Abstract
The use of cannabis preparations has steadily increased. Although cannabis was traditionally assumed to only have mild vegetative side effects, it has become evident in recent years that severe cardiovascular complications can occur. Cannabis use has recently even been added to the risk factors for myocardial infarction. This review is dedicated to pathogenetic factors contributing to cannabis-related myocardial infarction. Tachycardia is highly important in this respect, and we provide evidence that activation of CB1 receptors in brain regions important for cardiovascular regulation and of presynaptic CB1 receptors on sympathetic and/or parasympathetic nerve fibers are involved. The prototypical factors for myocardial infarction, i.e., thrombus formation and coronary constriction, have also been considered, but there is little evidence that they play a decisive role. On the other hand, an increase in the formation of carboxyhemoglobin, impaired mitochondrial respiration, cardiotoxic reactions and tachyarrhythmias associated with the increased sympathetic tone are factors possibly intensifying myocardial infarction. A particularly important factor is that cannabis use is frequently accompanied by tobacco smoking. In conclusion, additional research is warranted to decipher the mechanisms involved, since cannabis use is being legalized increasingly and Δ9-tetrahydrocannabinol and its synthetic analogue nabilone are indicated for the treatment of various disease states.
1. Introduction
Cannabis is derived from leaves, stems or flowers of the Cannabis sativa and Cannabis indica plants, which have been known since ancient times, and like other primeval plants, e.g., opium poppy and ephedra, have been used over centuries and are still prominent today [1,2,3]. Mostly, dried flowers or leaves (marijuana) are inhaled through smoking or vaping, but edible products such as cookies, gummies or chocolate are used as well. Cannabis plants contain more than 400 different compounds and about 100 cannabinoids [4]; the latter ones, mainly Δ9-tetrahydrocannabinol (THC; previous name Δ1-tetrahydrocannabinol) are responsible for the biological effects of cannabis including euphoria, relaxation and changes in perceptions but also dysphoria, anxiety or psychotic symptoms. THC and the nonintoxicating cannabidiol (CBD) are the best-studied cannabinoids [4,5].
THC, CBD, its mixture and the synthetic cannabinoid nabilone are also available in purified or pure form and are used for medical purposes. Cesamet® (nabilone) and Marinol® (THC; IUPAC name: (–)-Δ9-trans-tetrahydrocannabinol; INN: dronabinol) are approved for anorexia and weight loss in HIV infection and for nausea and vomiting in cancer chemotherapy; Epidiolex® (cannabidiol) is indicated for Lennox–Gastaut and Dravet syndrome and Sativex® (mixture of CBD + THC, nabiximols) for neuropathic pain from multiple sclerosis and intractable cancer pain [6,7]. Synthetic agonists of cannabinoid receptors (CB-Rs), so-called synthetic cannabimimetics, are lipid-soluble compounds which consist of 20–26 carbon atoms; they volatilize readily during smoking and are undetectable with common drug-screening tests. Nowadays, they are manufactured in illegal laboratories under various brand names such as K2 or Spice and became popular for recreational purposes due to their increased availability in the market [8,9]. Their distribution is not controlled and they are easily available as “seemingly innocent, herbal products”. They possess structural and biochemical similarities and exert even more potent and deleterious adverse effects in comparison to THC due to their greater affinity for the CB1-R, the CB-R subtype responsible for the psychotropic effects of cannabis (for affinities of THC and other cannabinoids considered in this review, see Figure 1) [10,11,12,13,14].
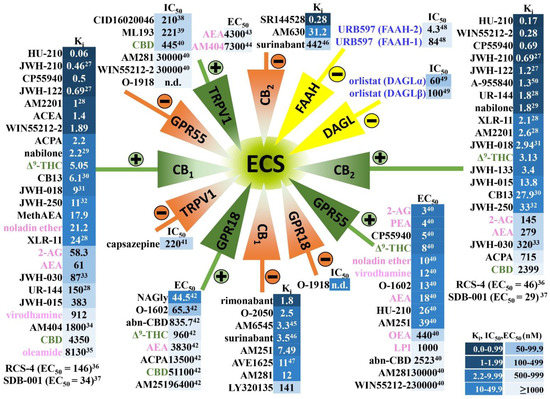
Figure 1.
Cannabinoids and their affinities to the classical cannabinoid CB1 and CB2 receptors and to other receptors sensitive to cannabinoids, as well as to inhibitors of enzymes involved in the synthesis and/or degradation of AEA and 2-AG. Note that the numbers in the superscript indicate the appropriate reference [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The figure presents only phytocannabinoids (green font), synthetic cannabinoids and other compounds discussed in this article (black font), endogenous cannabinoids (pink font) and inhibitors of the endocannabinoid synthesis and degradation (blue font) that have been considered in this review. ECS, endocannabinoid system; the “plus sign” indicates agonism and the “minus sign” antagonism, inverse agonism or inhibition versus the respective receptors/enzymes. The intensity of blue color next to the compound is higher the lower the values of Ki, IC50 or EC50 are (expressed in nM). Based on Pertwee et al. [39] unless stated otherwise (superscript). WIN55212-3, inactive S(–)enantiomer of WIN55212-2 [40]; AM404, an inhibitor of anandamide transport [41]. Abbreviations: Δ9-THC, Δ9-tetrahydrocannabinol; 2-AG, 2-arachidonoylglycerol; abn-CBD, abnormal cannabidiol; ACEA, arachidonoyl-2’-chlorethylamide; ACPA, arachidonylcyclopropylamide; AEA, anandamide; CB1, cannabinoid CB1 receptor; CB2, cannabinoid CB2 receptor; CBD, cannabidiol; DAGL, diacylglycerol lipase; ECS, endocannabinoid system; FAAH, fatty-acid amide hydrolase; GPR18, G protein-coupled receptor 18; GPR55, G protein-coupled receptor 55; LPI, L-alpha-lysophosphatidylinositol; MethAEA, methanandamide; n.d., not determined; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; TRPV1 transient receptor-potential cation-channel subfamily V member 1; URB597, inhibitor of fatty-acid amide hydrolase.
A significant increase in global intake of both plant and synthetic cannabinoids has been observed in recent decades. In 2016, 192.2 million people aged 15–64 years used cannabis for nonmedical purposes across the globe (INCB 2018). The underlying reasons of this effect are growing legalization (e.g., in U.S. the number of states legalizing marijuana for recreational purposes is still rising [42]); the impact of mass culture with the common occurrence of marijuana and its symbols in everyday products; and recently, the overall increase in the use of psychoactive substances during the lockdown of the COVID-19 pandemic [43,44,45]. Moreover, in 2016 the WHO reported a dramatic (about tenfold) increase in the THC content of marijuana [11].
In an early review about the cardiovascular safety of THC [46], the conclusion was reached that harmful effects occur in people with pre-existing heart disease only. In more recent studies, acute exposure even of young healthy people to cannabis was reported to lead to severe cardiovascular events including myocardial infarction (MI), sudden cardiac death, cardiomyopathy, transient ischemic attack and stroke (e.g., [47,48]. For example, Bachs and Mørland [49] reported six cases of cardiac death in young adults in which THC was present in postmortem blood samples. One of the first convincing studies suggesting that marijuana acts as a trigger for myocardial infarction (MI) was performed by Mittleman et al. [50] who showed that marijuana smokers had a 4.8-fold increased risk of developing MI in the first hour after cannabis exposure. Moreover, a French study reported that cardiovascular disorders, including MI and fatal stroke, were observed among 9.5% of 200 cannabis-related hospitalizations [51]. Therefore, it was recommended that individuals with pre-existing cardiovascular conditions should avoid cannabis [48].
In the past five years, cannabis use has been listed among the risk factors of MI (also associated with an increased risk of cardiovascular mortality) in younger patients [52,53] and at least twelve reviews have appeared drawing attention to adverse cardiac effects of cannabinoids (Table 1). They are based on numerous case reports, epidemiologic or retrospective cohort studies encompassing millions of cannabis users, patients or hospitalizations. Their authors conclude that (1) cannabis use is an independent predictor of MI, heart failure and cerebrovascular accidents; (2) young cannabis users are more at risk with respect to hospitalizations for acute MI, arrhythmia and stroke; (3) medical cannabis authorization was associated with an increased risk of visits at emergency departments or hospitalizations for cardiovascular events including stroke and acute coronary syndrome; (4) screening for marijuana use should be performed in young patients with cardiovascular disease; and (5) the increasing risk of MI and other acute cardiovascular events among young cannabis users strongly needs further studies (including clinical trials) to assess cannabis-related cardiovascular implications and to determine the detailed pathophysiology of cardiac adverse events of cannabis (Table 1).

Table 1.
Reviews from the past 5 years highlighting the potential impact of cannabis intake on the increase in risk of myocardial infarction and other severe cardiovascular disorders.
The aims of this review are (1) to give a short introduction of the endocannabinoid system, including cannabinoid receptors and their distribution in areas relevant for cardiovascular effects; and (2) to discuss sites and mechanisms responsible for tachycardia associated with cannabis use in humans. Moreover, we are going to assess whether cannabis use affects (3) the two prototypical pathogenetic mechanisms involved in the development of MI, i.e., thrombus formation and coronary constriction; and (4) further mechanisms which may worsen MI, such as a decrease in energy supply, an increase in energy demand, as well as proarrhythmogenic and cardiotoxic effects.
2. Endocannabinoid System in Humans and in Experimental Animals—Components and Anatomical Distribution
The main components of the endocannabinoid system (ECS) are shown in Figure 1. It consists of (i) the endogenous cannabinoids (endocannabinoids) such as anandamide (AEA), 2-arachidonoylglycerol (2-AG), noladin ether, virodhamine, oleamide or endocannabinoid-like compounds such as palmitoylethanolamide (PEA) or oleoylethanolamide (OEA), (ii) their receptors and (iii) the enzymes involved in their synthesis and degradation [63,64]. Cannabinoids act via two main types of cannabinoid receptors, CB1-R and CB2-R, that belong to the G protein-coupled receptor (GPCR) superfamily. Other targets of endocannabinoids are the orphan G-protein-coupled receptors GPR18 and GPR55. GPR18, which shares low sequence homology with the cannabinoid receptors, is also activated by endogenous L-α-lysophosphatidylinositol (LPI; [65]). Another important cannabinoid-sensitive receptor, which, does not, however, belong to the GPCR superfamily, is the transient receptor-potential vanilloid-type 1 (TRPV1). AEA is among others synthesized by the N-acyl-phosphatidylethanolamine (NAPE) phospholipase D. For 2-AG synthesis, two isoforms of diacylglycerol lipase (DAGL), α and β, are necessary. Fatty-acid amide hydrolases (isoforms FAAH-1 and FAAH-2) and monoacylglycerol lipase (MAGL) are responsible for degradation of AEA and 2-AG, respectively. For removal of AEA, an AEA transporter may also be relevant [64,66].
With respect to THC, two extreme differences to the ECS have to be considered. First, THC has an affinity for CB1, CB2, GPR18 and GPR55 receptors only (without binding to e.g., TRPV1 receptors; Figure 1). Second, degradation of THC does not involve enzymes such as FAAH or MAGL. For this reason, data with TRPV1 receptors will not be considered in this article at all and results with FAAH and MAGL inhibitors will be discussed only if experiments with appropriate antagonists have revealed that CB1, CB2, GPR18 and GPR55 receptors are involved.
To compare acute cardiac functional effects (and their potential mechanisms) in human and in experimental animals we have concentrated on the comparison of the distribution of CB-Rs in the heart, coronary artery, platelets and in brain regions involved in cardiovascular regulation. In the human heart, gene and/or protein expression of CB1-Rs and CB2-Rs occurs in the left ventricle [67,68,69], right atrium [70] and epicardial adipose tissue [71]. CB1-Rs and CB2-Rs were also identified in hearts of the guinea pig [72], rat [73,74,75], and mouse [76,77]. In detail, these receptors occur in the left ventricle [78,79] and left atrium of the rat [80], and in the left ventricle of the dog [81] and the mouse [67,82,83].
The occurrence of the GPR55 was described in human [84] and mouse [85] hearts, in rat neonatal cardiomyocytes [86] and left ventricles [79] and in mouse ventricles [87]. The GPR18 occurs in rat left ventricles [79] and in rat fetal cardiac tissue, but not the maternal heart [88].
Receptors sensitive to cannabinoids were also found in coronary arteries of humans (CB1-Rs, CB2-Rs; [89]) and rats (CB1-Rs; [90]). In human platelets, the occurrence of both classical CB-Rs [91] and the GPR55 [84] was shown.
CB1 receptors were detected in high densities in many brain regions of humans and experimental animals [92]. They also occur in brain regions involved in cardiovascular regulation, including the rostral ventrolateral medulla (RVLM; [93,94]), the bed nucleus of the stria terminalis (BNST; [95,96]), the ventral medial prefrontal cortex (vMPFC; [97]), the paraventricular nucleus of the hypothalamus (PVN; [98]), the nucleus tractus solitarii (NTS; [99]) and the dorsal periaqueductal gray (dPAG; [97,100]).
Receptors sensitive to cannabinoids have so far not been identified in hearts of rhesus monkeys, cats and rabbits. Moreover, the components of the ECS have not been detected in the cardiac conduction system of humans or experimental animals. To the best of our knowledge, GPR18 has not been identified in human cardiac tissue.
3. Tachycardia—General
Zuurman et al. [101] clearly showed in their review analyzing 165 articles, which are dedicated to the acute effects of cannabis or THC on the central nervous system (CNS) and HR in healthy volunteers and are based on 318 tests (or test variants), that an increase in HR is the most reliable biomarker to study the effects of cannabis. Tachycardia leading to complex adverse cardiac consequences such as a decrease in cardiac-stroke volume or myocardial oxygen supply–demand imbalance is regarded as a potent predictor of cardiovascular morbidity and mortality [102,103]. In this context, it is of interest that a reduction in heart rate (HR) induced by various drugs has a beneficial effect in patients with MI [103].
Table 2 shows that a THC-induced increase in HR occurs after:
- smoking of cigarettes (joints) [104,105,106,107,108,109,110,111,112,113,114];
- inhalation by vaporizer [106,111,115,116,117,118];
- peroral intake [111,119,120] (the synthetic form of THC, nabilone, has to be listed here as well (Cesamet®; [121]);
- intravenous (i.v.) injection [122,123,124,125,126];
- administration of an oromucosal spray containing THC and cannabidiol (Sativex®; [127]).

Table 2.
Cardiac effects of acute cannabinoid administration in humans.
Table 2.
