Brain Microvascular Pericytes—More than Bystanders in Breast Cancer Brain Metastasis
Abstract
1. Breast Cancer Brain Metastasis
2. Brain Colonization of Breast Cancer Cells
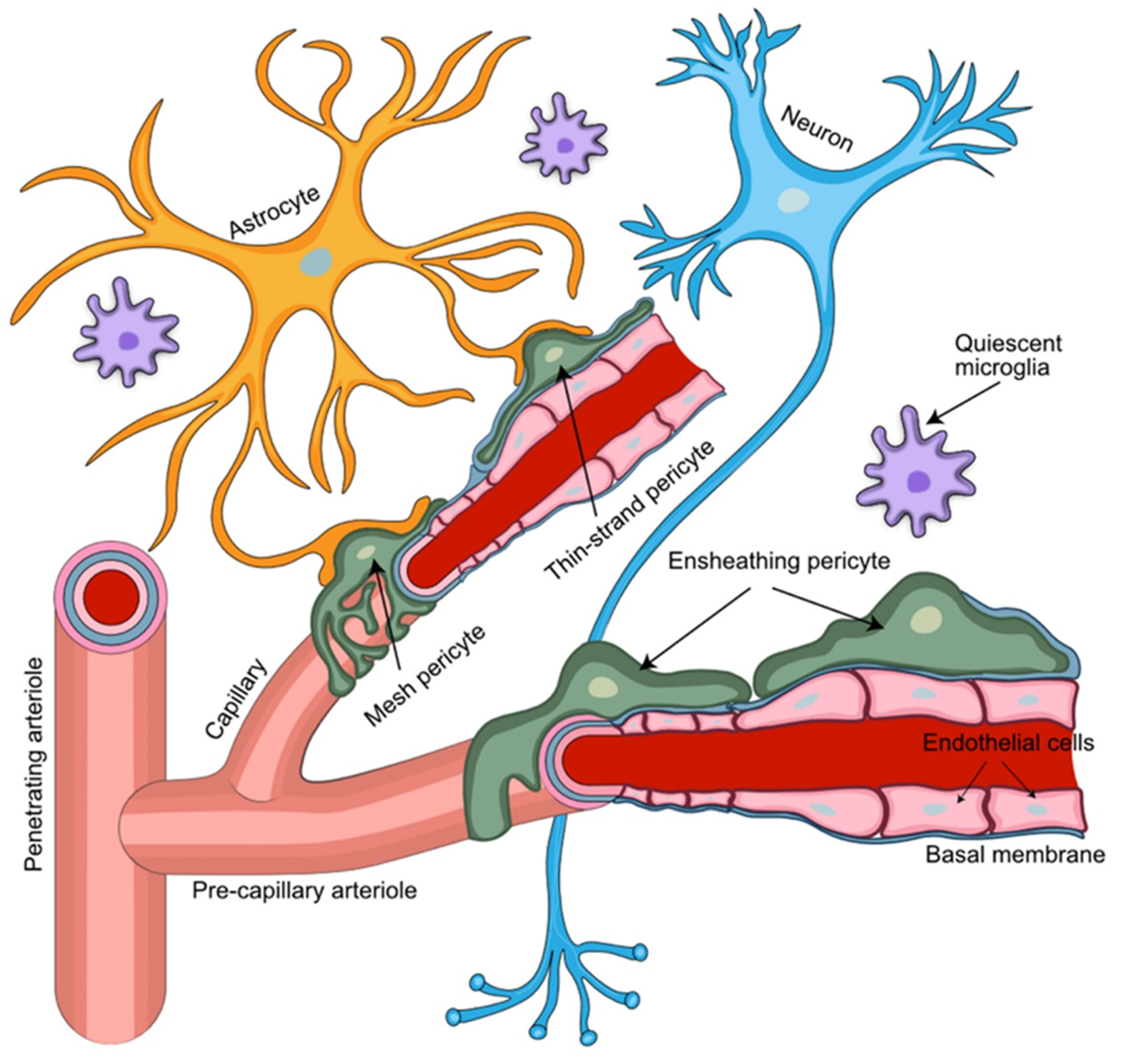
3. Breast Cancer Brain Metastatic Cells and the Neurovascular Unit (NVU)
4. Pericytes Provide Key Functional Support for the NVU
5. Pericyte Markers
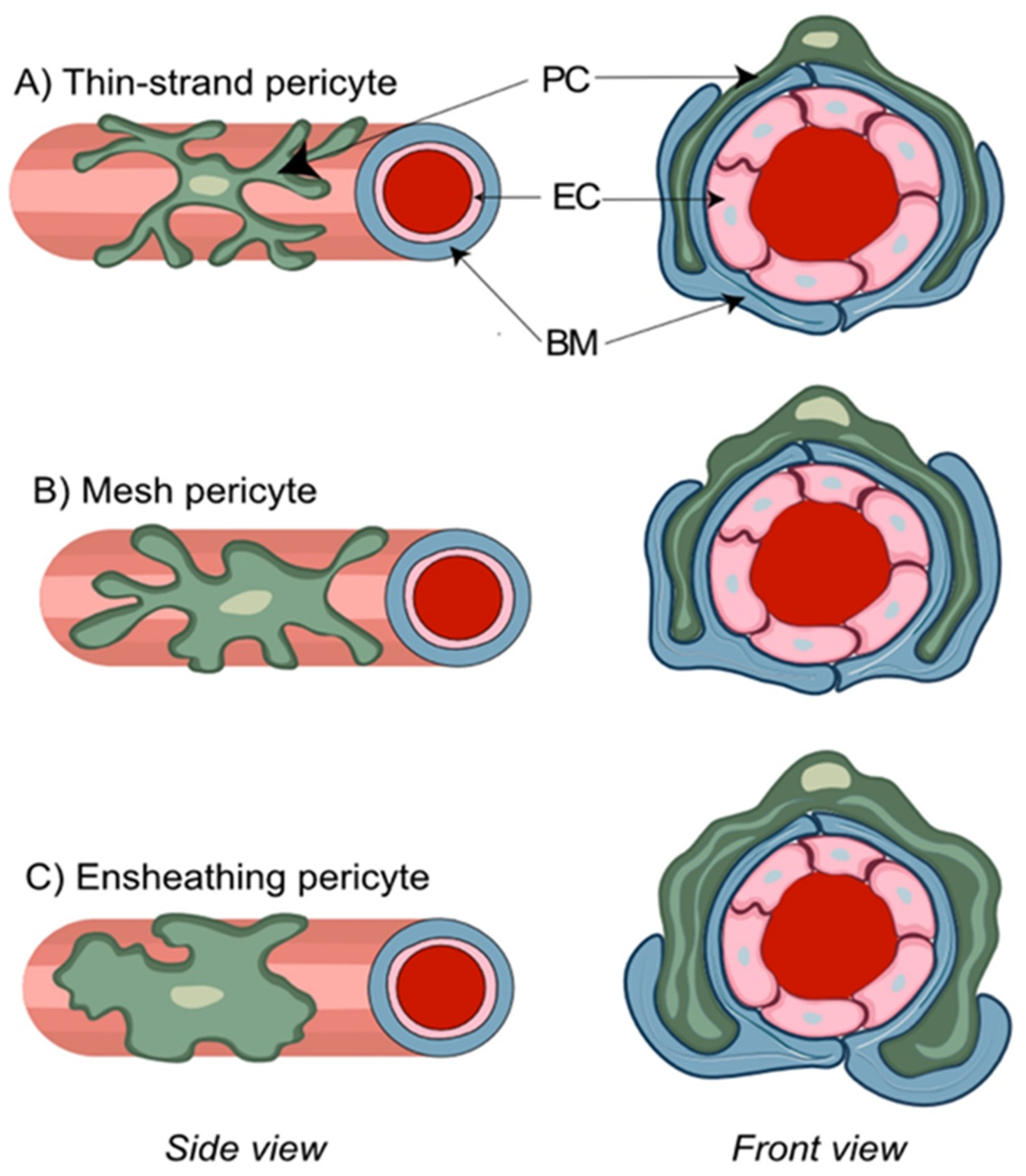
6. The NVU Contains Distinct Pericyte Populations
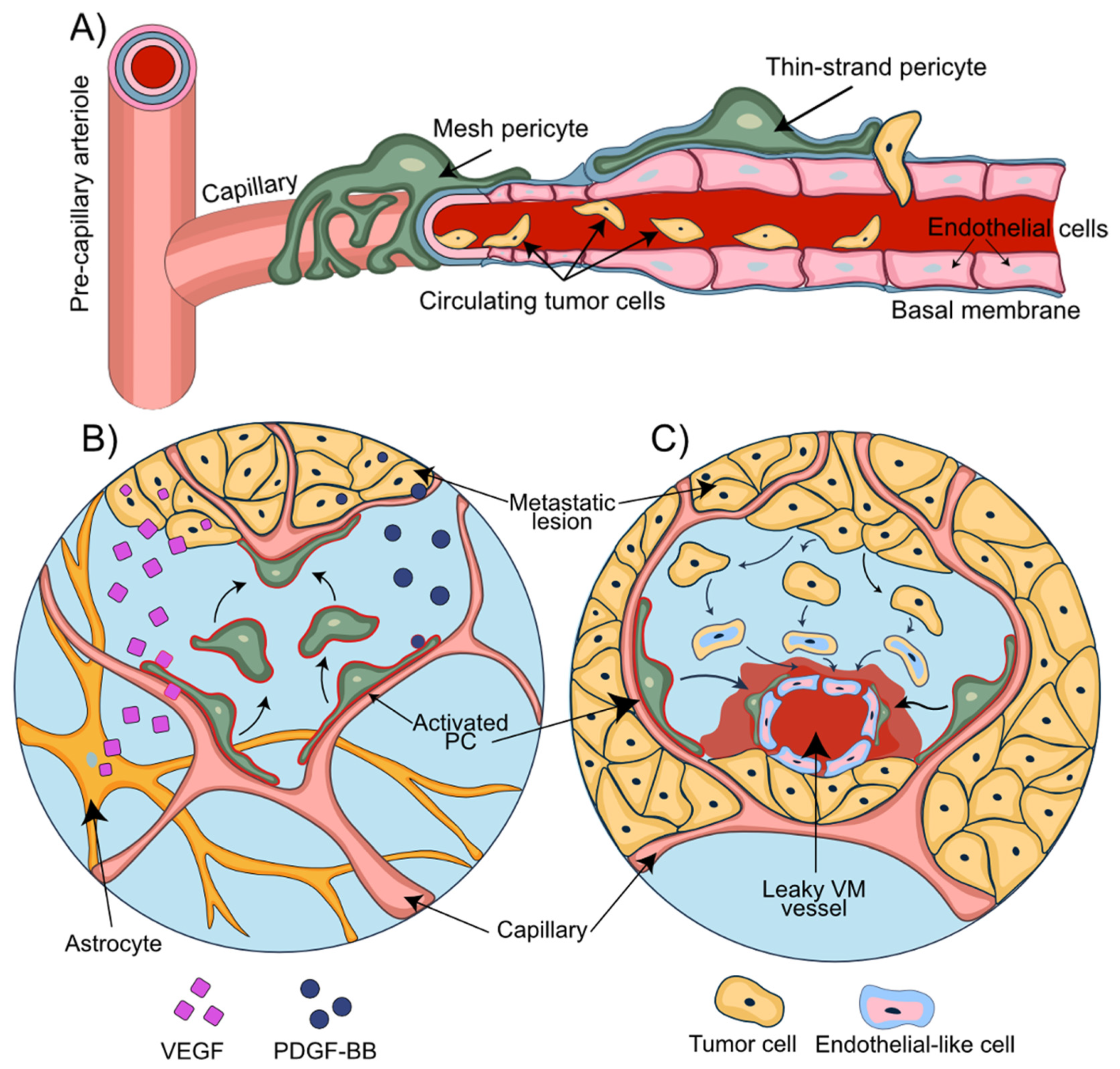
7. The NVU in Breast-to-Brain Metastasis
8. Role of Pericytes in the Establishment of Metastatic Lesions
9. Pericytes and Endoplasmatic Reticulum (ER) Stress—An Emerging Science
10. Pericytes and Metastatic Invasion Patterns
11. In Vitro and In Vivo Models in Pericyte Research
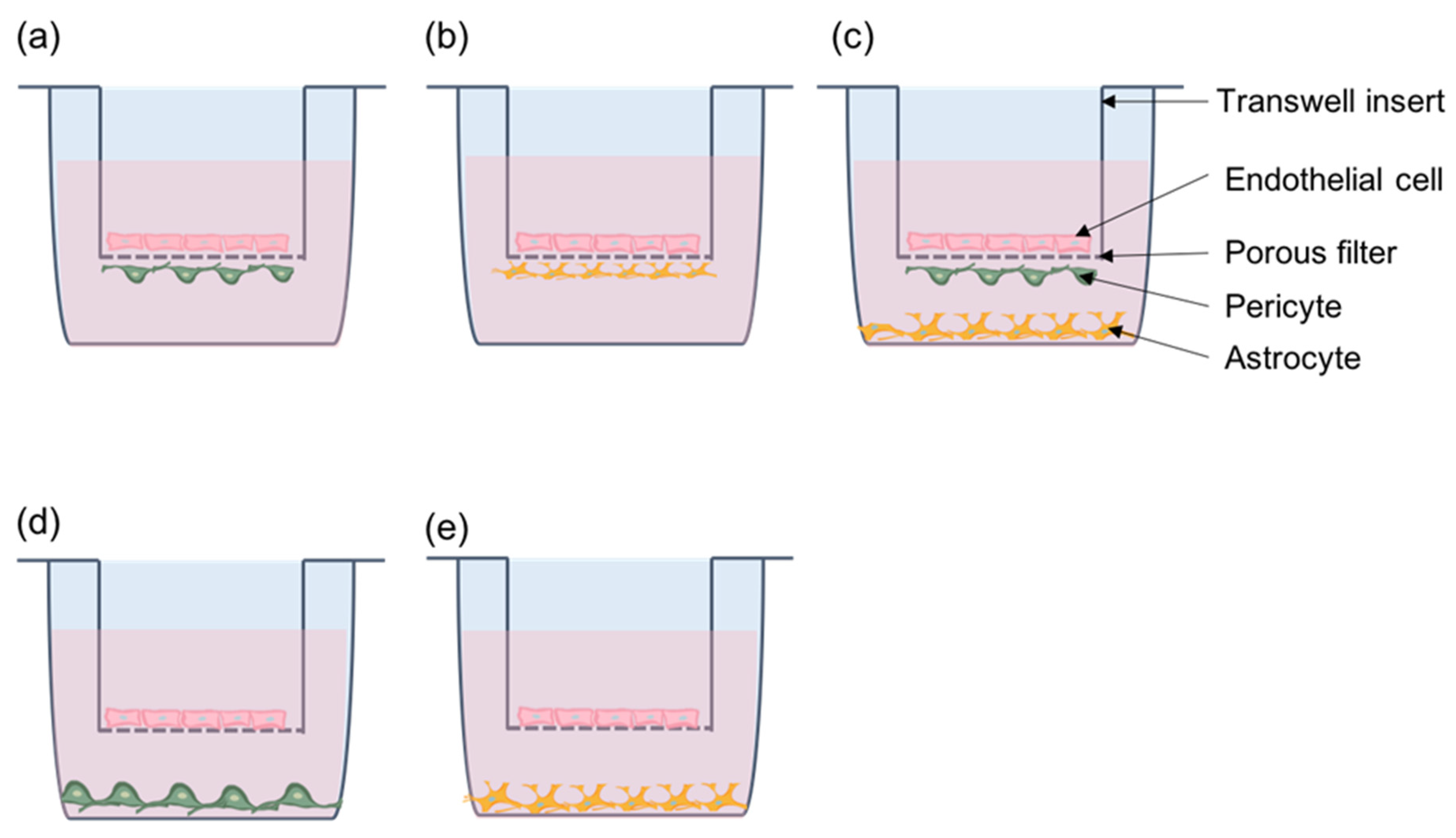
11.1. Pericytes Co-Culture Models
11.2. Microfluidics—Dynamic In Vitro Modeling of the Brain Microvasculature
11.3. In Vivo Monitoring of Pericytes
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar]
- DeSantis, C.; Ma, J.; Gaudet, M.; Newman, L.; Miller, K.; Goding Sauer, A.; Jemal, A.; Siegel, R. Breast cancer statistics. 2019. CA A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics. 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Giglio, P.; Gilbert, M. Neurologic complications of cancer and its treatment. Curr. Oncol. Rep. 2010, 12, 50–59. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.; Wen, P. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Saha, A.; Ghosh, S.; Roy, C.; Choudhury, K.; Chakrabarty, B.; Sarkar, R. Demographic and clinical profile of patients with brain metastases: A retrospective study. Asian J. Neurosurg. 2013, 8, 157–161. [Google Scholar] [CrossRef]
- Miller, K.; Weathers, T.; Haney, L.; Timmerman, R.; Dickler, M.; Shen, J.; Sledge, G. Occult central nervous system involvement in patients with metastatic breast cancer: Prevalence, predictive factors and impact on overall survival. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2003, 14, 1072–1077. [Google Scholar] [CrossRef]
- Niwinska, A.; Tacikowska, M.; Pienkowski, T. Occult brain metastases in HER2-positive breast cancer patients: Frequency and response to radiotherapy. Acta Oncol. Stockh. Swed. 2007, 46, 1027–1029. [Google Scholar] [CrossRef]
- Tsukada, Y.; Fouad, A.; Pickren, J.; Lane, W. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 1983, 52, 2349–2354. [Google Scholar] [CrossRef]
- Carter, C.; Allen, C.; Henson, D. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989, 63, 181–187. [Google Scholar] [CrossRef]
- Page, D. Prognosis and breast cancer. Recognition of lethal and favorable prognostic types. Am. J. Surg. Pathol. 1991, 15, 334–349. [Google Scholar] [CrossRef]
- Perou, C.; Sørlie, T.; Eisen, M.; van de Rijn, M.; Jeffrey, S.; Rees, C.; Pollack, J.; Ross, D.; Johnsen, H.; Akslen, L.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Lin, N.; Amiri-Kordestani, L.; Palmieri, D.; Liewehr, D.; Steeg, P. CNS metastases in breast cancer: Old challenge, new frontiers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 6404–6418. [Google Scholar] [CrossRef]
- Lin, N.; Winer, E. Brain metastases: The HER2 paradigm. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 1648–1655. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast Edinb. Scotl. 2015, 24 (Suppl. S2), S26–S35. [Google Scholar] [CrossRef]
- Darlix, A.; Louvel, G.; Fraisse, J.; Jacot, W.; Brain, E.; Debled, M.; Mouret-Reynier, M.; Goncalves, A.; Dalenc, F.; Delaloge, S.; et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br. J. Cancer 2019, 121, 991–1000. [Google Scholar] [CrossRef]
- Rostami, R.; Mittal, S.; Rostami, P.; Tavassoli, F.; Jabbari, B. Brain metastasis in breast cancer: A comprehensive literature review. J. Neuro-Oncol. 2016, 127, 407–414. [Google Scholar] [CrossRef]
- Graf, A.; Buchberger, W.; Langmayr, H.; Schmid, K. Site preference of metastatic tumours of the brain. Virchows Arch. A Pathol. Anat. Histopathol. 1988, 412, 493–498. [Google Scholar] [CrossRef]
- Puppa, A.D.; Pos, S.D.; Zovato, S.; Orvieto, E.; Ciccarino, P.; Manara, R.; Zustovich, F.; Berti, F.; Gardiman, M.; Scienza, R. Solitary intra-ventricular brain metastasis from a breast carcinoma. J. Neuro-Oncol. 2010, 97, 123–126. [Google Scholar] [CrossRef]
