRef]
- von Tempelhoff, G.-F.; Schelkunov, O.; Demirhan, A.; Tsikouras, P.; Rath, W.; Velten, E.; Csorba, R. Correlation between Blood Rheological Properties and Red Blood Cell Indices(MCH, MCV, MCHC) in Healthy Women. Clin. Hemorheol. Micro 2016, 62, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellory, J.C.; Hall, A.C. Human Red Cell Volume Regulation in Hypotonic Media. Comp. Biochem. Physiol. A Comp. Physiol. 1988, 90, 533–537. [Google Scholar] [CrossRef]
- Sorette, M.; Shiffer, K.; Clark, M. Improved Isolation of Normal Human Reticulocytes via Exploitation of Chloride-Dependent Potassium Transport. Blood 1992, 80, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, P.A.; Sachs, F. Piezo1: Properties of a Cation Selective Mechanical Channel. Channels 2012, 6, 214–219. [Google Scholar] [CrossRef]
- Kaestner, L.; Egée, S. Commentary: Voltage Gating of Mechanosensitive PIEZO Channels. Front. Physiol. 2018, 9, 1565. [Google Scholar] [CrossRef]
- Bogdanova, A.; Makhro, A.; Wang, J.; Lipp, P.; Kaestner, L. Calcium in Red Blood Cells-a Perilous Balance. Int. J. Mol. Sci. 2013, 14, 9848–9872. [Google Scholar] [CrossRef] [Green Version]
- Bogdanova, A.; Kaestner, L.; Simionato, G.; Wickrema, A.; Makhro, A. Heterogeneity of Red Blood Cells: Causes and Consequences. Front. Physiol. 2020, 11, 392. [Google Scholar] [CrossRef]
- Weems, H.B.; Lessin, L.S. Erythrocyte Density Distribution in Sickle Cell Anemia. Acta Haematol.-Basel 1984, 71, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L.; Bookchin, R.M. Ion Transport Pathology in the Mechanism of Sickle Cell Dehydration. Physiol. Rev. 2005, 85, 179–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, F.; John, T.; Makhro, A.; Bogdanova, A.; Minetti, G.; Wagner, C.; Kaestner, L. Continuous Percoll Gradient Centrifugation of Erythrocytes—Explanation of Cellular Bands and Compromised Age Separation. Cells 2022, 11, 1296. https://doi.org/10.3390/cells11081296
Maurer F, John T, Makhro A, Bogdanova A, Minetti G, Wagner C, Kaestner L. Continuous Percoll Gradient Centrifugation of Erythrocytes—Explanation of Cellular Bands and Compromised Age Separation. Cells. 2022; 11(8):1296. https://doi.org/10.3390/cells11081296
Chicago/Turabian StyleMaurer, Felix, Thomas John, Asya Makhro, Anna Bogdanova, Giampaolo Minetti, Christian Wagner, and Lars Kaestner. 2022. "Continuous Percoll Gradient Centrifugation of Erythrocytes—Explanation of Cellular Bands and Compromised Age Separation" Cells 11, no. 8: 1296. https://doi.org/10.3390/cells11081296
APA StyleMaurer, F., John, T., Makhro, A., Bogdanova, A., Minetti, G., Wagner, C., & Kaestner, L. (2022). Continuous Percoll Gradient Centrifugation of Erythrocytes—Explanation of Cellular Bands and Compromised Age Separation. Cells, 11(8), 1296. https://doi.org/10.3390/cells11081296