Cardiac effects of acute cannabinoid administration in humans.
| Number (Characterization, Age in Years) | Agonist | Dose (mg) * | Application | Cardiac Effects | Comments/Suggested Mechanisms and Involvement of CB1-Rs | References |
|---|---|---|---|---|---|---|
| 10 healthy volunteers (21–33) | THC | 1–40 | cigarette | dose-dependent ↑HR; ↑BP | changes in BP better correlated to HR than to doses; antagonists not studied | [104] |
| 16 healthy volunteers (18–42) | THC | 25 | cigarette | ↑HR; ↓BP: normotensive < hypertensive persons | n.a. | [105] |
| 6 healthy volunteers (18–30) | THC | 10 | cigarette | ↑HR; ↑BP | tachycardia resulting from β-AR activation (diminished by propranolol 120 mg p.o.) | [107] |
| 10 healthy volunteers(30–40) | THC | 10 | cigarette | ↑HR; ECG: ↑amplitude and ↓width of P wave in Lead 2 and inversion of T wave in Lead 3 | tachycardia is mediated via β-ARs, since it was prevented by propranolol (40 mg/kg p.o.) but not by atropine (0.6 mg/kg s.c.) | [108] |
| 14 healthy volunteers (20–31) | THC | 6 | cigarette | ↑HR and ↑left ventricular performance (mean rate of internal diameter shortening) | tachycardia not accompanied by ↑plasma NA levels, since the respective maximal increases took place at 10 and 30 min, respectively | [110] |
| 21 experienced users of cannabis (21–45) | THC | 20–60 | 1 to 3 cigarettes | ↑HR; ↑cardiac output ↔stroke volume ↔ejection fraction | marijuana has no significant effect on myocardial contractility independent of its effect on HR | [113] |
| 91 cannabis users (19–25); double-blind, placebo-controlled, parallel-group randomized clinical trial | THC | 94 | cigarette | ↑HR; peak HR (~40 beats/min) and plasma THC concentration (~55 ng/mL) at 5 min; ↑HR different until +4 h | n.a. | [112] |
| 42 volunteers (mean age of 29); randomized, double blind, parallel group design | cannabis | 2.8% THC | cigarette | ↑HR | acute tachycardia depends on CB1-Rs since it was diminished by acute rimonabant (90 mg/kg p.o.) or its chronic application (40 mg/kg for 8 and 15 days) | [109] |
| 16 healthy volunteers (mean age of 28) | THC UR-144 | 1, 1.5 10, 20 | joints (smoking) | ↑HR, ↑BP ↑HR, ↑BP | (THC and the preferential CB2-R agonist UR-144 were administered in joints containing tobacco.) | [114] |
| 16 healthy volunteers (mean age of 30) | JWH-122 JWH-210 | 1 1.25 | joints (smoking) | ↑HR, ↑BP ↔HR, ↔BP | (compounds with high potency at CB1-/CB2-Rs, respectively) | [128] |
| 17 healthy volunteers (mean age of 27) | THC | 10 25 | smoked or vaporized | ↑HR ↑HR | THC-induced tachycardia slightly higher in the case of vaporization | [106] |
| 36 healthy volunteers (18–31) | THC | 2, 4 and 6 | inhalation by vaporizer | ↑HR in a dose-dependent manner | THC-induced tachycardia inhibited by the CB1-R antagonist AVE1625 (20, 60, 120 mg p.o.) | [116] |
| 30 healthy volunteers (18–45); double-blind, placebo-controlled, randomized, four-period six-sequence crossover study | THC | 2, 4 and 6 | inhalation by vaporizer | ↑HR | THC-induced tachycardia was inhibited by the CB1-R antagonist surinabant 20 and 60 mg p.o. | [118] |
| 12 healthy volunteers (21–27) | THC | 2, 4, 6 and 8 | vaporized | sharp ↑HR and rapid decline | THC-induced tachycardia is dose-dependent | [115] |
| 12 healthy volunteers | THC | 2, 4, 6 and 8 | vaporized | ↑HR | different sites of action for cardiac and CNS responses suggested: average population equilibration half-life for HR 8 min and for CNS 39–85 min | [117] |
| 11 frequent and 9 occasional cannabis smokers (mean age of 27 and 29, respectively) | THC | ~54 | smoked vaporized oral | ↑HR; ↑carbon monoxide ↑HR ↑HR | smoking produced higher increase in carbon monoxide compared to vaporization | [129] |
| 84 healthy volunteers (mean age of 32), naturalistic, ad libitum use | THC | average 51 average 16 | smoked or vaporized (flower cannabis), oral (edible cannabis) | ↑HR ↑HR | the flower group started with lower basal HR than the edible group at pre-use but had higher average HR at post-use | [111] |
| 16 healthy volunteers (mean age of 26) | THC CBD | 10 600 | capsules | ↑HR ↔HR | tachycardia induced by THC but not by CBD (has low affinity to CB1-Rs) | [119] |
| 14 healthy volunteers (21–45); randomized, double-blind design | nabilone Cesamet® dronabinol Marinol® | 4, 6, 8 10, 20 | capsules capsules | dose-dependent ↑HR, ↓systolic BP ↔HR, ↓systolic BP | nabilone has better bioavailability than dronabinol (THC) | [121] |
| 37 healthy volunteers (18–35) | THC | 7.5, 15 | capsules | ↑HR, ↓heart rate variability, ↔pre-ejection period, ↔BP | tachycardia results from parasympathetic inhibition; no changes in sympathetic tone | [120] |
| 9 healthy volunteers (mean age of 21.4) | THC (Namisol®) | 6.5 and 8.0 | tablets | slight ↑HR (by about 5 beats/min) | n.a. | [130] |
| 11 healthy volunteers (≥65) | THC (Namisol®) | 3, 5 or 6.5 | tablets | no clinically relevant changes in HR and ECG parameters | n.a. | [131] |
| 5 volunteers (22–29); regular marijuana smokers (at least once a day) | THC | 30 μg/kg | i.v. | ↑HR | tachycardia results from sympathetic stimulation and parasympathetic inhibition (diminished by i.v. propranolol and atropine 0.2 and 0.04 mg/kg, respectively) | [122] |
| 20 healthy male volunteers (22–30) | THC | 25 μg/kg ≈ 5 mg in one marijuana cigarette | i.v. | ↑HR, ↑total electromechanical systole; ↑left ventricular ejection time; ↓pre-ejection period | THC-induced changes in cardiac performance via the autonomic nervous system: partially diminished by propranolol 0.1 mg/kg i.v. and totally by propranolol 0.1 mg/kg plus atropine 2 mg/kg i.v. | [123,124] |
| 21 healthy volunteers | THC CBD | 0.2 mg/min 1.8 mg/min | i.v. | ↑HR ↑HR | CBD increases HR only at a much higher dose than THC | [126] |
| 6 patients undergoing diagnostic ECG evaluation | THC | 25 μg/kg | i.v. | ↓sinus length; ↓mean sinus node recovery, ↓maximal sinus node recovery times; ↓mean calculated sinoatrial conduction; ↓mean A-V nodal effective and functional refractory periods | (25 µg/kg i.v. correspond to ≈5 mg in one marijuana cigarette) enhancement of sinus automaticity and facilitation of sinoatrial and A-V nodal conduction | [125] |
| 9 cannabis users | THC Sativex®: THC: CBD | 5, 15 16.2:15.0 | oromucosal sprays | comparable ↑HR induced by THC and Sativex® | CBD fails to diminish the THC-induced tachycardia | [127] |
This table does not include individual case reports because of the marked heterogeneity in terms of dose, concomitant diseases or concurrent drugs and stimulants (which, in turn, might lead to adverse cardiac reactions). * if not stated otherwise; A-V, atrioventricular; β-AR, β-adrenergic receptor; BP, blood pressure; CB1-R, CB2-R, cannabinoid CB1, CB2 receptor; CBD, cannabidiol; CNS, central nervous system; ECG, electrocardiography; HR, heart rate; i.v., intravenous(ly); NA, noradrenaline; n.a., not applicable; p.o., per os; s.c., subcutaneous(ly); THC, Δ9-tetrahydrocannabinol.
Significant tachycardia also occurs after smoking of UR-144, JWH-122 and JWH-210 [114,128] and other synthetic cannabimimetics (reviewed by Zawilska et al. [8] and Chung et al. [9]). Nonetheless, quantitative or even qualitative differences exist, depending on the route of administration, the compound under consideration and the pharmaceutical formulation. Thus, comparable increases in HR took place 0.5 h after smoking or vaporization but only 1.5 h after oral administration of the same dose of THC [129]. An increase in HR was induced by nabilone (Cesamet®) but not by higher doses of THC (Marinol®), because of the better bioavailability of nabilone compared to THC [121]. Namisol®, another oral formulation of THC, led to only slight and not clinically relevant changes in HR [130,131].
Cannabinoid-induced tachycardia in humans is mediated by cannabinoid CB1 receptors (Table 2), since it was antagonized by three different CB1-R antagonists including rimonabant [109], AVE1625 [116] and surinabant [118]. Moreover, cannabidiol (CBD), which possesses very low affinity to CB receptors (see Figure 1), failed to modify HR [119] or increased HR only at a much higher dose than THC did [126]. CBD (believed to be an antagonistic principle against some of the effects of THC; [132]) did not modify the tachycardia induced by THC [127].
In order to determine the mechanism and site of action of the tachycardic effect of THC, it is of interest to include experiments on experimental animals since many studies in humans are not possible for ethical reasons. Since THC was less frequently used in experiments on animals than in humans, not only cardiac effects of THC but also of various other CB-R ligands have been considered in Table 3, Table 4 and Table 5.

Table 3.
Cardiac effects of acute cannabinoid administration in experimental animals in vivo.
Importantly, THC injected i.v. increased HR only in one early publication performed on conscious rhesus monkeys [133]. In two other publications on conscious rhesus monkeys THC given intraperitoneally (i.p.) and intramuscularly (i.m.) induced bradycardia [134,135] probably via CB1-Rs (Table 3). The above receptors are probably activated by endogenous cannabinoids or are constitutively active, since rimonabant i.v., which diminished the THC-elicited bradycardia [135], produced tachycardia by itself in conscious rhesus monkeys [136]. THC-induced bradycardia in conscious animals also occurred independent from the route of its administration in other species such as mongrel dogs (i.v., [137]), rats (i.p., [142]) and mice (i.p., [25]). Sativex®, administered as spray, had no effect in beagle dogs [138].
How can we explain such a drastic difference in the cardiac effects of THC between humans and experimental animals? Of course, species differences may be responsible. In this context, one should keep in mind that the low resting HR (~70 beats/min) in humans results from strong parasympathetic dominance [165]. By contrast, basal HR in the experimental animals listed in Table 3 were higher than in humans, pointing to differences in the balance between sympathetic and parasympathetic tone. It amounted to (in beats/min) 100–130 [133], ~130 [136], 140–160 [134] and 190–230 [135] in conscious rhesus monkeys, ~90 in mongrel dogs [137], 350–400 in rats [142] and ~400 in mice [25]. Thus, we cannot exclude the possibility that THC (and/or other cannabinoids) increases HR only in the case of a low basal HR such as in humans.
The cardiovascular effects of cannabinoids have also been studied in anaesthetized animals (for review, see Malinowska et al. [166]; Table 3). Regarding changes in HR, THC i.v. increased HR in mongrel dogs anaesthetized with morphine plus chloralose, but reduced it in conscious animals [137]. Tachycardia was also induced by the highest dose of THC (30 mg/kg i.v.) in anaesthetized rats [155]. In other cases, decreases in HR were reported (i) for THC in anaesthetized cats [151,152] and rats [142,155], (ii) for THC and ∆8-THC (previous name Δ6-THC) and the synthetic cannabinoids WIN55212-2, CP55940, HU-210, JWH-030, JWH-015 and ACPA in anaesthetized rats [152,155,156,157,159] and (iii) for AEA in anaesthetized mice (CB1 receptor-mediated Phase II; [158]). The cannabinoid-induced bradycardia in anaesthetized rats, as in conscious animals, is mediated via CB1-Rs since these responses were diminished by rimonabant [155,157,159] but not by the CB2-R antagonist SR144528 [157].
4. Tachycardia—Mechanisms
The site/mechanism of action involved in the tachycardic effect of THC in humans may be
- i.
- the heart itself;
- ii.
- the autonomic nervous system;
- iii.
- the central nervous system;
- iv.
- the baroreceptor reflex.
4.1. Heart
The possibility that THC elicits tachycardia via activation of CB1-Rs in the sinoatrial node is not plausible, since these receptors are Gi/o protein-coupled, i.e., inhibitory. The possibility that THC acts via the β1-adrenoceptors activated by noradrenaline (NA) can be excluded, since THC is devoid of a sufficient affinity for this type of receptors [149,163].
Another possibility might be that THC affects the β1-adrenoceptor-mediated effect of NA via allosteric modulation. Maggo and Ashton [167] found in isolated rat right atria that WIN55212-2 and MethAEA slightly increased the chronotropic effect of NA. The authors suggest the potential involvement of CB1-Rs (as opposed to CB2-Rs) in this effect, but they did not use any antagonists.

Table 4.
Effects of cannabinoid-receptor agonists on heart preparations and platelets of humans and experimental animals in vitro.
Table 4.