- Franzoi, M.; Hortobagyi, G. Leptomeningeal carcinomatosis in patients with breast cancer. Crit. Rev. Oncol. Hematol. 2019, 135, 85–94. [Google Scholar] [CrossRef]
- Nathanson, S.; Krag, D.; Kuerer, H.; Newman, L.; Brown, M.; Kerjaschki, D.; Pereira, E.; Padera, T. Breast cancer metastasis through the lympho-vascular system. Clin. Exp. Metastasis 2018, 35, 443–454. [Google Scholar] [CrossRef]
- Lorger, M.; Felding-Habermann, B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 2010, 176, 2958–2971. [Google Scholar] [CrossRef]
- Steeg, P. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Custódio-Santos, T.; Videira, M.; Brito, M. Brain metastasization of breast cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 132–147. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Underly, R.G.; Grant, R.I.; Watson, A.N.; Lindner, V.; Shih, A.Y. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015, 2, 041402. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Lorger, M. Tumor microenvironment in the brain. Cancers 2012, 4, 218–243. [Google Scholar] [CrossRef]
- Wang, L.; Cossette, S.; Rarick, K.; Gershan, J.; Dwinell, M.; Harder, D.; Ramchandran, R. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS ONE 2013, 8, e80933. [Google Scholar] [CrossRef]
- Sá-Pereira, I.; Brites, D.; Brito, M. Neurovascular unit: A focus on pericytes. Mol. Neurobiol. 2012, 45, 327–347. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Gage, P.; Rhoades, W.; Prucka, S.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4200–4208. [Google Scholar] [CrossRef]
- Trost, A.; Schroedl, F.; Lange, S.; Rivera, F.; Tempfer, H.; Korntner, S.; Stolt, C.; Wegner, M.; Bogner, B.; Kaser-Eichberger, A.; et al. Neural crest origin of retinal and choroidal pericytes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7910–7921. [Google Scholar] [CrossRef]
- Faal, T.; Phan, D.T.T.; Davtyan, H.; Scarfone, V.M.; Varady, E.; Blurton-Jones, M.; Hughes, C.C.W.; Inlay, M.A. Induction of Mesoderm and Neural Crest-Derived Pericytes from Human Pluripotent Stem Cells to Study Blood-Brain Barrier Interactions. Stem Cell Rep. 2019, 12, 451–460. [Google Scholar] [CrossRef]
- Etchevers, H.; Vincent, C.; Le Douarin, N.; Couly, G. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Dev. Camb. Engl. 2001, 128, 1059–1068. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood-Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Hall, C.; Reynelln, B.; Hamilton, N.; Mishra, A.; Sutherland, B.; O’Farrell, F.; Buchan, A.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef]
- Heymans, M.; Figueiredo, R.; Dehouck, L.; Francisco, D.; Sano, Y.; Shimizu, F.; Kanda, T.; Bruggmann, R.; Engelhardt, B.; Winter, P.; et al. Contribution of brain pericytes in blood-brain barrier formation and maintenance: A transcriptomic study of cocultured human endothelial cells derived from hematopoietic stem cells. Fluids Barriers CNS 2020, 17, 1–28. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Caporarello, N.; D’Angeli, F.; Cambria, M.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.; et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef]
- ElAli, A.; Thériault, P.; Rivest, S. The Role of Pericytes in Neurovascular Unit Remodeling in Brain Disorders. Int. J. Mol. Sci. 2014, 15, 6453–6474. [Google Scholar] [CrossRef]
- Sweeney, M.; Ayyadurai, S.; Zlokovic, B. Pericytes of the Neurovascular Unit: Key Functions and Signaling Pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; Larue, B.; Deane, R.; Zlokovic, B.V. Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Hellström, M.; Gerhardt, H.; Kalén, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001, 153, 543–554. [Google Scholar] [CrossRef]
- Sattiraju, A.; Mintz, A. Pericytes in Glioblastomas: Multifaceted Role Within Tumor Microenvironments and Potential for Therapeutic Interventions. Adv. Exp. Med. Biol. 2019, 1147, 65–91. [Google Scholar] [CrossRef]
- Abramsson, A.; Berlin, O.; Papayan, H.; Paulin, D.; Shani, M.; Betsholtz, C. Analysis of mural cell recruitment to tumor vessels. Circulation 2002, 105, 112–117. [Google Scholar] [CrossRef]
- Furuhashi, M.; Sjöblom, T.; Abramsson, A.; Ellingsen, J.; Micke, P.; Li, H.; Bergsten-Folestad, E.; Eriksson, U.; Heuchel, R.; Betsholtz, C. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004, 64, 2725–2733. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Zhou, H.-N.; He, S.-S.; Xue, M.-Y.; Li, T.; Liu, L.-M. Research advances in pericyte function and their roles in diseases. Chin. J. Traumatol. 2020, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain Vascular Pericytes Following Ischemia Have Multipotential Stem Cell Activity to Differentiate Into Neural and Vascular Lineage Cells. Stem Cells 2015, 33, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.; Bechmann, I. CNS pericytes: Concepts, misconceptions, and a way out. Glia 2010, 58, 1–10. [Google Scholar] [CrossRef]
- Jindatip, D.; Fujiwara, K.; Kouki, T.; Yashiro, T. Transmission and scanning electron microscopy study of the characteristics and morphology of pericytes and novel desmin-immunopositive perivascular cells before and after castration in rat anterior pituitary gland. Anat. Sci. Int. 2012, 87, 165–173. [Google Scholar] [CrossRef]
- Birbrair, A. Pericyte Biology: Development, Homeostasis, and Disease. Adv. Exp. Med. Biol. 2018, 1109, 1–3. [Google Scholar] [CrossRef]
- Grant, R.; Hartmann, D.; Underly, R.; Berthiaume, A.; Bhat, N.; Shih, A. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 39, 411–425. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 2010, 5, 32. [Google Scholar] [CrossRef]
- Alliot, F.; Rutin, J.; Leenen, P.; Pessac, B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J. Neurosci. Res. 1999, 58, 367–378. [Google Scholar] [CrossRef]
- Bandopadhyay, R.; Orte, C.; Lawrenson, J.; Reid, A.; De Silva, S.; Allt, G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J. Neurocytol. 2001, 30, 35–44. [Google Scholar] [CrossRef]
- Ozerdem, U.; Grako, K.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 222, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Hui, H.; Cai, T.; Huang, H.; Yang, F.; Feng, J.; Zhang, J.; Yan, X. CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proc. Natl. Acad. Sci. USA 2017, 114, E7622–E7631. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Muramatsu, M.; Azuma, E.; Ikutani, M.; Nagai, Y.; Sagara, H.; Koo, B.; Kita, S.; O’Donnell, E.; Osawa, T.; et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dore-Duffy, P.; Katychev, A.; Wang, X.; Van Buren, E. CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2006, 26, 613–624. [Google Scholar] [CrossRef]
- Dore-Duffy, P. Pericytes: Pluripotent cells of the blood brain barrier. Curr. Pharm. Des. 2008, 14, 1581–1593. [Google Scholar] [CrossRef]
- Smyth, L.; Rustenhoven, J.; Scotter, E.; Schweder, P.; Faull, R.; Park, T.; Dragunow, M. Markers for Human Brain Pericytes and Smooth Muscle Cells. J. Chem. Neuroanat. 2018, 92, 48–60. [Google Scholar] [CrossRef]
- Damisah, E.; Hill, R.; Tong, L.; Murray, K.; Grutzendler, J. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat. Neurosci. 2017, 20, 1023–1032. [Google Scholar] [CrossRef]
- Dore-Duffy, P. Isolation and characterization of cerebral microvascular pericytes. Methods Mol. Med. 2003, 89, 375–382. [Google Scholar] [CrossRef]
- Hill, R.; Tong, L.; Yuan, P.; Murikinati, S.; Gupta, S.; Grutzendler, J. Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron 2015, 87, 95–110. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mäe, M.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Esen, N. The Microvascular Pericyte: Approaches to Isolation, Characterization, and Cultivation. Adv. Exp. Med. Biol. 2018, 1109, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Garbelli, R.; de Bock, F.; Medici, V.; Rousset, M.; Villani, F.; Boussadia, B.; Arango-Lievano, M.; Jeanneteau, F.; Daneman, R.; Bartolomei, F.; et al. PDGFRβ(+) cells in human and experimental neuro-vascular dysplasia and seizures. Neuroscience 2015, 306, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.; Byman, E.; Fex, M.; Wennström, M. Amylin alters human brain pericyte viability and NG2 expression. J. Cereb. Blood Flow Metab. 2017, 37, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Chan-Ling, T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Feisst, V.; Brooks, A.; Rustenhoven, J.; Monzo, H.; Feng, S.; Mee, E.; Bergin, P.; Oldfield, R.; Graham, E.; et al. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci. Rep. 2016, 6, 26587. [Google Scholar] [CrossRef] [PubMed]