Effects of cannabinoid-receptor agonists on heart preparations and platelets of humans and experimental animals in vitro.
| Species | Isolated Heart Preparation | CB-R Ligands, Concentrations 1 | Effects | Possible Mechanisms (CB1-Rs, CB2-Rs, Others) if Determined | References |
|---|---|---|---|---|---|
| humans | right atrial muscle | AEA, MethAEA and HU-210 AM251 | ↓contractility ↑contractility | CB1-R-mediated negative inotropic effect of AEA (antagonized by AM251 but not by AM630); endogenous tone at CB1-Rs | [70] |
| right atrial appendages | AEA and CP | ↓ electrically stimulated [3H]-NA release | presynaptic inhibitory CB1-Rs (effect antagonized by RIM and LY320135) | [168] | |
| Wistar rats | perfused heart 2 | THC | ↑HR, ↓CF, ↓ cardiac activity | cardiotoxicity, antagonists not used | [169] |
| SD rats | perfused heart 2 | THC ∆8-THC | ↑HR, ↓force of contraction ↔HR (arrhythmia), ↓force of contraction | antagonists not used | [170] |
| Wistar rats | perfused heart 2 | HU-210 | ↓HR | antagonists not used | [171] |
| Wistar rats | perfused heart 2 | HU-210, RIM and SR144528 | ↔HR, ↓LVDP, ↓dp/dt max, ↓dp/dt min | negative inotropism, mechanism unclear; partial agonism of RIM and SR144528? | [172] |
| Wistar rats | perfused heart 2 | HU-210 | ↓HR, ↓LVDP, ↓MRC, ↓MRR, ↓LVEDP alone and after ISO (100 nM) | negative chrono- and inotropism (mechanism?); ↓cardiostimulatory effect of ISO | [157,173] |
| SD rat | perfused heart 2 | AEA, MethAEA and ACEA PEA and JWH-015 | ↓LVDP, ↑CF ↔ LVDP, ↔CF | novel sites mediating AEA-induced negative inotropism (reduced by RIM and SR144528 but not AM251, AM630, CAPZ) and coronary vasodilation (reduced by RIM, SR144528 and AM251, but not AM630 and CAPZ) | [174] |
| Wistar rats | perfused heart 2 | AEA | ↔HR, ↔CF, ↓dp/dt max, ↓LVSP | antagonists not used | [175] |
| Wistar rats | perfused heart 2 | oleamide | ↑CF | CB1-R suggested but no proven | [176] |
| Wistar rats | perfused heart 2 with VP-induced coronary preconstriction | AEA or ACEA JWH-133 THC | ↔HR, ↑CF, ↑LVSP ↑CF, ↑LVSP ↓CF, ↓LVSP | coronary vasodilation and positive inotropism of AEA and ACEA (but not of THC and JWH-133) mediated via CB1-Rs (reduced by RIM and AM251 but not by SR144528 and O-1918); effects of THC and JWH-133 not modified by AM251 or AM630 | [175] |
| SD rats | perfused heart 2 | 2-AG WIN-2 | ↑CF, ↔LVDP ↑CF, ↓LVDP | coronary vasodilation is mediated via CB1-Rs; diminished by O-2050; negative inotropic effect by WIN-2 but not by 2-AG | [77] |
| SD rats | perfused heart 2 | O-2050 and orlistat | ↓effects induced by Ang II including ↓LVDP, ↓dp/dt max, ↓dp/dt min and ↓CF | 2-AG reduces negative inotropism + coronary constriction of Ang II via CB1-Rs | [77] |
| SD rats | perfused heart 2 | JWH-030 30 (but not 3) µM | ↓LVEDP, ↔LVSP, ↔LVDP, ↔HR | JWH-030 reduces cell viability via CB2-Rs (effect of AM630; no effect of RIM) | [156] |
| SD rats | perfused heart 2 or tachypaced | CB13 | ↔ dp/dt max, dp/dt min, HR, AV interval, LVDP, ↓tachypa- cing-induced shortening of the atrial effective refractory period | no effects on basal hemodynamic properties; beneficial effects against atrial fibrillation (antagonists not used) | [80] |
| Wistar rats | intramural coronary resistance artery | WIN-2 | relaxation | coronary vasodilation mediated by CB1-Rs (antagonized by O-2050 and AM251); CB1-R blockade enhanced myogenic tone | [90] |
| guinea pigs | atria | WIN-2 abn-CBD | ↓electrically stimulated [3H]-NA release ↔ electrically stimulated [3H]-NA release | presynaptic CB1-R (antagonized by RIM) but no GPR18 on sympathetic nerve endings | [177] |
| SD rats | atria | AEA, THC, PEA, JWH-015 | ↓stimulated [3H]-NA release | presynaptic CB1-Rs on sympathetic nerve endings (antagonism by RIM) | [178] |
| Wistar rats | right atria | WIN-2, MethAEA JWH-133 | ↔ HR; ↑chronotropic effect of NA ↔HR; ↔chronotropic effect of NA | slight enhancement of chronotropic effect of NA (antagonists not used) | [167] |
| Wistar rats | right atria | CP, CBD AM251 or AM630 | ↔chronotropic effect and ↑inotropic effect of ISO chronotropic effect of ISO ↑ 1 µM; ↓ 3 µM | mechanism of the effect of CP, AM251 and AM630 on the positive inotropism and/or chronotropism of ISO unclear | [179] |
| Wistar rats | left atria | AEA AEA ACEA JWH-015 | ↔dF/dt + AM251: ↑dF/dt + AM630: ↓dF/dt ↓dF/dt ↑dF/dt | negative inotropic effect via CB1-Rs and positive inotropic effect via CB2-Rs (AM251 and AM630 reduced the effects of ACEA and JWH-015, respectively) | [180] |
| rabbits | left ventricular myocytes | A-955840 | ↓FS, ↓dL/dt max, ↓L-type calcium current | CB2-R agonist A-955840 has negative inotropic effect independent of CB1-Rs and CB2-Rs (not modified by RIM and SR144528) | [38] |
| SD rats | ventricular myocytes | CB13 | ↔ contractile function | no inotropic effect | [18] |
| mice GPR55-/- | left ventricular cardiomyocyte | GPR55 deletion | ↑diastole sarcomere length ↔systole sarcomere length ↑transient Ca2+ amplitude ↓time from peak contraction to 50% and 90% relaxation | contractile changes dependent of GPR55 | [85] |
| SD rats | homogenized ventricles | THC ∆8-THC | ↓adenylate cyclase activity ↔adenylate cyclase activity | ↓adenylate cyclase activity may lead to cardiac depressant action of THC | [181] |
| humans | platelets | THC | ↑expression of glycoprotein IIb-IIIa and P-selectin involved in platelet activation | antagonists not used | [91] |
| platelets | THC | ↓adrenaline- or ADP-induced aggregation | antagonists not used | [182] | |
| platelets | AEA | ↑platelet activation ↑intracellular Ca2+ concentration | AA metabolites not involved (lack of effect of ASA and an FAAH inhibitor) | [183] | |
| platelets | AEA | ↔aggregation ↓aggregation induced by collagen, ADP, AA and TXA2 analogue but not by thrombin | antagonists not used | [184] | |
| platelets | 2-AG | ↑aggregation; ↑intracellular Ca2+ concentration; ↑AA and TXB2 release | CB1-Rs involved (inhibition by RIM but not by SR144528 and AA derivatives) | [185] | |
| platelets | THC 2-AG AM251 | ↔aggregation ↑aggregation ↔aggregation induced by ADP and thrombin | 2-AG induced aggregation was independent from CB1- and CB2-Rs (not antagonized by AM251 and AM630) but dependent on the conversion to AA (inhibited by MAGL inhibitor and ASA) | [186] | |
| platelets | 2-AG ACEA, JWH-015 | ↑aggregation, platelet activation ↔aggregation | platelet aggregation induced by 2-AG is independent from CB1- and CB2-Rs (not antagonized by RIM or SR144528 but connected with ↑TXA2) | [187] | |
| platelets | 2-AG, virodhamine AEA, ACEA, JWH015 | ↑aggregation, platelet activation ↔aggregation | platelet aggregation induced by 2-AG and virodhamine independent from CB1- and CB2-Rs but inhibited by MAGL inhibitor, ASA and TXA2-R antagonist | [188] | |
| platelets | AM251 or AM630 | ↔platelet count ↔aggregation induced by collagen, AA and ADP | platelet aggregation is independent of CB1- and CB2-Rs | [189] | |
| platelets | LPI | ↔aggregation ↓aggregation induced by ADP | GPR55 involved (effect of LPI reversed by the GPR55 antagonist CID16020046) | [26] | |
| rabbits | platelets | THC | ↓aggregation induced by ADP and PAF; ↓5-HT release from platelets | antagonists not used | [182] |
| rabbits | platelets | AEA HU-210 | ↑aggregation ↔aggregation | platelet aggregation induced by AEA independent from CB1-Rs (not antagonized by RIM but reduced by ASA) | [190] |
| mice | homogenised hearts | THC 100 µM | ↓oxygen consumption | antagonists not used | [191] |
| mice | cardiac mitochondria | THC 0.1 and 0.2 µM | ↓oxygen consumption ↓mitochondria coupled respiration | ↓oxygen consumption; not dependent on CB1-Rs (similar changes in CB1−/− mice) | [192] |
| beef | cardiacmitochondria | THC 120 µM | ↓respiration ↓ oxygen consumption | ↓mitochondrial oxygen consumption; antagonists not used | [193] |
| rats | cardiac mitochondria | THC, HU-210, AEA THC and HU-210 | ↓oxygen consumption and ↓mitochondrial membrane potential ↑mitochondrial hydrogen peroxide production | ↓mitochondrial oxygen consumption; antagonists not used | [194] |
| Wistar rats | cardiac mitochondria | THC up to 500 µM | ↔ROS production, no mitochondrial swelling ↔membrane potential, no oxidative stress, no lipid peroxidation | THC is not directly toxic in isolated cardiac mitochondria, and may even be helpful in reducing mitochondrial toxicity | [195] |
| SD rats | neonatal ventricular myocytes | CB13 | prevents ET1–induced ↓mitochondrial bioenergetics and mitochondrial membrane depolarization | improvement in cardiac mitochondrial function (precise mechanism unclear) | [196] |
| sheep | Purkinje fibers | THC | ↑APD90 | antagonists not used | [197] |
| rabbits | Purkinje fibers | JWH-030 JWH-210 | ↓APD90, ↔resting membrane potential ↔APD90, ↔resting membrane potential | only the highest concentration of JWH-030 reduces APD (mechanism unclear) | [156] |
| rabbits | sinoatrial node samples | AEA | ↓AP duration and ↓AP amplitude | ↓AP duration and ↓AP amplitude in SAN pacemaker cells via CB1-Rs (blocked by AM251 but not by AM630) | [198] |
| Wistar rats | papillary muscles; ventricular myocytes | AEA | ↓AP duration ↓AP amplitude ↓L-type Ca2+ current | antiarrhythmic properties; ↓AP and ↓L-type Ca2+ current through CB1-Rs (antagonized by AM251 but not AM630) | [199] |
| Wistar rats | ventricular myocytes | AEA | ↓Ito, ↑IKATP, ↔Iss, ↔IK1 | antiarrhythmic and cardioprotective properties: ↓Ito independent of CB1- and CB2-Rs; ↑IKATP mediated via CB2-Rs (antagonized by AM630 but not AM251) | [200] |
| Wistar rats | ventricular myocytes | AEA JWH-133 | ↔NCX1 [Ca2+]i: normal conditions; ↓NCX1 and [Ca2+]i: ischemia ↓NCX1 and [Ca2+]i: ischemia | ↓calcium overload through ↓NCX1 during ischemia via CB2-Rs (antagonized by AM630 but not by AM251; mimicked by JWH-133) | [201] |
| SD rats | neonatal ventricular myocytes | AEA, MethAEA, JWH-133 and CB13 | ↓ET1-induced induction of markers of hypertrophy | antihypertrophic properties via CB1- and CB2-Rs (antagonism by AM251/AM281 and AM630, respectively) | [18] |
| SD rats | neonatal ventricular myocytes | extracellular LPI administration intracellular LPI administration | ↑Ca2+ entry via L-type Ca2+ channels, long-lasting membrane depolarization ↑Ca2+ release from endolysosomal Ca2+ channels, short-lived membrane hyperpolarization | GPR55 Rs at the sarcolemma: ↑Ca2+ entry via L-type Ca2+ channels, leading to depolarization; GPR55 Rs at membranes of intracellular organelles: ↑intracellular Ca2+ release, leading to hyperpolarization (all effects blocked by ML193) | [86] |
| guinea pigs | ventricular cardiac nuclei | AEA | ↓IP3-mediated nuclear Ca2+ release | involves CB1- and CB2-Rs (effect reversed by AM251 and AM630, respectively) | [72] |
| rat cardiomyoblast cell line | H9c2 cells | THC-OH and THC-COOH THC | ↑cell migration and proliferation, ↑cell death and significant deterioration in cellular architecture ↔cell morphology or viability | the key metabolites of THC, as opposed to THC itself, elicit toxic cardiac effects (note that THC does not undergo metabolism in H9c2 cells) | [202] |
| rat cardiomyoblast cell line | H9c2 cells | JWH-030, JWH-210, JWH-250 or RCS-4 | all 0.1–100 µM: ↓cell viability, ↑cell apoptosis | synthetic cannabinoids induce cardiotoxicity via CB2-Rs (reduced by AM630 but not RIM) | [156] |
| mice | HL-1 atrial cardiomyocyte | THC 10 and 30 µM | ↑ER stress and apoptosis | cardiotoxicity independent of CB1-/CB2-Rs (not blocked by AM251 and AM630) | [203] |
Unless stated otherwise, antagonists did not modify cardiac parameters. 1 concentrations of drugs (µM) usually not given; 2 spontaneously beating. ↑increase; ↓decrease; ↔no effect; [3H]-NA, [3H]-noradrenaline; [Ca2+]i, intracellular Ca2+ concentration; Δ8-THC, Δ8-tetrahydrocannabinol (formerly Δ6-THC); 2-AG, 2-arachidonoylglycerol; 5-HT, 5-hydroxytryptamine; AA, arachidonic acid; abn-CBD, abnormal cannabidiol; ACEA, arachidonoyl-2′-chlorethylamide; ADP, adenosine diphosphate; AEA, anandamide; Ang II, angiotensin II; AP, action potential; APD, action potential duration; APD90, action potential duration at 90% repolarization; ASA, acetylsalicylic acid; AV, atrioventricular; CAPZ, capsazepine; CB1-R, cannabinoid CB1 receptor; CB2-R, cannabinoid CB2 receptor; CBD, cannabidiol; CF, coronary flow; CP, CP55940; dF/dt, contractility; dL/dt max, maximal shortening velocity; dp/dt max, maximum rates of contraction; dp/dt min, maximum rates of relaxation; ER, endoplasmic reticulum; ET1, endothelin-1; FAAH, fatty-acid amide hydrolase; FS, fractional shortening; GPR18, G protein-coupled receptor 18; GPR55, G protein-coupled receptor 55; HR, heart rate; IK1, inward-rectifier potassium current; IKATP, ATP-sensitive potassium current; IP3, inositol 1,4,5-trisphosphate receptor; ISO, isoprenaline; Iss, steady-state outward potassium current; Ito, transient outward potassium current; LPI, L-α-lysophosphatidylinositol; LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure; MAGL, monoacylglycerol lipase; MethAEA, methanandamide; MRC, maximum rate of contraction; MRR, maximum rate of relaxation; NA, noradrenaline; NCX1, Na+/Ca2+exchanger; PAF, platelet-activating factor; PEA, palmitoylethanolamide; RIM, rimonabant; ROS, reactive oxygen species; Rs, receptors; SAN, sinoatrial node; SD, Sprague Dawley; THC, Δ9-tetrahydrocannabinol (formerly Δ1-THC); THC-OH, 11-hydroxy-Δ9-THC; THC-COOH, 11-nor-9-carboxy-Δ⁹-tetrahydrocannabinol; TXA2, thromboxane A2; TXA2-R, thromboxane A2 receptor; TXB2, thromboxane B2; VP, vasopressin; WIN-2, WIN55,212-2.
In a similar study on rat atria [179], CP55940 (or CBD) did not affect the positive chronotropic effect of isoprenaline, an unselective β-adrenoceptor agonist. By contrast, both AM251 (CB1-R antagonist) and AM630 (CB2-R antagonist) increased the effect of isoprenaline at 1 µM and decreased it at 3 µM. It must be recalled in this context that a negative and positive inotropic effect occurs following CB1- and CB2-R activation in the isolated left atrium of the rat, respectively [180]. It is surprising that both antagonists influenced the effect of isoprenaline in an identical concentration-dependent manner in the study of Weresa et al. [179] and it is unclear whether the results obtained for the antagonists are of interest for the effect of THC.
However, THC and/or synthetic cannabinoids failed to modify the tachycardia induced by isoprenaline in spinal dogs [149], pithed rats [40,163] and rabbits [139]. They also failed to affect the bradycardia induced by the muscarinic (M) receptor agonist methacholine in pithed rats [40].