- Ramsauer, M.; Kunz, J.; Krause, D.; Dermietzel, R. Regulation of a blood-brain barrier-specific enzyme expressed by cerebral pericytes (pericytic aminopeptidase N/pAPN) under cell culture conditions. J. Cereb. Blood Flow Metab. 1998, 18, 1270–1281. [Google Scholar] [CrossRef]
- Hutter-Schmid, B.; Humpel, C. Platelet-derived Growth Factor Receptor-beta is Differentially Regulated in Primary Mouse Pericytes and Brain Slices. Curr. Neurovascul. Res. 2016, 13, 1. [Google Scholar] [CrossRef]
- Sagare, A.; Sweeney, M.; Makshanoff, J.; Zlokovic, B. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci. Lett. 2015, 607, 97–101. [Google Scholar] [CrossRef]
- Crouch, E.E.; Doetsch, F. FACS isolation of endothelial cells and pericytes from mouse brain microregions. Nat. Protoc. 2018, 13, 738–751. [Google Scholar] [CrossRef]
- Téglási, V.; Csűry, D.; Dezső, K.; Bugyik, E.; Szabó, V.; Szállási, Z.; Paku, S.; Reiniger, L. Origin and Distribution of Connective Tissue and Pericytes Impacting Vascularization in Brain Metastases With Different Growth Patterns. J. Neuropathol. Exp. Neurol. 2019, 78, 326–339. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time Imaging Reveals the Single Steps of Brain Metastasis Formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, Z.; Sinha, D.; Kaur, P. Pericytes in Metastasis. Adv. Exp. Med. Biol. 2019, 1147, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E5618–E5627. [Google Scholar] [CrossRef] [PubMed]
- Aalders, K.; Tryfonidis, K.; Senkus, E.; Cardoso, F. Anti-angiogenic treatment in breast cancer: Facts, successes, failures and future perspectives. Cancer Treat. Rev. 2017, 53, 98–110. [Google Scholar] [CrossRef]
- Bos, R.; Zhong, H.; Hanrahan, C.; Mommers, E.; Semenza, G.; Pinedo, H.; Abeloff, M.; Simons, J.; van Diest, P.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef]
- Sinha, D.; Chong, L.; George, J.; Schlüter, H.; Mönchgesang, S.; Mills, S.; Li, J.; Parish, C.; Bowtell, D.; Kaur, P. Pericytes Promote Malignant Ovarian Cancer Progression in Mice and Predict Poor Prognosis in Serous Ovarian Cancer Patients. Clin. Cancer Res. 2015, 22, 1813–1824. [Google Scholar] [CrossRef]
- Bos, P.; Zhang, X.; Nadal, C.; Shu, W.; Gomis, R.; Nguyen, D.; Minn, A.; van de Vijver, M.; Gerald, W.; Foekens, J.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef]
- Panse, J.; Friedrichs, K.; Marx, A.; Hildebrandt, Y.; Luetkens, T.; Barrels, K.; Horn, C.; Stahl, T.; Cao, Y.; Milde-Langosch, K.; et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br. J. Cancer 2008, 99, 930–938. [Google Scholar] [CrossRef]
- Curtaz, C.; Schmitt, C.; Herbert, S.; Feldheim, J.; Schlegel, N.; Gosselet, F.; Hagemann, C.; Roewer, N.; Meybohm, P.; Wöckel, A.; et al. Serum-derived factors of breast cancer patients with brain metastases alter permeability of a human blood-brain barrier model. Fluids Barriers CNS 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Maniotis, A.; Folberg, R.; Hess, A.; Seftor, E.; Gardner, L.; Pe’er, J.; Trent, J.; Meltzer, P.; Hendrix, M. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Hendrix, M.; Seftor, E.; Hess, A.; Seftor, R. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat. Rev. Cancer 2003, 3, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Döme, B.; Hendrix, M.; Paku, S.; Tóvári, J.; Tímár, J. Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications. Am. J. Pathol. 2007, 170, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Angara, K.; Borin, T.F.; Arbab, A.S. Vascular Mimicry: A Novel Neovascularization Mechanism Driving Anti-Angiogenic Therapy (AAT) Resistance in Glioblastoma. Transl. Oncol. 2017, 10, 650–660. [Google Scholar] [CrossRef]
- Tang, W.; Yu, F.; Yao, H.; Cui, X.; Jiao, Y.; Lin, L.; Chen, J.; Yin, D.; Song, E.; Liu, Q. MiR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene 2014, 33, 2629–2638. [Google Scholar] [CrossRef]
- Thijssen, V.; Paulis, Y.; Nowak-Sliwinska, P.; Deumelandt, K.; Hosaka, K.; Soetekouw, P.; Cimpean, A.; Raica, M.; Pauwels, P.; van den Oord, J.; et al. Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. J. Pathol. 2018, 246, 447–458. [Google Scholar] [CrossRef]
- Bentolila, L.; Prakash, R.; Mihic-Probst, D.; Wadehra, M.; Kleinman, H.; Carmichael, T.; Péault, B.; Barnhill, R.; Lugassy, C. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci. Rep. 2016, 6, 23834. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Nakao, S.; Honda, M.; Hayashi, K.; Nakaoke, R.; Kataoka, Y.; Niwa, M. Pericytes from Brain Microvessels Strengthen the Barrier Integrity in Primary Cultures of Rat Brain Endothelial Cells. Cell. Mol. Neurobiol. 2007, 27, 687–694. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, Á.; Tanaka, K.; Niwa, M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef]
- Fujimoto, T.; Nakagawa, S.; Morofuji, Y.; Watanabe, D.; Ujifuku, K.; Horie, N.; Izumo, T.; Niwa, M.; Banks, W.A.; Deli, M.A.; et al. Pericytes Suppress Brain Metastasis from Lung Cancer In Vitro. Cell. Mol. Neurobiol. 2020, 40, 113–121. [Google Scholar] [CrossRef]
- Cooke, V.; LeBleu, V.; Keskin, D.; Khan, Z.; O’Connell, J.; Teng, Y.; Duncan, M.; Xie, L.; Maeda, G.; Vong, S.; et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012, 21, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Babak, M.; Zalutsky, M.; Balyasnikova, I. Heterogeneity and vascular permeability of breast cancer brain metastases. Cancer Lett. 2020, 489, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Ono, M.; Shimizu, C.; Hirakawa, A.; Hasegawa, T.; Hatanaka, Y.; Narita, Y.; Shibui, S.; Fujiwara, Y. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010, 116, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Müller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. BCR 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lyle, L.; Lockman, P.; Adkins, C.; Mohammad, A.; Sechrest, E.; Hua, E.; Palmieri, D.; Liewehr, D.; Steinberg, S.; Kloc, W.; et al. Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin. Cancer Res. 2016, 22, 5287–5299. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Xue, Y.; Hedlund, E.; Zhong, Z.; Tritsaris, K.; Tondelli, B.; Lucchini, F.; Zhu, Z.; Dissing, S.; Cao, Y. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc. Natl. Acad. Sci. USA 2010, 107, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeheimi, N.; Naik, A.; Bakheit, C.; Al Riyami, M.; Al Ajarrah, A.; Al Badi, S.; Al Baimani, K.; Malik, K.; Al Habsi, Z.; Al Moundhri, M.; et al. Neoadjuvant Chemotherapy Alters Neuropilin-1, PlGF, and SNAI1 Expression Levels and Predicts Breast Cancer Patients Response. Front. Oncol. 2019, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Eilken, H.; Diéguez-Hurtado, R.; Schmidt, I.; Nakayama, M.; Jeong, H.; Arf, H.; Adams, S.; Ferrara, N.; Adams, R. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Arshad, F.; Wang, L.; Sy, C.; Avraham, S.; Avraham, H. Blood-brain barrier integrity and breast cancer metastasis to the brain. Pathol. Res. Int. 2010, 2011, 1–12. [Google Scholar] [CrossRef]
- Fitzgerald, D.; Palmieri, D.; Hua, E.; Hargrave, E.; Herring, J.; Qian, Y.; Vega-Valle, E.; Weil, R.; Stark, A.; Vortmeyer, A.; et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metastasis 2008, 25, 799–810. [Google Scholar] [CrossRef]
- Termini, J.; Neman, J.; Jandial, R. Role of the neural niche in brain metastatic cancer. Cancer Res. 2014, 74, 4011–4015. [Google Scholar] [CrossRef]
- Sierra, A.; Price, J.; García-Ramirez, M.; Méndez, O.; López, L.; Fabra, A. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab. Investig. A J. Tech. Methods Pathol. 1997, 77, 357–368. [Google Scholar]
- Bachelder, R.; Crago, A.; Chung, J.; Wendt, M.; Shaw, L.; Robinson, G.; Mercurio, A. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001, 61, 5736–5740. [Google Scholar] [PubMed]
- Momeny, M.; Saunus, J.; Marturana, F.; McCart Reed, A.; Black, D.; Sala, G.; Iacobelli, S.; Holland, J.; Yu, D.; Da Silva, L.; et al. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget 2015, 6, 3932–3946. [Google Scholar] [CrossRef] [PubMed]
- Lacroix-Fralish, M.; Tawfik, V.; Nutile-McMenemy, N.; Harris, B.; Deleo, J. Differential regulation of neuregulin 1 expression by progesterone in astrocytes and neurons. Neuron Glia Biol. 2006, 2, 227–234. [Google Scholar] [CrossRef][Green Version]
- Silva, L.D.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010, 12, R46. [Google Scholar] [CrossRef]
- Pukrop, T.; Dehghani, F.; Chuang, H.; Lohaus, R.; Bayanga, K.; Heermann, S.; Regen, T.; Van Rossum, D.; Klemm, F.; Schulz, M.; et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 2010, 58, 1477–1489. [Google Scholar] [CrossRef]