In conclusion, there is not much evidence that the conduction system of the heart plays a role in the THC-induced tachycardia.
4.2. Autonomic System
Several studies shown in Table 2 suggest that the tachycardia induced by THC in humans might result from sympathetic stimulation and/or parasympathetic inhibition, since it was diminished by previous administration of the β-adrenoceptor antagonist propranolol and the M receptor antagonist atropine [107,122,123,124]. However, these results are not consistent. Thus, the THC-induced tachycardia led to a decrease in high-frequency heart-rate variability (HF-HRV), a measure of parasympathetic cardiac control; but to no changes in the pre-ejection period (PEP), a measure of sympathetic cardiac functioning [120]. However, THC shortened PEP in the early study by Kanakis et al. [123]. Moreover, Beaconsfield et al. [108] described an inhibitory effect by propranolol but not by atropine.
The question is whether THC directly acts via the autonomic system and/or via a central mechanism. The results by Gash et al. [110], who found that the maximal increases in HR and plasma noradrenaline (NA) level took place 10 and 30 min after THC smoking, respectively, suggest but do not prove that tachycardia is related to a peripheral mechanism. This conclusion may also be reached from the study by Strougo et al. [117], in which the average population equilibration half-lifes for HR and CNS responses were < 10 min and 40–85 min, respectively.
Provided that the autonomic system is the site involved in THC-induced tachycardia, a direct activation of the sympathetic nervous system and/or an inhibition of the parasympathetic system should occur. To clarify the mechanisms, again in vivo experiments on animals and in vitro experiments on tissues from humans and experimental animals have to be considered.
The possibility that the effect of THC on HR directly involves the peripheral autonomic system was examined in dogs, cats, rabbits and rats. In anaesthetized dogs [149], the THC-induced bradycardia was partially inhibited by spinal section at C2-C4 or by bilateral vagotomy (to destroy the sympathetic and parasympathetic parts of the autonomic nervous system, respectively) and abolished by the combination of both procedures. Vollmer et al. [151] showed that the THC-induced bradycardia in anaesthetized cats was diminished by cervical cardiac denervation but not by vagotomy. In pithed rabbits [139] and in pithed and vagotomized rats [40,160,161,162,163], in which the CNS is mechanically destroyed, neither THC nor one of the synthetic or endogenous cannabinoids under study (i.e., WIN55212-2, CP55940, AEA and MetAEA) produced a fall in HR.
The study by Cavero et al. [149] on dogs also excludes that THC acts via autonomic ganglia. Another two mechanisms have been considered in in vitro studies. Thus, THC might lead to an increased availability of NA due to the inhibition of the neuronal NA transporter or to facilitation of carrier-mediated NA release. The latter two mechanisms are involved in the peripheral effects of cocaine and methamphetamine, respectively, which, as with THC, can elicit a marked tachycardia, sometimes even associated with MI [204,205]. However, an inhibitory effect of THC on the NA transporter in rat hypothalamic synaptosomes occurred at very high concentrations only [206] and THC, CP55940, WIN55212-2, AEA and 2-AG did not facilitate carrier-mediated NA release in rat and mouse renal tissue at all [207]. The results of the latter studies carried out on extracardiac tissues can be transferred to the heart, since the properties of the NA transporter do not differ between tissues.
Although there are some pieces of evidence that THC does not directly act via the autonomic nervous system, more recent data show that there is a direct effect anyway, i.e., via presynaptic receptors. These types of receptors were almost unknown when Cavero et al. [149] and Vollmer et al. [151] carried out their experiments in dogs and cats, respectively. In pithed animal preparations, presynaptic receptors on sympathetic and/or parasympathetic nerve fibers will be overlooked since an impulse flow along the neurones does no longer occur. When, however, the sympathetic outflow of pithed rats [40,160,161] and rabbits [139] was stimulated electrically, a CB1 receptor-mediated inhibition of the neurogenic tachycardic response could be demonstrated. In pithed rabbits, a CB1 receptor-mediated inhibition of vagal neuroeffector transmission in the heart was also described [139]. In harmony with the latter study, methylatropine, an M-receptor antagonist that does not penetrate the blood–brain barrier, diminished the bradycardia induced by WIN55212-2 in anaesthetized rats [159]. Presynaptic CB1-Rs leading to inhibition of atrial NA release have also been identified in vitro in atrial tissue from humans [168], guinea pigs [177] and rats [178]. Presynaptic facilitatory CB1-Rs cannot be expected, since CB1-Rs are Gi/o protein-coupled and only Gs and Gq protein-coupled receptors lead to facilitation of neurotransmitter release [208]. The other three receptor entities activated by THC, i.e., CB2, GPR18 [177] and GPR55, do not serve as presynaptic receptors.
One can expect that sympathetic hyperactivity and reduced parasympathetic transmission are accompanied by several cellular pathologies, typical of MI-induced cardiac injury. Examples are oxidative stress, infiltration of inflammatory cells to the myocardium and peripheral ganglia, elevation of proinflammatory cytokines and nerve growth factor, and activation of satellite glial cells [209].
In conclusion, presynaptic inhibitory CB1-Rs are present on the cardiac sympathetic neurones of humans and animals and on the parasympathetic neurones of rabbits; their activation would lead to a decrease and increase in HR. The bradycardia usually elicited by THC in animals might be related to a predominant action on the presynaptic CB1-Rs on the sympathetic neurones or by a combined activation of CB1-Rs in the brain and in the autonomic system. The fact that tachycardia occurs in humans instead may be related to a stimulatory input from the CNS which overrides the brake due to the inhibitory CB1 receptors on the sympathetic nerve endings. The potential role of presynaptic inhibitory CB1-Rs on the cardiac human parasympathetic neurons has so far not been examined.
4.3. Central Nervous System
In order to obtain a deeper insight into the brain mechanisms involved in the effect of THC on HR, experiments are of interest in which THC or another cannabinoid was administered to the cerebrospinal fluid (CSF) or directly into brain sites involved in cardiovascular regulation. In studies on dogs [149], cats [151], rabbits [140,141] and rats [210,211,212] in which THC or another cannabinoid agonist was injected into the cerebral circulation or into the CSF, a bradycardia occurred consistently (Table 5). Only in one study on anaesthetized rats, an agonist with preference for CB1 receptors did not affect HR at all [210].

Table 5.
Cardiovascular effects of acute cannabinoid administration into the cerebral circulation, the cerebrospinal fluid or directly into selected brain areas.
Table 5.
Cardiovascular effects of acute cannabinoid administration into the cerebral circulation, the cerebrospinal fluid or directly into selected brain areas.
| Species | Conscious/ Anaesthetized with | Site of Injection | Drug under Study | Dose (nmol/rat), if Not Stated Otherwise | Effects | Possible/Suggested Mechanisms and Involvement of CB1-Rs/CB2-Rs/Others if Determined 1 | References |
|---|---|---|---|---|---|---|---|
| rabbits | conscious | intracisternal | CP or WIN-2 CP or WIN-2 WIN-3 | 0.1 and 1 µg/kg 10 µg/kg 0.1, 1 or 10 µg/kg | ↓HR, ↑RSNA, ↑plasma NA ↓HR, ↑RSNA, ↑plasma NA + ↑BP no effects | ↓HR and ↑BP, related to central CB1-Rs (diminished by i.v. RIM, not shared by inactive WIN-3) ↓HR related to muscarine receptors (also reduced by atropine) | [140,141] |
| mongrel dogs | pentobarbital | head circulation | THC | 2.5 mg/kg | ↓HR | THC-induced bradycardia has a central origin and involves an alteration of the central autonomic outflow regulating HR | [149] |
| cats | chloralose | lateral cerebral ventricle | THC | 2 mg/kg | ↓HR; ↔ BP | THC-induced bradycardia mediated centrally and not associated with a substantial reduction in BP | [151] |
| WKY | urethane | i.c.v. | ACEA | 1400 | ↔ HR, ↔ BP, ↔plasma NA and Adr | [210] | |
| Wistar rats | urethane | intracisternal | WIN-2 WIN-3 | both 1, 3, 10 and 30 µg/kg | WIN-2 unlike WIN-3: ↓HR, ↑BP and ↑plasma NA | CB1-Rs in the brain stem enhance cardiac vagal tone and sympathetic tone (all effects diminished by RIM) | [211] |
| Sprague Dawley rats | conscious | intracisternal | WIN-2 | 23 and 70 | immediate ↓HR but delayed ↑BP and ↑plasma NA (maximum at 10 min) | ↓HR, ↑BP and ↑plasma NA depend on CB1-Rs (reduced by AM251); ↑BP and ↑plasma NA but not ↓HR reduced by GABAA-R agonist muscimol | [212] |
| Sprague Dawley rats | conscious | RVLM | WIN-2 | 0.1, 0.2, 0.3 | ↑HR, ↑BP | difference in the HR response between Ibrahim and Abdel-Rahman [204] vs. [205] might be caused by the localized effect of WIN-2 within the RVLM compared to a more widespread effect after intracisternal administration | [213] |
| Wistar rats | conscious | RVLM | ACEA AM251 | 0.00005 0.00025 | ↑HR, ↑BP, ↑RSNA ↓HR, ↓BP, ↓RSNA | ↑HR and ↑BP mediated by CB1-Rs (reduced by AM251); CB1-Rs tonically active (AM had effects opposite in direction to those of ACEA) | [94] |
| Wistar rats | conscious | RVLM | AM251 | 0.00025 | ↓HR and ↓BP | CB1-Rs activated tonically by eCBs (cf. study by Wang et al. [94]) | [214] |
| Sprague Dawley rats | conscious | RVLM | abn-CBD NAGly O-1918 | 0.65, 1.3, 2.5 1.4, 2.8, 5.5, 11 0.7, 1.4, 2.8 | ↑HR, ↓BP ↔ HR, small ↓BP ↓HR, ↑BP | GPR18-Rs might mediate tachycardia and hypotension; probably activated by eCBs (O-1918 had effects opposite in direction to those of abn-CBD) | [215] |
| Sprague Dawley rats | urethane | RVLM | WIN-2 HU-210 | 0.00005, 0.0005 or 0.005 0.0005 | both agonists: ↔ HR, ↑ BP and ↑ sSNA | central sympathoexcitation mediated by CB1-Rs (reduced by AM281) | [216] |
| Wistar rats | urethane | RVLM | WIN-2 | 12 | ↔HR; slight ↓BP; ↔plasma NA | not examined | [159] |
| Sprague Dawley rats | pentobarbital | dPAG | AEA | 0.0018 | ↑HR, ↑BP, ↑RSNA; higher baseline HR connected with increased AEA content and decreased FAAH activity | eCBs can lead to sympathoexcitation via modulation of GABAergic inhibition by CB1-Rs at the level of the dPAG (responses reduced by AM281 and the GABAA-R antagonist gabazine) | [217,218,219] |
| Wistar rats | urethane | PVN | MethAEA CP MethAEA +AM251 or CP +AM251 or +AM6545 | 10 or 0.1 doses of agonists as above; antagonist doses (mg/kg): AM251 1.7 i.v. AM6545 8.3 i.p. | ↓HR, ↓BP ↑HR, ↑BP | the centrally induced ↑HR and ↑BP is mediated by CB1-Rs in the PVN (reduced by AM251 given into the PVN) and can be masked by peripheral CB1-Rs; the direction of the response (↑ or ↓ of HR and BP) probably depends on the sympathetic tone | [220,221] |
| Wistar rats | urethane | PVN | CP + AM251 1.7 mg/kg i.v. | 10 | ↑HR, ↑BP | pressor response of CP (after blockade of peripheral CB1-Rs by AM251) mediated via NMDA-, GABAA-, β2-, TP-, AT1-Rs and NO (antagonized by the respective inhibitors given i.v.) | [220,221] |
| Wistar rats | conscious | BNST | AM251 URB597 | 0.001, 0.03, 0.1 0.03 | ↑HR but not ↑BP induced by restraint stress increased by AM251 and decreased by URB597 | CB1-Rs and eCBs play a role in cardiac responses during stress via modulation of NMDA receptors in BNST and GABAA-Rs in the lateral hypothalamus; amplificatory effect of AM251 reduced by the respective antagonists LY235959 and SR95531 | [222,223,224] |
| dog | α-chloralose + urethane | NTS | WIN-2 RIM | 1.25–1.50 pmol 2.5–3.0 pmol | ↔BP, ↔BRS ↔BP, ↔BRS | [225] | |
| Sprague Dawley rats | urethane | NTS | WIN-2 CP AM281 | 10 10 14 | ↔ HR, ↓BP ↔ HR, ↓BP, ↔BRS ↔ HR, ↔ BP, ↔BRS | CB1-Rs in NTS do not modulate HR and baroreflex sensitivity | [226] |
| Sprague Dawley rats | pentobarbital | NTS | AEA AM404 (AEA transport inhibitor) | 0.0025 0.0035 | both drugs: ↔HR; ↔BP ↑BRS | CB1-Rs activated by eCBs in the NTS may have presynaptically attenuated GABA release, leading to enhanced BRS (effects of AEA inhibited by RIM and GABAA-R antagonist bicuculline) | [41,227] |
| Wistar rats | conscious | vMPFC | AM251 | 0.1 | ↔ HR and ↔ BP by itself; ↑BRS | CB1-Rs reduce the cardiac responses of the baroreflex | [228] |
1 CB-R antagonists were mentioned only if their cardiac effects were determined independent of CB-R agonists. Antagonists did not modify cardiac parameters by themselves, unless stated otherwise. ↑increase; ↓decrease; ↔no effect; β2-R, β2-adrenergic receptor; abn-CBD, abnormal cannabidiol; ACEA, arachidonyl-2-chloroethylamide; Adr, adrenaline; AEA, anandamide; AT1-R, angiotensin II receptor type 1; BNST, bed nucleus of stria terminalis; BP, blood pressure; BRS, baroreceptor-reflex sensitivity expressed as the ratio of the HR change over the change in the mean BP; CB1-R, cannabinoid CB1 receptor; CB2-R, cannabinoid CB2 receptor; CP, CP55940; dPAG, dorsal periaqueductal gray; eCBs, endocannabinoids; FAAH, fatty-acid amide hydrolase; GABA, γ-aminobutyric acid; GABAA-R, γ-aminobutyric acid type A receptor; GPR18, G protein-coupled receptor 18; HR, heart rate; i.c.v., intracerebroventricular; i.p., intraperitoneal; i.v., intravenous; MethAEA, methanandamide; NA, noradrenaline; NAGly, N-arachidonoyl glycine; NMDA-R, N-methyl-D-aspartate receptor; NTS, nucleus tractus solitarii; PVN, paraventricular nucleus of hypothalamus; R, receptor; RIM, rimonabant; RSNA, renal sympathetic nerve activity; RVLM, rostral ventrolateral medulla; sSNA, splanchnic nerve activity; THC, Δ9-tetrahydrocannabinol; TP, thromboxane receptor; URB597, inhibitor of fatty-acid amide hydrolase; vMPFC, ventromedial prefrontal cortex, WIN-2, WIN55212-2; WIN-3, WIN55212-3; WKY, Wistar-Kyoto rats.