- Stratoulias, V.; Venero, J.; Tremblay, M.; Joseph, B. Microglial subtypes: Diversity within the microglial community. EMBO J. 2019, 38, e101997. [Google Scholar] [CrossRef]
- Ghajar, C.M.; Peinado, H.; Mori, H.; Matei, I.R.; Evason, K.J.; Brazier, H.; Almeida, D.; Koller, A.; Hajjar, K.A.; Stainier, D.Y.; et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013, 15, 807–817. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Seki, T.; Nakamura, M.; Andersson, P.; Rouhi, P.; Yang, X.; Jensen, L.; Lim, S.; Feng, N.; et al. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat. Commun. 2013, 4, 2129. [Google Scholar] [CrossRef]
- Sakuma, R.; Kawahara, M.; Nakano-Doi, A.; Takahashi, A.; Tanaka, Y.; Narita, A.; Kuwahara-Otani, S.; Hayakawa, T.; Yagi, H.; Matsuyama, T.; et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflamm. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, D.; Su, Y.; Yu, D.; Wang, Q.; Wang, T.; Zhou, Q.; Ran, X.; Zou, Z. Hyperplasia of pericytes is one of the main characteristics of microvascular architecture in malignant glioma. PLoS ONE 2014, 9, e114246. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.; Semenza, G. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013, 9, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Patkar, S.; Ogunshola, O. Cell-specific blood-brain barrier regulation in health and disease: A focus on hypoxia. Br. J. Pharmacol. 2014, 171, 1210–1230. [Google Scholar] [CrossRef]
- Kaur, C.; Sivakumar, V.; Zhang, Y.; Ling, E. Hypoxia-induced astrocytic reaction and increased vascular permeability in the rat cerebellum. Glia 2006, 54, 826–839. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.; Jo, I. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc. Res. 2003, 65, 125–131. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Wang, X.; Mehedi, A.; Kreipke, C.; Rafols, J. Differential expression of capillary VEGF isoforms following traumatic brain injury. Neurol. Res. 2007, 29, 395–403. [Google Scholar] [CrossRef]
- McGrath, E.P.; Logue, S.E.; Mnich, K.; Deegan, S.; Jäger, R.; Gorman, A.M.; Samali, A. The Unfolded Protein Response in Breast Cancer. Cancers 2018, 10, 344. [Google Scholar] [CrossRef]
- Ikesugi, K.; Mulhern, M.L.; Madson, C.J.; Hosoya, K.-I.; Terasaki, T.; Kador, P.F.; Shinohara, T. Induction of Endoplasmic Reticulum Stress in Retinal Pericytes by Glucose Deprivation. Curr. Eye Res. 2006, 31, 947–953. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, G.; Huang, L.; Yuan, Y.; Wu, C.; Li, Y. Transmissible Endoplasmic Reticulum Stress: A Novel Perspective on Tumor Immunity. Front. Cell Dev. Biol. 2020, 8, 846. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, J.J.; Zhang, S.X. Intermittent but Not Constant High Glucose Induces ER Stress and Inflammation in Human Retinal Pericytes. In Retinal Degenerative Diseases; LaVail, M.M., Ash, J.D., Anderson, R.E., Hollyfield, J.G., Grimm, C., Eds.; Springer: Boston, MA, USA, 2012; Volume 723, pp. 285–292. [Google Scholar]
- Sena, I.F.G.; Paiva, A.E.; Prazeres, P.; Azevedo, P.O.; Lousado, L.; Bhutia, S.K.; Salmina, A.B.; Mintz, A.; Birbrair, A. Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med. 2018, 7, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Valdor, R.; García-Bernal, D.; Riquelme, D.; Martinez, C.M.; Moraleda, J.M.; Cuervo, A.M.; Macian, F.; Martinez, S. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 2019, 116, 20655–20665. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Tuncman, G.; Calay, E.S.; Rathaus, M.; Ron, I.; Tirosh, A.; Yalcin, A.; Lee, Y.G.; Livne, R.; Ron, S.; et al. Intercellular Transmission of Hepatic ER Stress in Obesity Disrupts Systemic Metabolism. Cell Metab. 2021, 33, 1716. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.; Goodman, S.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro-Oncology 2013, 15, 1664–1672. [Google Scholar] [CrossRef]
- Bronzert, D.; Pantazis, P.; Antoniades, H.; Kasid, A.; Davidson, N.; Dickson, R.; Lippman, M. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc. Natl. Acad. Sci. USA 1987, 84, 5763–5767. [Google Scholar] [CrossRef]
- Yi, B.; Williams, P.; Niewolna, M.; Wang, Y.; Yoneda, T. Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 2002, 62, 917–923. [Google Scholar]
- Molnár, K.; Mészáros, Á.; Fazakas, C.; Kozma, M.; Győri, F.; Reisz, Z.; Tiszlavicz, L.; Farkas, A.; Nyúl-Tóth, Á.; Haskó, J.; et al. Pericyte-secreted IGF2 promotes breast cancer brain metastasis formation. Mol. Oncol. 2020, 14, 2040–2057. [Google Scholar] [CrossRef]
- Tilling, T.; Engelbertz, C.; Decker, S.; Korte, D.; Hüwel, S.; Galla, H. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. 2002, 310, 19–29. [Google Scholar] [CrossRef]
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Burkhart, A.; Moos, T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS ONE 2015, 10, e0134765. [Google Scholar] [CrossRef]
- Wolff, A.; Antfolk, M.; Brodin, B.; Tenje, M. In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J. Pharm. Sci. 2015, 104, 2727–2746. [Google Scholar] [CrossRef] [PubMed]
- Malina, K.C.-K.; Cooper, I.; Teichberg, V.I. Closing the gap between the in-vivo and in-vitro blood-brain barrier tightness. Brain Res. 2009, 1284, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasundaram, G.; Pieper, C.; Lischper, M.; Galla, H.J. Regulation of the blood-brain barrier integrity by pericytes via matrix metalloproteinases mediated activation of vascular endothelial growth factor in vitro. Brain Res. 2010, 1347, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef]
- Stebbins, M.J.; Gastfriend, B.D.; Canfield, S.G.; Lee, M.S.; Richards, D.; Faubion, M.G.; Li, W.J.; Daneman, R.; Palecek, S.P.; Shusta, E.V. Human pluripotent stem cell–derived brain pericyte-like cells induce blood-brain barrier properties. Sci. Adv. 2019, 5, 7375–7388. [Google Scholar] [CrossRef]
- Abbott, N.J.; Dolman, D.E.M.; Yusof, S.R.; Reichel, A. In vitro models of CNS barriers. AAPS Adv. Pharm. Sci. Ser. 2014, 10, 163–197. [Google Scholar] [CrossRef]
- Perides, G.; Zhuge, Y.; Lin, T.; Stins, M.F.; Bronson, R.T.; Wu, J.K. The fibrinolytic system facilitates tumor cell migration across the blood-brain barrier in experimental melanoma brain metastasis. BMC Cancer 2006, 6, 56. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Abdelkarim, M.; Dillenburg-Pilla, P.; Luissint, A.C.; di-Tommaso, A.; Deshayes, F.; Pontes, C.L.S.; Molina, A.; Cagnard, N.; Letourneur, F.; et al. Angiotensin ii facilitates breast cancer cell migration and metastasis. PLoS ONE 2012, 7, e35667. [Google Scholar] [CrossRef]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in Microfluidic Blood-Brain Barrier (BBB) Models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R. 3D Self-Organized Microvascular Model of the Human Blood-Brain Barrier With Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, M.; Lee, S.R.; Jeon, N.L. 3D brain angiogenesis model to reconstitute functional human blood–brain barrier in vitro. Biotechnol. Bioeng. 2020, 117, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Noorani, B.; Bhalerao, A.; Raut, S.; Nozohouri, E.; Bickel, U.; Cucullo, L. A Quasi-Physiological Microfluidic Blood-Brain Barrier Model for Brain Permeability Studies. Pharmaceutics 2021, 13, 1474. [Google Scholar] [CrossRef] [PubMed]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kumar-Kanojia, A.; Hombach-Klonisch, S.; Klonisch, T.; Lin, F. A radial microfluidic platform for higher throughput chemotaxis studies with individual gradient control. Lab Chip 2018, 18, 3855–3864. [Google Scholar] [CrossRef]
- Saliba, J.; Daou, A.; Damiati, S.; Saliba, J.; El-Sabban, M.; Mhanna, R. Development of Microplatforms to Mimic the In Vivo Architecture of CNS and PNS Physiology and Their Diseases. Genes 2018, 9, 285. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef]
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 2016, 13, 306. [Google Scholar] [CrossRef]
- Meza-Resillas, J.; Ahmadpour, N.; Stobart, M.; Stobart, J. Brain Pericyte Calcium and Hemodynamic Imaging in Transgenic Mice In Vivo. J. Vis. Exp. 2021, e62725. [Google Scholar] [CrossRef]
- Arango-Lievano, M.; Dromard, Y.; Fontanaud, P.; Lafont, C.; Mollard, P.; Jeanneteau, F. Regeneration of the neurogliovascular unit visualized in vivo by transcranial live-cell imaging. J. Neurosci. Methods 2020, 343, 108808. [Google Scholar] [CrossRef]