An entirely different picture emerged when THC or an agonist was injected into brain areas relevant for cardiovascular regulation; all studies of that kind were performed on rats (Table 5; experiments involving the nucleus tractus solitarii (NTS) and the ventral medial prefrontal cortex (vMPFC) will be discussed under Section 4.4). Microinjection of the cannabinoid into the rostral ventrolateral medulla (RVLM) caused tachycardia [94,213], bradycardia [214] or did not affect HR at all [159,216]. In one study, injection of a GPR18 agonist into the RVLM increased HR [215]. HR was increased following microinjection of AEA into the dorsal periaqueductal grey (dPAG; [217,218,219]) but decreased upon microinjection of an inhibitor of AEA degradation into the bed nucleus of the stria terminalis (BNST; [222,223,224]); both effects were mediated via CB1-Rs.
In our own studies on the paraventricular nucleus of the hypothalamus (PVN; [220,221]; Table 5), cannabinoids led to brady- or tachycardia, dependent on the experimental conditions. Microinjection of the cannabinoid CP55940 into this brain region led to bradycardia, which was antagonized by the CB1-R antagonist AM251 given into the PVN. When AM6545, a CB1-R antagonist which does not penetrate the blood–brain barrier, was injected i.v., CP55940 microinjected into the PVN elicited tachycardia instead. One interpretation of these findings might be that CP55940 increases the sympathetic outflow, which is, however, inhibited by cardiac presynaptic inhibitory CB1-Rs, eventually leading to bradycardia. If the “brake” is removed by blockade of these receptors, tachycardia will occur instead. The latter studies are somewhat reminiscent of the paper by Szabo et al. [139] on conscious rabbits, in which WIN55212-2 injected i.v. to conscious rabbits produced bradycardia at lower doses (0.005 and 0.05 mg/kg) but tachycardia at the highest dose (0.5 mg/kg). One might assume that the bradycardia is the result of the central activation of the parasympathetic outflow but is reversed into a tachycardia when the presynaptic CB1-Rs on the vagal nerves are strongly activated.
THC does not only act via the autonomic nervous system but also activates the hypothalamus/pituitary/adrenal cortex (HPA) axis, eventually leading to an increased cortisol secretion [229]. Cortisol in turn sensitizes the adrenoceptors which lead to tachycardia and BP increase. THC acts via different pathways in the brain, namely via noradrenergic neurones projecting from the locus coeruleus and serotoninergic neurones projecting from the raphe nuclei to the PVN. In addition, the PVN, which represents the origin of the HPA axis, is also activated by neurones originating in higher brain regions (reviewed in El Dahan et al. [230]).
In conclusion, there is good evidence that the effect of THC on HR is the result of a combined action between central CB1 receptors in areas of the brain involved in cardiovascular regulation and peripheral presynaptic inhibitory CB1 receptors on sympathetic and/or parasympathetic nerve fibers. It is interesting that even within the same animal species cannabinoids can elicit either brady- or tachycardia. In humans, the interplay between central and peripheral CB1 receptors appears to be such that tachycardia will be the only effect. Tachycardia is further increased by a THC-driven rise in cortisol secretion.
4.4. Baroreceptor Reflex
Administration of conventional (rapid release) formulations of the Ca2+ channel blocker nifedipine led to marked tachycardia sometimes associated with MI [231]; this side effect is related to unloading of the baroreceptor reflex due to an abrupt fall in blood pressure. Table 2 shows that cannabinoids, although they decreased blood pressure in two studies on conscious humans, usually increase blood pressure or leave it unaffected. Moreover, the dose-dependent increases in BP induced by THC are better correlated to changes in HR than to the dose [104].
There are few studies on anaesthetized animals in which the effect of topical administration of cannabinoids into the NTS on HR and on baroreceptor sensitivity was examined (Table 5). HR was not affected in rats [41,226,227]. Baroreceptor sensitivity was not affected in dogs [225] and in rats in the study by Durakoglugil and Orer [226], increased in the studies by Seagard et al. [227] and Brozoski et al. [41] and even decreased in the study by Lagatta et al. [228]. In the latter study, which was performed on conscious rats and in which the CB1-R antagonist AM251 was topically administered to the vMPFC, HR remained constant but baroreceptor sensitivity increased, suggesting that the CB1-Rs in this brain region decrease its sensitivity [228].
In conclusion, it is unlikely that the baroreceptor reflex plays a role in the tachycardia elicited by THC and other cannabinoids.
5. Thrombus Formation and Coronary Constriction?
Since THC can lead to MI associated with tachycardia, the question arises whether THC also influences the two major causes of MI, i.e., thrombus formation and coronary constriction.
Multiple case reports have linked marijuana to thrombus formation, leading to acute MI [13], and the authors quote the publication by Deusch et al. [91] on human platelets in vitro in which CB1- and CB2-Rs are expressed and THC enhances expression of the platelet fibrinogen receptor (glycoprotein IIb-IIIa) and P-selectin; experiments with CB-R antagonists were not performed in that study. In other studies, THC by itself failed to modify the aggregation of human platelets [186] and even decreased aggregation of human and rabbit platelets induced by various factors [182]. Agonists such as ACEA, HU-210 and JWH-015 also failed to modify the aggregation of human [187,188] or rabbit platelets [190]; the same held true for the CB1- and CB2-R antagonists AM251 and AM630 [186,189] and for LPI (endogenous ligand of the GPR55), which, in addition, decreased the ADP-induced platelet aggregation [26]. Only the endocannabinoids 2-AG, AEA and virodhamine clearly activated human platelets and stimulated their aggregation [185,186,187,188]; however, in a manner dependent on thromboxane A2, which cannot be formed from THC and synthetic cannabinoids (for details, see Table 4). In the only in vivo study, WIN55212-2 given i.p. failed to affect thrombus formation in the ear venules of hairless mice whereas AEA reduced it, again by an arachidonic acid-derived agent ([164]; Table 3).
The possibility that THC leads to alterations of the vascular wall and eventually to atherogenesis and atherotic plaque rupture was considered in human cells. CB1-R activation increases the formation of reactive oxygen species (ROS) and accumulation of lipid droplets in macrophages, ROS production and injury of endothelial cells and ROS formation and migration of vascular smooth-muscle cells. By contrast, CB2-Rs usually influence the three cell types in an opposite direction, i.e., they counteract the formation of lipid droplets in macrophages, inhibit the adhesive and infiltrative properties of endothelial cells and inhibit migration of vascular smooth-muscle cells (reviewed in El Dahan et al. [230]).
In conclusion, taking into account the experimental studies discussed above, there is not much evidence to suggest that THC and synthetic cannabinoids (unlike endocannabinoids) can lead to platelet aggregation as a process eventually leading to thrombus formation, but they may promote atherogenesis and atherotic plaque rupture as a prerequisite of thrombus formation. Whether a detrimental effect on the wall of the coronary arteries will really occur in vivo is, however, unclear since CB2-R activation, unlike CB1-R, has effects in the opposite direction.
Coronary flow (CF) is impaired by tachycardia, since blood supply to the coronary arteries can occur only during the diastole of the heart action. Consequently, in the rat perfused spontaneously beating heart, THC caused tachycardia that was accompanied by a decrease in coronary flow [169]. In this section, we will consider the possibility that THC and related compounds have a direct effect on the coronary arteries.
THC reduced CF in the rat perfused heart in which vasopressin (VP) was given to induce coronary tone [175]. By contrast, other cannabinoids enhanced CF both under standard conditions [77,174,176] and under VP ([175]; for details, see Table 4). The increase in CF was mainly connected to changes in ventricular performance measured as a decrease in left ventricular developed pressure (LVDP, which is obtained by subtracting the end-diastolic pressure from the left ventricular systolic pressure; LVSP); this phenomenon might explain the increase in CF [77,174]. However, the increase in CF in coronary arteries preconstricted with VP was associated with an enhanced LVSP [175].
Which receptors are involved in the above effects? Surprisingly, the decrease in CF by THC was not antagonized by CB1- and CB2-R antagonists. On the other hand, CB1-Rs are involved in the enhancement of coronary vasodilation, since CB1-R antagonists such as rimonabant, AM251 and O-2050 but not the CB2-R antagonist SR144528 or the GPR18 antagonist O-1918 showed an antagonistic effect [77,175]. For the ACEA-induced increase in CF, Ford et al. [174] suggest the involvement of a novel site since this effect was reduced by rimonabant, AM281 (CB1-R antagonists) and SR144528 (CB2-R antagonist), but not by AM251 (CB1-R antagonist) and AM630 (CB2-R antagonist).
The results of experiments on isolated rat coronary arteries [90] are consistent with those obtained on isolated hearts. Thus, WIN55212-2 elicited vasodilation and this effect was antagonized by two CB1-R antagonists [90].
In conclusion, a direct dilatory effect of cannabinoids on coronary arteries has been shown in one in vitro model of the rat only. Although most cannabinoids increase CF, THC itself showed an inhibitory effect.
6. Increased Energy Demand and Decreased Energy Supply?
Tachycardia, thrombus formation and/or coronary constriction have been discussed as factors involved in the development of acute MI accompanying the use of THC or related compounds. The question arises whether other factors may contribute. An increase in energy demand and/or a decrease in energy supply may play a role.
An increase in energy demand might be caused by a positive inotropic effect of cannabinoids. Indeed, in isolated rat left atria the CB2-R agonist JWH-015 and AEA, examined in the presence of a CB1-R antagonist, induced a positive inotropic effect which is mediated by CB2-Rs [180]. Moreover, Walsh et al. [87] concluded from their experiments on GPR55-deficient mice that GPR55 increases the adrenoceptor-mediated positive inotropic response.
By contrast, a negative inotropic effect of THC, ∆8-THC and HU-210 was obtained in the perfused rat heart (Table 4; [157,170,173]); in those studies, however, the authors did not use CB-R antagonists to determine the type of receptors involved. In the study by Sterin-Borda et al. [180] in rat left atria, the CB1-R agonist ACEA alone, as well as AEA in the presence of a CB2-R antagonist, had a negative inotropic effect. Likewise, AEA had a negative inotropic effect in human right atrial muscle, which was mimicked by its stable analogue MethAEA and by HU-210 and antagonized by the CB1-R antagonist AM251 [70]. The latter increased contractility of human right atrial muscles by itself [70], suggesting that these receptors are activated by endocannabinoids or are constitutively active. The lack of a positive inotropic effect of AEA in isolated human, as opposed to rat, cardiac tissue [70] may be due to species differences. However, Bonz et al. [70] have not examined the effect of AEA in the presence of a CB1-R antagonist or of a selective CB2-R agonist. An opposite influence of CB1-R and CB2-R activation in the rat heart might be the reason for the lack of changes in contractile function of the isolated rat heart in response to AEA alone [180] and in rat ventricular myocytes in response to the CB1-/CB2-R dual agonist CB13 [18]. Another two studies are difficult to interpret. Thus, in the rat perfused heart, both a CB1- and a CB2-R antagonist showed a negative inotropic effect [172] and in rabbit left ventricular myocytes the CB2-R agonist A-955840 had a CB1-R- and CB2-R-independent negative inotropic effect [38].
In conclusion, there is no evidence that THC and other cannabinoids elicit a positive inotropic effect in the human heart.
A decrease in energy supply might be caused by an impairment of oxygen transport by cannabis use. Since tobacco smoking is associated with an increased carboxyhemoglobin level resulting in decreased cardiac oxygen supply (reviewed in Dorey et al. [232]), the frequent combination of tobacco and cannabis smoking can explain the impairment of oxygen supply in many instances. However, cannabis use per se can also lead to significantly increased expired carbon oxide concentrations, provided that THC was administered by smoking but not when it was vaporized or taken orally ([129]; Table 2). An increase in serum carboxyhemoglobin level was also observed in an animal study, i.e., in mice “smoking” cannabis cigarettes via a special smoke-inhalation system ([148]; Table 3).
Smoking cannabis can decrease the oxygen transport to the heart and, in this respect, changes in mitochondrial oxygen consumption in response to cannabinoids are of interest. However, the results obtained so far are contradictory. Thus, on the one hand, THC or AEA and HU-210 not only at high (100–120 or 1–20 μM) but even at low (0.1 or 0.2 μM) concentrations led to a decrease in oxygen consumption in bovine [193], rat [194] and mouse [191,192] cardiac tissue or mitochondria. The latter was connected with a lower mitochondrial membrane potential and an enhanced mitochondrial hydrogen peroxide production (Table 4). The detailed mechanism(s) of the above changes have so far not been examined. Although CB1Rs were detected in cardiac mitochondria, Mendizabal-Zubiaga et al. [192] excluded CB1-Rs, since similar changes were observed in CB1–/– and CB1+/+ mice. By contrast, a detailed analysis of the toxic effects of a wide range of THC concentrations (1–500 μM) on isolated rat-heart mitochondria failed to detect any changes regarding an enhanced production of reactive oxygen species or lipid-peroxidation products, mitochondrial swelling or changes in mitochondrial membrane potential [195]; the authors even concluded that THC may be helpful in reducing mitochondrial toxicity. Moreover, the dual CB1/CB2 receptor agonist CB13 prevented cardiac mitochondrial dysfunction (such as membrane depolarization and decreased mitochondrial bioenergetics) induced by endothelin-1 in neonatal rat ventricular myocytes [196].
Even if a direct effect of marijuana or cannabimimetics on mitochondrial function is controversial, indirect effects should be considered as well. Thus, all potential mechanisms involved in cardiac injury such as tachycardia, constriction of coronary artery and platelet aggregation, changes in action potential (mainly disturbances in calcium homeostasis), i.e., pathological conditions characterized by the deprivation of oxygen supply to cardiomyocytes, may impact adversely on mitochondrial function [233]. There is increasing evidence for an important role of cardiac (e.g., [234,235,236]) and coronary microvascular [237,238] mitochondria in MI.
In conclusion, independent from tobacco smoking, cannabis smoking can lead to an increase in carboxyhemoglobin and a subsequent reduction in oxygen transport. Although controversial data exist as to whether THC affects mitochondrial respiration directly, an indirect detrimental effect (e.g., due to tachycardia) is very likely.
7. Other Factors
MI can lead to life-threatening arrhythmias, and for this reason possible effects of THC and other cannabinoids on the conduction system of the heart are of great relevance.
In the study by Miller et al. ([125], Table 2), i.v. THC enhanced sinus automaticity and facilitated sinoatrial and atrioventricular nodal conduction in humans in vivo, most probably representing the positive chronotropic and positive dromotropic effect elicited by noradrenaline as a result of sympathetic stimulation; intra-atrial and intraventricular conduction was not affected. It is very plausible that noradrenaline will stimulate the cardiac conductive system, thereby eventually leading to a proarrhythmogenic effect under pathological conditions.