- Bahrami, N.; Childs, S.J. Pericyte Biology in Zebrafish. Adv. Exp. Med. Biol. 2018, 1109, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Fukuhara, S.; Izumi, N.; Nakajima, H.; Fukui, H.; Kelsh, R.N.; Mochizuki, N. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 2016, 143, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.W.; Douek, A.M.; Loosli, F.; Kaslin, J. A Whole Brain Staining, Embedding, and Clearing Pipeline for Adult Zebrafish to Visualize Cell Proliferation and Morphology in 3-Dimensions. Front. Neurosci. 2017, 11, 750. [Google Scholar] [CrossRef] [PubMed]



| Model | Species | Cells in Co-Culture | Reference |
|---|---|---|---|
| Transwell | Rat | Primary cerebral pericytes Primary brain capillary EC Primary cerebral astrocytes | Nakagawa et al., 2009 |
| Transwell | Porcine | Primary brain EC Primary astrocytes | Malina et al., 2009 |
| Transwell | Porcine | Primary brain capillary pericytes Primary brain capillary EC | Thanabalasundaram et al., 2010 |
| Transwell | Porcine | Primary cerebral pericytes Primary brain EC Primary astrocytes | Thomsen et al., 2015 |
| Transwell | Human | Primary brain vascular pericytes Primary cerebral microvascular EC Cerebral astrocytes | Hatherell et al., 2011 |
| Transwell | Human | Primary fetal brain pericytes hPSC-derived brain microvascular EC Differentiated neural progenitor cells | Lippman et al., 2014 |
| Transwell | Human | Primary brain pericytes hPSC-derived pericytes hPSC-derived neural crest stem cells iPSC-derived brain microvascular EC | Stebbins et al., 2019 |
| Microfluidic | Mixed | iPSC-derived human brain microvascular EC Primary rat astrocytes | Wang et al., 2016 |
| Microfluidic | Human | Primary brain pericytes iPSC-derived EC Primary brain astrocytes | Campisi et al., 2018 |
| Microfluidic | Human | Primary placental pericytes Primary brain microvascular EC Primary umbilical vein EC Primary astrocytes | Lee et al., 2019 |
| Microfluidic | Human | Primary brain pericytes iPSC-derived brain microvascular EC Primary astrocytes | Park et al., 2019 |
| Microfluidic | Human | Primary brain pericytes iPSC-derived brain microvascular EC Primary astrocytes | Noorani et al., 2021 |
| Brain-on-chip | Human | Primary brain pericytes, vascular endothelial cells and astrocytes Human neurons differentiated from neuronal stem cells | Maoz et al., 2018 |
| Brain-on-chip | Human | Primary brain pericytes, vascular endothelial cells and astrocytes iPSC-derived cortical neurons and astrocytes | Brown et al., 2016 |
| Brain-on-chip | Human | Different combinations of human brain and NVU cells in review | Saliba et al., 2018 |
| Model | Species | PC Visualization | Reference |
|---|---|---|---|
| Cranial window | Mouse | Transgenic mice; αSMA promoter to label ensheathing pericytes; 2-Photon microscopy | Meza-Resillas et al., 2021 |
| Transcranial imaging | Mouse | Neurotrace™ labeling of pericytes; multimodal optical transcranial imaging | Arango-Lievano et al., 2020 |
| Whole brain imaging | Zebrafish | Transgenic zebrafish; confocal microscopy | Bahrami et al., 2018 |
| Whole mount imaging | Zebrafish | pdgfrb promoter transgenic zebrafish; confocal upright fluorescence imaging | Ando et al., 2016 |
| Cranial window | Mouse | αSMA-mCherry transgenic reporter mice; NeuroTrace 500/525 and TO-PRO-3 PC labeling; optogenetic manipulation of PC in rhodopsin transgenic mice | Tong at al., 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippolitov, D.; Arreza, L.; Munir, M.N.; Hombach-Klonisch, S. Brain Microvascular Pericytes—More than Bystanders in Breast Cancer Brain Metastasis. Cells 2022, 11, 1263. https://doi.org/10.3390/cells11081263
Ippolitov D, Arreza L, Munir MN, Hombach-Klonisch S. Brain Microvascular Pericytes—More than Bystanders in Breast Cancer Brain Metastasis. Cells. 2022; 11(8):1263. https://doi.org/10.3390/cells11081263
Chicago/Turabian StyleIppolitov, Danyyl, Leanne Arreza, Maliha Nuzhat Munir, and Sabine Hombach-Klonisch. 2022. "Brain Microvascular Pericytes—More than Bystanders in Breast Cancer Brain Metastasis" Cells 11, no. 8: 1263. https://doi.org/10.3390/cells11081263
APA StyleIppolitov, D., Arreza, L., Munir, M. N., & Hombach-Klonisch, S. (2022). Brain Microvascular Pericytes—More than Bystanders in Breast Cancer Brain Metastasis. Cells, 11(8), 1263. https://doi.org/10.3390/cells11081263