On the other hand, some effects on cardiac ion channels in cardiomyocytes may be beneficial in tachyarrhythmias. As shown in Table 4, cannabinoids might exert an antiarrhythmic effect related to the inhibition of cardiac voltage-gated inward L-type Ca2+ currents; this verapamil-like effect was shown in rat tissue and is related to the activation of CB1-Rs [199]. The suppression of cardiac Na+/Ca2+ exchanger (NCX1)-mediated currents in rat ventricular cardiomyocytes may contribute to an antiarrhythmogenic effect of JWH-133 under ischemic conditions (mediated via CB2-Rs; [201]). Moreover, prolongation of the AP in response to THC was found in sheep Purkinje fibers (receptor not determined; [197]). The synthetic agonist CB13 inhibited the tachypacing-induced shortening of the rat atrial effective refractory period protecting against atrial fibrillation (receptor not identified; [80]).
By contrast, a decrease in AP duration was observed in rabbit sinoatrial-node samples in response to AEA (CB1-Rs involved; [198]) and in rabbit Purkinje fibers in response to a high concentration of JWH-030 (mechanism unclear; [156]). Moreover, the endogenous agonist of GPR55 receptors, l-α-lysophosphatidylinositol (LPI), increased Ca2+ entry via L-type Ca2+ channels in rat cardiomyocytes [86]; the involvement of GPR55 receptors has been proven using an appropriate antagonist (Table 4).
In conclusion, although THC may lead to tachyarrhythmia as a result of a high NA level, evidence for direct anti- and proarrhythmogenic effects is restricted to in vitro studies on preparations from animals.
There may be other (adverse) cardiac effects of THC or synthetic cannabinoids suggested by in vitro experiments (Table 4): (1) THC, at a high concentration of 30 μM, acted cardiotoxically and stopped cardiac activity in the perfused rat heart [169]; (2) an enhanced apoptosis caused by endoplasmic reticulum stress in mouse HL-1 atrial cardiomyocytes occurred after treatment with high THC concentrations of 10 and 30 μM [203]; (3) cell viability in H9c2 cells (rat cardiomyoblast cell line) decreased in response to the synthetic cannabinoids JWH-030, JWH-210, JWH-250 and RCS-4 [156] and (4) the primary metabolites of THC i.e., 11-hydroxy-Δ9-THC (THC-OH) and 11-nor-9-carboxy-Δ⁹-tetrahydrocannabinol (THC-COOH), but not the parent compound THC itself [202].
The mechanism(s) of cardiotoxicity are still not entirely clear. When the study by Nahas and Trouve [169] appeared, CB-Rs had not yet been deciphered. The use of selective CB1- and CB2-R antagonists revealed that the apoptotic effect of THC is neither CB1- nor CB2-R-related [203] whereas CB2-Rs are involved in the effect of JWH-030 on cell viability [156]. Although THC is devoid of an inhibitory effect on cell viability in H9c2 cells, it may have such an effect in vivo. One can expect that, unlike in a cell line, THC is metabolized to THC-OH and THC-COOH. The metabolites, however, do not possess any affinity for CB1- or CB2-Rs.
In conclusion, some interesting cardiotoxic effects of THC and synthetic cannabinoids have been shown in vitro but it is unclear to which extent they play a role in humans in vivo.
8. General Conclusions
Cannabis contains Δ9-tetrahydrocannabinol as its major psychotropic principle and, with respect to vegetative effects, its use for recreational purposes has been considered safe over a long time period. In recent years, however, an increasing number of studies revealed serious cardiovascular effects, even including acute myocardial infarction (MI) in healthy young people; indeed, cannabis has been listed among the risk factors of MI. The potential mechanisms induced by exposure to THC and other cannabimimetics triggering MI are shown in Figure 2. MI related to cannabis use is associated with tachycardia. Tachycardia is the most reliable biomarker of cannabis use and occurs independent of the route of administration. The reason why cannabis elicits tachycardia in humans but almost exclusively bradycardia in animals is unclear but may have to do with the relatively low heart rate level in humans. One explanation for the difference between humans and animals might be that the cannabinoid CB1 receptor-driven central stimulation of the sympathetic system is inhibited markedly by presynaptic inhibitory CB1 receptors on the sympathetic nerve fibers in animals but only slightly in humans. Cannabis use is frequently associated with tobacco smoking, thereby increasing the risk to develop MI. However, it is questionable whether the two most typical pathogenetic factors for the development of MI, i.e., thrombus formation and coronary constriction, play a role in the case of cannabis-related MI, at least not on the basis of the few studies available. Administration of cannabis by smoking but not by other routes impairs energy supply by increasing the formation of carboxyhemoglobin; impairment of mitochondrial respiration is an additional factor. Worsening of MI by an increased energy demand because of a positive inotropic effect is unlikely. Proarrhythmogenic effects of cannabis per se are unlikely but may appear as a consequence of increased noradrenaline levels associated with tachycardia. The increasing use of cannabis preparations for recreational but also for therapeutic purposes warrants each effort to further elucidate cardiovascular mechanisms, in order to avoid severe side effects.
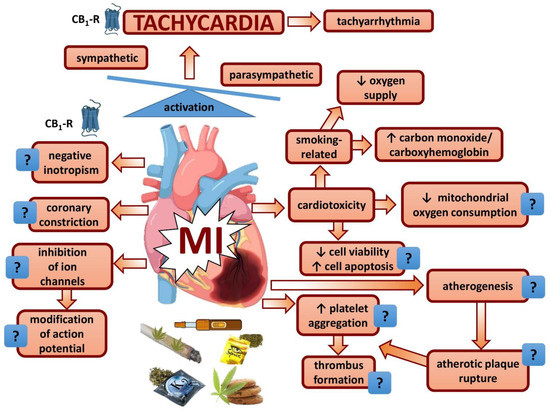
Figure 2.
Effects of cannabinoids on the heart possibly implicated in myocardial infarction. ?, pathophysiological relevance plausible but not supported by appropriate studies; CB1-R, cannabinoid CB1 receptor; MI, myocardial infarction.
Author Contributions
Conceptualization: J.W., B.M. and E.S.; data acquisition: J.W., A.P.-B., K.M., B.M. and E.S.; writing—original draft preparation: J.W., A.P.-B., K.M., B.M. and E.S.; writing—review and editing: J.W., A.P.-B., B.M. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Medical University of Białystok (Poland, grant No SUB/2/DN/22/004/2213 end SUB/2/DN/22/001/2213).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol. Sci. 2006, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Tang, Z.; Wu, X.; Spengler, R.; Jiang, H.; Yang, Y.; Boivin, N. The origins of cannabis smoking: Chemical residue evidence from the first millennium BCE in the Pamirs. Sci. Adv. 2019, 5, eaaw1391. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Hallak, J.E.C.; Crippa, J.A.S. Neuropharmacological effects of the main phytocannabinoids: A narrative review. Adv. Exp. Med. Biol. 2021, 1264, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.F.; Greene, S.J.; Fudim, M.; Dewald, T.; Mentz, R.J. Drugs of abuse and heart failure. J. Card. Fail. 2021, 27, 1260–1275. [Google Scholar] [CrossRef]
- Page, R.L., 2nd; Allen, L.A.; Kloner, R.A.; Carriker, C.R.; Martel, C.; Morris, A.A.; Piano, M.R.; Rana, J.S.; Saucedo, J.F.; American Heart Association Clinical Pharmacology Committee; et al. Medical marijuana, recreational cannabis, and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2020, 142, e131–e152. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Available online: https://go.drugbank.com/ (accessed on 25 January 2022).
- Zawilska, J.B. “Legal Highs”-An Emerging Epidemic of Novel Psychoactive Substances. Int. Rev. Neurobiol. 2015, 120, 273–300. [Google Scholar] [CrossRef]
- Chung, E.Y.; Cha, H.J.; Min, H.K.; Yun, J. Pharmacology and adverse effects of new psychoactive substances: Synthetic cannabinoid receptor agonists. Arch. Pharm. Res. 2021, 44, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Derakhshandeh, R.; Liu, J.; Narayan, S.; Nabavizadeh, P.; Le, S.; Danforth, O.M.; Pinnamaneni, K.; Rodriguez, H.J.; Luu, E.; et al. One Minute of Marijuana Secondhand Smoke Exposure Substantially Impairs Vascular Endothelial Function. J. Am. Heart Assoc. 2016, 5, e003858. [Google Scholar] [CrossRef]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- Drummer, O.H.; Gerostamoulos, D.; Woodford, N.W. Cannabis as a cause of death: A review. Forensic Sci. Int. 2019, 298, 298–306. [Google Scholar] [CrossRef]
- Chetty, K.; Lavoie, A.; Deghani, P. A literature review of cannabis and myocardial infarction-What clinicians may not be aware of. CJC Open 2020, 3, 12–21. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Bajaj, N.S.; Singh, A.; Malloy, R.; Givertz, M.M.; Blankstein, R.; Bhatt, D.L.; Vaduganathan, M. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.W.; Zengin, G.; Wu, M.J.; Lu, J.; Hynd, G.; Bushell, K.; Thompson, A.L.; Bushell, S.; Tartal, C.; Hurst, D.P.; et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: Steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 2005, 13, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Banister, S.D.; Stuart, J.; Kevin, R.C.; Edington, A.; Longworth, M.; Wilkinson, S.M.; Beinat, C.; Buchanan, A.S.; Hibbs, D.E.; Glass, M.; et al. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem. Neurosci. 2015, 6, 1445–1458. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Śniecikowska, J.; Fajkis, N.; Paśko, P.; Franczyk, W.; Kołaczkowski, M. Management of dementia-related psychosis, agitation and aggression: A review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs 2020, 34, 243–268. [Google Scholar] [CrossRef]
- Lu, Y.; Akinwumi, B.C.; Shao, Z.; Anderson, H.D. Ligand activation of cannabinoid receptors attenuates hypertrophy of neonatal rat cardiomyocytes. J. Cardiovasc. Pharmacol. 2014, 64, 420–430. [Google Scholar] [CrossRef]
- Aung, M.M.; Griffin, G.; Huffman, J.W.; Wu, M.; Keel, C.; Yang, B.; Showalter, V.M.; Abood, M.E.; Martin, B.R. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000, 60, 133–140. [Google Scholar] [CrossRef]
- Wiley, J.L.; Marusich, J.A.; Martin, B.R.; Huffman, J.W. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012, 123, 148–153. [Google Scholar] [CrossRef]
- Gu, S.M.; Lee, H.J.; Lee, T.H.; Song, Y.J.; Kim, Y.H.; Han, K.M.; Shin, J.; Park, H.K.; Kim, H.S.; Cha, H.J.; et al. A synthetic cannabinoid JWH-210 reduces lymphoid organ weights and T-cell activator levels in mice via CB2 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1201–1209. [Google Scholar] [CrossRef]
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.Y.; Makriyannis, A.; Piomelli, D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997, 277, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.M.; Dart, M.J.; Tietje, K.R.; Garrison, T.R.; Grayson, G.K.; Daza, A.V.; El-Kouhen, O.F.; Yao, B.B.; Hsieh, G.C.; Pai, M.; et al. Indol-3-ylcycloalkyl ketones: Effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J. Med. Chem. 2010, 53, 295–315. [Google Scholar] [CrossRef]
- Banister, S.D.; Stuart, J.; Conroy, T.; Longworth, M.; Manohar, M.; Beinat, C.; Wilkinson, S.M.; Kevin, R.C.; Hibbs, D.E.; Glass, M.; et al. Structure–activity relationships of synthetic cannabinoid designer drug RCS-4 and its regioisomers and C4 homologues. Forensic Toxicol. 2015, 33, 355–366. [Google Scholar] [CrossRef]
- Banister, S.D.; Wilkinson, S.M.; Longworth, M.; Stuart, J.; Apetz, N.; English, K.; Brooker, L.; Goebel, C.; Hibbs, D.E.; Glass, M. The synthesis and pharmacological evaluation of adamantane-derived indoles: Cannabimimetic drugs of abuse. ACS Chem. Neurosci. 2013, 4, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Brown, A.J.; Andersen, L.; Dorn, G.; Schicho, R.; Waldhoer, M.; Heinemann, A. A selective antagonist reveals a potential role of G protein-coupled receptor 55 in platelet and endothelial cell function. J. Pharmacol. Exp. Ther. 2013, 346, 54–66. [Google Scholar] [CrossRef]
- Heynen-Genel, S.; Dahl, R.; Shi, S.; Milan, L.; Hariharan, S.; Sergienko, E.; Hedrick, M.; Dad, S.; Stonich, D.; Su, Y.; et al. Screening for Selective Ligands for GPR55—Antagonists. In Probe Reports from the NIH Molecular Libraries Program [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010; pp. 1–24. [Google Scholar]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- McIntyre, P.; McLatchie, L.M.; Chambers, A.; Phillips, E.; Clarke, M.; Savidge, J.; Toms, C.; Peacock, M.; Shah, K.; Winter, J.; et al. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br. J. Pharmacol. 2001, 132, 1084–1094. [Google Scholar] [CrossRef]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef]
- Heblinski, M.; Santiago, M.; Fletcher, C.; Stuart, J.; Connor, M.; McGregor, I.S.; Arnold, J.C. Terpenoids commonly found in Cannabis sativa do not modulate the actions of phytocannabinoids or endocannabinoids on TRPA1 and TRPV1 channels. Cannabis Cannabinoid Res. 2020, 5, 305–317. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Chuang, H.; Movahed, P.; Julius, D.; Högestätt, E.D. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000, 396, 39–42. [Google Scholar] [CrossRef]
- Bowles, N.P.; Karatsoreos, I.N.; Li, X.; Vemuri, V.K.; Wood, J.A.; Li, Z.; Tamashiro, K.L.; Schwartz, G.J.; Makriyannis, A.M.; Kunos, G.; et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi-Carmona, M.; Barth, F.; Congy, C.; Martinez, S.; Oustric, D.; Pério, A.; Poncelet, M.; Maruani, J.; Arnone, M.; Finance, O.; et al. SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)1H-pyrazole-3-carb-oxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: Biochemical and pharmacological characterization. J. Pharmacol. Exp. Ther. 2004, 310, 905–914. [Google Scholar] [CrossRef]
- Black, M.D.; Stevens, R.J.; Rogacki, N.; Featherstone, R.E.; Senyah, Y.; Giardino, O.; Borowsky, B.; Stemmelin, J.; Cohen, C.; Pichat, P.; et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: Improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacology 2011, 215, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.Q.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Su, Z.; Preusser, L.; Diaz, G.; Green, J.; Liu, X.; Polakowski, J.; Dart, M. Negative inotropic effect of a CB2 agonist A-955840 in isolated rabbit ventricular myocytes is independent of CB1 and CB2 receptors. Curr. Drug Saf. 2011, 6, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Malinowska, B.; Piszcz, J.; Koneczny, B.; Hryniewicz, A.; Schlicker, E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 233–241. [Google Scholar] [CrossRef]
- Brozoski, D.T.; Dean, C.; Hopp, F.A.; Seagard, J.L. Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res. 2005, 1059, 197–202. [Google Scholar] [CrossRef]
- Legality of Cannabis. Available online: https://en.wikipedia.org/wiki/Legality_of_cannabis (accessed on 25 January 2022).
- Latif, Z.; Garg, N. The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. J. Clin. Med. 2020, 9, 1925. [Google Scholar] [CrossRef] [PubMed]
- Motyka, M.A.; Al-Imam, A. Representations of psychoactive drugs’ use in mass culture and their impact on audiences. Int. J. Environ. Res. Public Health 2021, 18, 6000. [Google Scholar] [CrossRef] [PubMed]
- Borgonhi, E.M.; Volpatto, V.L.; Ornell, F.; Rabelo-da-Ponte, F.D.; Kessler, F.H.P. Multiple clinical risks for cannabis users during the COVID-19 pandemic. Addict. Sci. Clin. Pract. 2021, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E. Health aspects of cannabis. Pharmacol Rev. 1986, 38, 1–20. [Google Scholar] [CrossRef]
- Tait, R.J.; Caldicott, D.; Mountain, D.; Hill, S.L.; Lenton, S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin. Toxicol. 2016, 54, 783–784. [Google Scholar] [CrossRef]
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190. [Google Scholar] [CrossRef]
- Bachs, L.; Mørland, H. Acute cardiovascular fatalities following cannabis use. Forensic Sci. Int. 2001, 124, 200–203. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering myocardial infarction by marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar] [CrossRef]
- Jouanjus, E.; Leymarie, F.; Tubery, M.; Lapeyre-Mestre, M. Cannabis-related hospitalizations: Unexpected serious events identified through hospital databases. Br. J. Clin. Pharmacol. 2011, 71, 758–765. [Google Scholar] [CrossRef]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with Myocardial Infarction. Cardiovasc. Res. 2021, cvab264. [Google Scholar] [CrossRef]
- Chami, T.; Kim, C.H. Cannabis abuse and elevated risk of myocardial infarction in the young: A population-based study. Mayo Clin. Proc. 2019, 94, 1647–1649. [Google Scholar] [CrossRef]
- Jouanjus, E.; Raymond, V.; Lapeyre-Mestre, M.; Wolff, V. What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr. Atheroscler. Rep. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Patel, U.; Sharma, S.; Amin, P.; Bhuva, R.; Patel, M.S.; Sharma, N.; Shah, M.; Patel, S.; Savani, S.; et al. Recreational marijuana use and acute myocardial infarction: Insights from Nationwide Inpatient Sample in the United States. Cureus 2017, 9, e1816. [Google Scholar] [CrossRef]
- Kalla, A.; Krishnamoorthy, P.M.; Gopalakrishnan, A.; Figueredo, V.M. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the National Inpatient Sample. J. Cardiovasc. Med. 2018, 19, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Fong, H.K.; Shah, K.; Kaur, V.P.; Savani, S.; Gangani, K.; Damarlapally, N.; Goyal, H. Rising trends in hospitalizations for cardiovascular events among young cannabis users (18-39 Years) without other substance abuse. Medicina 2019, 55, 438. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.K.; Lodhi, M.U.; Kothapudi, V.N.; Singh, S.; Desai, R. Alarming trends in the frequency of malignant hypertension among admissions with a known cannabis use disorder. Int. J. Cardiol. Heart Vasc. 2021, 33, 100729. [Google Scholar] [CrossRef]
- Zongo, A.; Lee, C.; Dyck, J.R.B.; El-Mourad, J.; Hyshka, E.; Hanlon, J.G.; Eurich, D.T. Medical cannabis authorization and the risk of cardiovascular events: A longitudinal cohort study. BMC Cardiovasc. Disord. 2021, 21, 426. [Google Scholar] [CrossRef]
- Yang, P.K.; Odom, E.C.; Patel, R.; Loustalot, F.; Coleman King, S. Nonmedical marijuana use and cardiovascular events: A systematic review. Public Health Rep. 2022, 137, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Skipina, T.M.; Upadhya, B.; Soliman, E.Z. Cannabis use and electrocardiographic myocardial injury. Am. J. Cardiol. 2021, 151, 100–104. [Google Scholar] [CrossRef]
- Ladha, K.S.; Mistry, N.; Wijeysundera, D.N.; Clarke, H.; Verma, S.; Hare, G.M.T.; Mazer, C.D. Recent cannabis use and myocardial infarction in young adults: A cross-sectional study. CMAJ 2021, 193, E1377–E1384. [Google Scholar] [CrossRef]
- Pertwee, R.G. Endocannabinoids and their pharmacological actions. Handb. Exp. Pharmacol. 2015, 231, 1–37. [Google Scholar] [CrossRef]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E.; Jagerovic, N. Therapeutic exploitation of GPR18: Beyond the cannabinoids? J. Med. Chem. 2020, 63, 14216–14227. [Google Scholar] [CrossRef] [PubMed]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The endocannabinoid system and cannabidiol: Past, present, and prospective for cardiovascular diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Valenta, I.; Varga, Z.V.; Valentine, H.; Cinar, R.; Horti, A.; Mathews, W.B.; Dannals, R.F.; Steele, K.; Kunos, G.; Wahl, R.L.; et al. Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: A translational approach. JACC Cardiovasc. Imaging 2018, 11, 320–332. [Google Scholar] [CrossRef]
- Van Esbroeck, A.; Varga, Z.V.; Di, X.; van Rooden, E.J.; Tóth, V.E.; Onódi, Z.; Kuśmierczyk, M.; Leszek, P.; Ferdinandy, P.; Hankemeier, T.; et al. Activity-based protein profiling of the human failing ischemic heart reveals alterations in hydrolase activities involving the endocannabinoid system. Pharmacol. Res. 2020, 151, 104578. [Google Scholar] [CrossRef]
- Weis, F.; Beiras-Fernandez, A.; Sodian, R.; Kaczmarek, I.; Reichart, B.; Beiras, A.; Schelling, G.; Kreth, S. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. J. Mol. Cell Cardiol. 2010, 48, 1187–1193. [Google Scholar] [CrossRef]
- Bonz, A.; Laser, M.; Küllmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef]
- Cappellano, G.; Uberti, F.; Caimmi, P.P.; Pietronave, S.; Mary, D.A.; Dianzani, C.; Micalizzi, E.; Melensi, M.; Boldorini, R.; Nicosia, G.; et al. Different expression and function of the endocannabinoid system in human epicardial adipose tissue in relation to heart disease. Can. J. Cardiol. 2013, 29, 499–509. [Google Scholar] [CrossRef]
- Currie, S.; Rainbow, R.D.; Ewart, M.A.; Kitson, S.; Pliego, E.H.; Kane, K.A.; McCarron, J.G. IP(3)R-mediated Ca2+ release is modulated by anandamide in isolated cardiac nuclei. J. Mol. Cell Cardiol. 2008, 45, 804–811. [Google Scholar] [CrossRef]
- Wagner, J.A.; Hu, K.; Karcher, J.; Bauersachs, J.; Schäfer, A.; Laser, M.; Han, H.; Ertl, G. CB(1) cannabinoid receptor antagonism promotes remodeling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction. Br. J. Pharmacol. 2003, 138, 1251–1258. [Google Scholar] [CrossRef]
- Sun, H.J.; Lu, Y.; Wang, H.W.; Zhang, H.; Wang, S.R.; Xu, W.Y.; Fu, H.L.; Yao, X.Y.; Yang, F.; Yuan, H.B. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment-induced Cardioprotection against ischemia-reperfusion injury in rats. Oxid. Med. Cell. Longev. 2017, 2017, 2186383. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, M.; Łuczaj, W.; Jarocka-Karpowicz, I.; Ambrożewicz, E.; Toczek, M.; Skrzydlewska, E. The effect of long-term administration of fatty acid amide hydrolase inhibitor URB597 on oxidative metabolism in the heart of rats with primary and secondary hypertension. Molecules 2018, 23, 2350. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Czifra, N.; Harvey-White, J.; Haskó, G.; Zsengeller, Z.; Gerard, N.P.; Liaudet, L.; Kunos, G.; et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. 2007, 50, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Miklós, Z.; Wafa, D.; Nádasy, G.L.; Tóth, Z.E.; Besztercei, B.; Dörnyei, G.; Laska, Z.; Benyó, Z.; Ivanics, T.; Hunyady, L.; et al. Angiotensin II-induced cardiac effects are modulated by endocannabinoid-mediated CB1 receptor activation. Cells 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Pędzińska-Betiuk, A.; Weresa, J.; Toczek, M.; Baranowska-Kuczko, M.; Kasacka, I.; Harasim-Symbor, E.; Malinowska, B. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br. J. Pharmacol. 2017, 174, 2114–2129. [Google Scholar] [CrossRef] [PubMed]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic cannabidiol administration fails to diminish blood pressure in rats with primary and secondary hypertension despite its effects on cardiac and plasma endocannabinoid system, oxidative stress and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef]
- Lee, D.I.; Murninkas, M.; Elyagon, S.; Etzion, Y.; Anderson, H.D. Cannabinoid receptor agonist inhibits atrial electrical remodeling in a tachypaced Ex Vivo rat model. Front. Pharmacol. 2021, 12, 642398. [Google Scholar] [CrossRef]
- Tomlinson, L.; Tirmenstein, M.A.; Janovitz, E.B.; Aranibar, N.; Ott, K.H.; Kozlosky, J.C.; Patrone, L.M.; Achanzar, W.E.; Augustine, K.A.; Brannen, K.C.; et al. Cannabinoid receptor antagonist-induced striated muscle toxicity and ethylmalonic-adipic aciduria in beagle dogs. Toxicol. Sci. 2012, 29, 268–279. [Google Scholar] [CrossRef]
- Wang, P.F.; Jiang, L.S.; Bu, J.; Huang, X.J.; Song, W.; Du, Y.P.; He, B. Cannabinoid-2 receptor activation protects against infarct and ischemia-reperfusion heart injury. J. Cardiovasc. Pharmacol. 2012, 59, 301–307. [Google Scholar] [CrossRef]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.S.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Balenga, N.A.; Kargl, J.; Andradas, C.; Brown, A.J.; Irving, A.; Sanchez, C.; Waldhoer, M. Minireview: Recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol. Endocrinol. 2011, 25, 1835–1848. [Google Scholar] [CrossRef]
- Puhl, S.L.; Hilby, M.; Kohlhaas, M.; Keidel, L.M.; Jansen, Y.; Hristov, M.; Schindler, J.; Maack, C.; Steffens, S. Haematopoietic and cardiac GPR55 synchronize post-myocardial infarction remodelling. Sci. Rep. 2021, 11, 14385. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Deliu, E.; Zhang, X.Q.; Hoffman, N.E.; Carter, R.L.; Grisanti, L.A.; Brailoiu, G.C.; Madesh, M.; Cheung, J.Y.; Force, T.; et al. Differential activation of cultured neonatal cardiomyocytes by plasmalemmal versus intracellular G protein-coupled receptor 55. J. Biol. Chem. 2013, 288, 22481–22492. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hector, E.E.; Andréasson, A.C.; Jönsson-Rylander, A.C.; Wainwright, C.L. GPR55 deletion in mice leads to age-related ventricular dysfunction and impaired adrenoceptor-mediated inotropic responses. PLoS ONE 2014, 9, e108999. [Google Scholar] [CrossRef]
- Sleep, S.L.; Shrestha, N.; Cuffe, J.; Holland, O.J.; Headrick, J.P.; McAinch, A.J.; Hryciw, D.H. The effect of high maternal linoleic acid on endocannabinoid signalling in rodent hearts. J. Dev. Orig. Health Dis. 2020, 11, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Huffman, J.W.; Csiszar, A.; Ungvari, Z.; Mackie, K.; Chatterjee, S.; et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2210–H2218. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Soltész-Katona, E.; Hunyady, L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018, 134, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Deusch, E.; Kress, H.G.; Kraft, B.; Kozek-Langenecker, S.A. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth. Analg. 2004, 99, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Abdel-Rahman, A.A. A pivotal role for enhanced brainstem Orexin receptor 1 signaling in the central cannabinoid receptor 1-mediated pressor response in conscious rats. Brain Res. 2015, 1622, 51–63. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Li, G.Q.; Zhang, H.P.; Zhang, Y.; Li, Q. Overactivation of cannabinoid receptor type 1 in rostral ventrolateral medulla promotes cardiovascular responses in spontaneously hypertensive rats. J. Hypertens. 2017, 35, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Puente, N.; Elezgarai, I.; Lafourcade, M.; Reguero, L.; Marsicano, G.; Georges, F.; Manzoni, O.J.; Grandes, P. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS ONE 2010, 5, e8869. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.D.; Daldrup, T.; Remmers, F.; Szkudlarek, H.J.; Lesting, J.; Guggenhuber, S.; Ruehle, S.; Jüngling, K.; Seidenbecher, T.; Lutz, B.; et al. Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol. Psychiatry 2017, 22, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Park, J.Y.; Oh, J.Y.; Bae, S.J.; Jang, H.; Jeon, S.; Kim, J.; Park, H.J. Novel analgesic effects of melanin-concentrating hormone on persistent neuropathic and inflammatory pain in mice. Sci. Rep. 2018, 8, 707. [Google Scholar] [CrossRef]
- Ruginsk, S.G.; Vechiato, F.M.; Uchoa, E.T.; Elias, L.L.; Antunes-Rodrigues, J. Type 1 cannabinoid receptor modulates water deprivation-induced homeostatic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1358–R1368. [Google Scholar] [CrossRef] [PubMed]
- Mostafeezur, R.M.; Zakir, H.M.; Takatsuji, H.; Yamada, Y.; Yamamura, K.; Kitagawa, J. Cannabinoids facilitate the swallowing reflex elicited by the superior laryngeal nerve stimulation in rats. PLoS ONE 2012, 7, e50703. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Terzian, A.L.; Aguiar, D.C.; Zangrossi, H.; Guimarães, F.S.; Wotjak, C.T.; Moreira, F.A. Opposing roles for cannabinoid receptor type-1 (CB1) and transient receptor potential vanilloid type-1 channel (TRPV1) on the modulation of panic-like responses in rats. Neuropsychopharmacology 2012, 37, 478–486. [Google Scholar] [CrossRef]
- Zuurman, L.; Ippel, A.E.; Moin, E.; van Gerven, J.M. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br. J. Clin. Pharmacol. 2009, 67, 5–21. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Etheridge, S.P.; Marchlinski, F.E.; Spinale, F.G.; Lakkireddy, D.; Olshansky, B. Arrhythmia-induced cardiomyopathies: Mechanisms, recognition, and management. J. Am. Coll. Cardiol. 2015, 66, 1714–1728. [Google Scholar] [CrossRef]
- Palatini, P.; Julius, S. Elevated heart rate: A major risk factor for cardiovascular disease. Clin. Exp. Hypertens. 2004, 26, 637–644. [Google Scholar] [CrossRef]
- Johnson, S.; Domino, E.F. Some cardiovascular effects of marihuana smoking in normal volunteers. Clin. Pharmacol. Ther. 1971, 12, 762–768. [Google Scholar] [CrossRef]
- Crawford, W.J.; Merritt, J.C. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 191–196. [Google Scholar]
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Netw. Open 2018, 1, e184841. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, A.; Vachon, L.; Rich, E.S., Jr. Propranolol effects on acute marihuana intoxication in man. Psychopharmacology 1977, 52, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Beaconsfield, P.; Ginsburg, J.; Rainsbury, R. Marihuana smoking: Cardiovascular effects in man and possible mechanisms. N. Engl. J. Med. 1972, 287, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Boyd, S.J.; Heishman, S.J.; Preston, K.L.; Bonnet, D.; Le Fur, G.; Gorelick, D.A. Single and multipmillmle doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology 2007, 194, 505–515. [Google Scholar] [CrossRef]
- Gash, A.; Karliner, J.S.; Janowsky, D.; Lake, C.R. Effects of smoking marihuana on left ventricular performance and plasma norepinephrine: Studies in normal men. Ann. Intern. Med. 1978, 89, 448–452. [Google Scholar] [CrossRef]
- Bidwell, L.C.; Karoly, H.C.; Torres, M.O.; Master, A.; Bryan, A.D.; Hutchison, K.E. A naturalistic study of orally administered vs. inhaled legal market cannabis: Cannabinoids exposure, intoxication, and impairment. Psychopharmacology 2021, 239, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Brands, B.; Mann, R.E.; Wickens, C.M.; Sproule, B.; Stoduto, G.; Sayer, G.S.; Burston, J.; Pan, J.F.; Matheson, J.; Stefan, C. Acute and residual effects of smoked cannabis: Impact on driving speed and lateral control, heart rate, and self-reported drug effects. Drug Alcohol Depend. 2019, 205, 107641. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Levisman, J.A.; Abbasi, A.S.; Shapiro, B.J.; Ellis, N.M. Short-term effects of smoked marihuana on left ventricular function in man. Chest 1977, 72, 20–26. [Google Scholar] [CrossRef]
- Maida, N.; Papaseit, E.; Martínez, L.; Pérez-Mañá, C.; Poyatos, L.; Pellegrini, M.; Pichini, S.; Pacifici, R.; Ventura, M.; Galindo, L.; et al. Acute pharmacological effects and oral fluid biomarkers of the synthetic cannabinoid UR-144 and THC in recreational users. Biology 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Zuurman, L.; Roy, C.; Schoemaker, R.C.; Hazekamp, A.; den Hartigh, J.; Bender, J.C.; Verpoorte, R.; Pinquier, J.L.; Cohen, A.F.; van Gerven, J.M. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J. Psychopharmacol. 2008, 22, 707–716. [Google Scholar] [CrossRef]
- Zuurman, L.; Roy, C.; Schoemaker, R.C.; Amatsaleh, A.; Guimaeres, L.; Pinquier, J.L.; Cohen, A.F.; van Gerven, J.M. Inhibition of THC-induced effects on the central nervous system and heart rate by a novel CB1 receptor antagonist AVE1625. J. Psychopharmacol. 2010, 24, 363–371. [Google Scholar] [CrossRef]
- Strougo, A.; Zuurman, L.; Roy, C.; Pinquier, J.L.; van Gerven, J.M.; Cohen, A.F.; Schoemaker, R.C. Modelling of the concentration—Effect relationship of THC on central nervous system parameters and heart rate—Insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J. Psychopharmacol. 2008, 22, 717–726. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Roy, C.; Ferron, G.; Turpault, S.; Poitiers, F.; Pinquier, J.L.; van Hasselt, J.G.; Zuurman, L.; Erwich, F.A.; van Gerven, J.M. Surinabant, a selective cannabinoid receptor type 1 antagonist, inhibits Δ9-tetrahydrocannabinol-induced central nervous system and heart rate effects in humans. Br. J. Clin. Pharmacol. 2013, 76, 65–77. [Google Scholar] [CrossRef]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farré, M.; et al. Acute effects of a single, oral dose of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef]
- Pabon, E.; Rockwood, F.; Norman, G.J.; de Wit, H. Acute effects of oral delta-9-tetrahydrocannabinol (THC) on autonomic cardiac activity and their relation to subjective and anxiogenic effects. Psychophysiology 2022, 59, e13955. [Google Scholar] [CrossRef]
- Bedi, G.; Cooper, Z.D.; Haney, M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict. Biol. 2013, 18, 872–881. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Rosenberg, J.; Rogers, W.; Bachman, J.; Jones, R.T. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: Autonomic nervous mechanisms. Clin. Pharmacol. Ther. 1979, 25, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C., Jr.; Pouget, J.M.; Rosen, K.M. The effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac performance with and without beta blockade. Circulation 1976, 53, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.; Pouget, M.; Rosen, K.M. Lack of cardiovascular effects of delta-9-tetrahydrocannabinol in chemically denervated men. Ann. Intern. Med. 1979, 91, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Dhingra, R.C.; Kanakis, C., Jr.; Amat-y-Leon, F.; Rosen, K.M. The electrophysiological effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac conduction in man. Am. Heart J. 1977, 94, 740–747. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Timmons, M.C.; Davis, K.H.; Wall, E.M. A comparison of the pharmacological activity in man of intravenously administered delta9-tetrahydrocannabinol, cannabinol, and cannabidiol. Experientia 1973, 29, 1368–1369. [Google Scholar] [CrossRef] [PubMed]
- Karschner, E.L.; Darwin, W.D.; McMahon, R.P.; Liu, F.; Wright, S.; Goodwin, R.S.; Huestis, M.A. Subjective and physiological effects after controlled Sativex and oral THC administration. Clin. Pharmacol. Ther. 2011, 89, 400–407. [Google Scholar] [CrossRef]
- Martínez, L.; La Maida, N.; Papaseit, E.; Pérez-Mañá, C.; Poyatos, L.; Pellegrini, M.; Pichini, S.; Ventura, M.; Galindo, L.; Busardò, F.P.; et al. Acute pharmacological effects and oral fluid concentrations of the synthetic cannabinoids JWH-122 and JWH-210 in humans after self-administration: An observational study. Front. Pharmacol. 2021, 12, 705643. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Swortwood, M.J.; Abulseoud, O.A.; Huestis, M.A. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 2017, 175, 67–76. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Beumer, T.L.; van Hasselt, J.G.; Lipplaa, A.; Karger, L.B.; Kleinloog, H.D.; Freijer, J.I.; de Kam, M.L.; van Gerven, J.M. Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects. Br. J. Clin. Pharmacol. 2012, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; van den Elsen, G.A.; Colbers, A.; van der Marck, M.A.; Burger, D.M.; Feuth, T.B.; Rikkert, M.; Kramers, C. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: A randomized controlled trial. Eur. Neuropsychopharmacol. 2014, 24, 1475–1482. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabinoids in the management of difficult to treat pain. Ther. Clin. Risk Manag. 2008, 4, 245–259. [Google Scholar] [CrossRef]
- Fredericks, A.B.; Benowitz, N.L.; Savanapridi, C.Y. The cardiovascular and autonomic effects of repeated administration of delta-9-tetrahydrocannabinol to rhesus monkeys. J. Pharmacol. Exp. Ther. 1981, 216, 247–253. [Google Scholar]
- Matsuzaki, M.; Casella, G.A.; Ratner, M. Δ9-Tetrahydrocannabinol: EEG changes, bradycardia and hypothermia in the rhesus monkey. Brain Res. Bull. 1987, 19, 223–229. [Google Scholar] [CrossRef]
- Vivian, J.A.; Kishioka, S.; Butelman, E.R.; Broadbear, J.; Lee, K.O.; Woods, J.H. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: Antagonist effects of SR141716A. J. Pharmacol. Exp. Ther. 1998, 286, 697–703. [Google Scholar] [PubMed]
- Stewart, J.L.; McMahon, L.R. Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: Discriminative stimulus effects and other withdrawal signs. J. Pharmacol. Exp. Ther. 2010, 334, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, B.S.; Hamed, A.T. Pulmonary and systemic hemodynamic effects of delta9-tetrahydrocannabinol in conscious and morphine-chloralose-anesthetized dogs: Anesthetic influence on drug action. Eur. J. Pharmacol. 1978, 53, 63–68. [Google Scholar] [CrossRef]
- Fernández-Trapero, M.; Pérez-Díaz, C.; Espejo-Porras, F.; de Lago, E.; Fernández-Ruiz, J. Pharmacokinetics of Sativex® in dogs: Towards a potential cannabinoid-based therapy for canine disorders. Biomolecules 2020, 10, 279. [Google Scholar] [CrossRef]
- Szabo, B.; Nordheim, U.; Niederhoffer, N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J. Pharmacol. Exp. Ther. 2001, 297, 819–826. [Google Scholar]
- Niederhoffer, N.; Szabo, B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999, 126, 457–466. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Szabo, B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000, 294, 707–713. [Google Scholar]
- Kawasaki, H.; Watanabe, S.; Oishi, R.; Ueki, S. Effects of delta-9-tetrahydrocannabinol on the cardiovascular system, and pressor and behavioral responses to brain stimulation in rats. Jpn. J. Pharmacol. 1980, 30, 493–502. [Google Scholar] [CrossRef]
- Gardiner, S.M.; March, J.E.; Kemp, P.A.; Bennett, T. Influence of the CB(1) receptor antagonist, AM 251, on the regional haemodynamic effects of WIN-55212-2 or HU 210 in conscious rats. Br. J. Pharmacol. 2002, 136, 581–587. [Google Scholar] [CrossRef][Green Version]
- Schindler, C.W.; Gramling, B.R.; Justinova, Z.; Thorndike, E.B.; Baumann, M.H. Synthetic cannabinoids found in “spice” products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depend. 2017, 179, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Szopa, A.; Serefko, A.; Poleszak, E. A Novel alternative in the treatment of detrusor overactivity? In vivo activity of O-1602, the newly synthesized agonist of GPR55 and GPR18 cannabinoid receptors. Molecules 2020, 25, 1384. [Google Scholar] [CrossRef] [PubMed]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F.; Böhme, G.A.; Imperato, A.; Pedrazzini, T.; Roques, B.P.; et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Berteotti, C.; Bastianini, S.; Cohen, G.; Lo Martire, V.; Mazza, R.; Pagotto, U.; Quarta, C.; Zoccoli, G. Cardiorespiratory anomalies in mice lacking CB1 cannabinoid receptors. PLoS ONE 2014, 9, e100536. [Google Scholar] [CrossRef]
- Fantauzzi, M.F.; Cass, S.P.; McGrath, J.J.C.; Thayaparan, D.; Wang, P.; Stampfli, M.R.; Hirota, J.A. Development and validation of a mouse model of contemporary cannabis smoke exposure. ERJ Open Res. 2021, 7, 00107–02021. [Google Scholar] [CrossRef] [PubMed]
- Cavero, I.; Buckley, J.P.; Jandhyala, B.S. Hemodynamic and myocardial effects of (-)-delta9-trans-tetrahydrocannabinol in anesthetized dogs. Eur. J. Pharmacol. 1973, 24, 243–251. [Google Scholar] [CrossRef]
- Cavero, I.; Lokhandwala, M.F.; Buckley, J.P.; Jandhyala, B.S. The effect of (minus)-delta 9-trans-tetrahydrocannibinol on myocardial contractility and venous return in anesthetized dogs. Eur. J. Pharmacol. 1974, 29, 74–82. [Google Scholar] [CrossRef]
- Vollmer, R.R.; Cavero, I.; Ertel, R.J.; Solomon, T.A.; Buckley, J.P. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (-)-delta 9-trans-tetrahydrocannabinol. J. Pharm. Pharmacol. 1974, 26, 186–192. [Google Scholar] [CrossRef]
- Graham, J.D.; Li, D.M. Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol. 1973, 49, 1–10. [Google Scholar] [CrossRef]
- Waldman, M.; Hochhauser, E.; Fishbein, M.; Aravot, D.; Shainberg, A.; Sarne, Y. An ultra-low dose of tetrahydrocannabinol provides cardioprotection. Biochem. Pharmacol. 2013, 85, 1626–1633. [Google Scholar] [CrossRef]
- Bátkai, S.; Pacher, P.; Járai, Z.; Wagner, J.A.; Kunos, G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H595–H600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lake, K.D.; Compton, D.R.; Varga, K.; Martin, B.R.; Kunos, G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997, 281, 1030–1037. [Google Scholar] [PubMed]
- Yun, J.; Yoon, K.S.; Lee, T.H.; Lee, H.; Gu, S.M.; Song, Y.J.; Cha, H.J.; Han, K.M.; Seo, H.; Shin, J.; et al. Synthetic cannabinoid, JWH-030, induces QT prolongation through hERG channel inhibition. Toxicol. Res. 2016, 5, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Ermakov, S.; Lasukova, O.V.; Barzakh, E.I.; Crawford, D.; Pertwee, R.G. Significance of cardiac cannabinoid receptors in regulation of cardiac rhythm, myocardial contractility, and electrophysiologic processes in heart. Izv. Akad. Nauk Ser. Biol. 2007, 34, 28–35. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004, 558, 647–657. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Schmid, K.; Szabo, B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 434–443. [Google Scholar] [CrossRef]
- Baranowska, U.; Göthert, M.; Rudz, R.; Malinowska, B. Methanandamide allosterically inhibits in vivo the function of peripheral nicotinic acetylcholine receptors containing the alpha 7-subunit. J. Pharmacol. Exp. Ther. 2008, 326, 912–919. [Google Scholar] [CrossRef]
- Kurz, C.; Baranowska, U.; Lupiński, S.; Göthert, M.; Malinowska, B.; Schlicker, E. Urethane, but not pentobarbitone, attenuates presynaptic receptor function in rats: A contribution to the choice of anaesthetic. Br. J. Pharmacol. 2009, 157, 1474–1482. [Google Scholar] [CrossRef]
- Zakrzeska, A.; Schlicker, E.; Baranowska, M.; Kozłowska, H.; Kwolek, G.; Malinowska, B. A cannabinoid receptor, sensitive to O-1918, is involved in the delayed hypotension induced by anandamide in anaesthetized rats. Br. J. Pharmacol. 2010, 160, 574–584. [Google Scholar] [CrossRef]
- Li, D.M. The lack of β-adrenoceptor involvement in the cardiac action of Δ1-tetrahydrocannabinol in rats. Clin. Exp. Pharmacol. Physiol. 1980, 7, 23–29. [Google Scholar] [CrossRef]
- Grambow, E.; Strüder, D.; Klar, E.; Hinz, B.; Vollmar, B. Differential effects of endogenous, phyto and synthetic cannabinoids on thrombogenesis and platelet activity. Biofactors 2016, 42, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, T.E.; Notenboom, R.G.E.; Wink, J.; Kiès, P.; Vliegen, H.W.; Egorova, A.D.; Schalij, M.J.; De Ruiter, M.C.; Jongbloed, M.R.M. Asymmetry and heterogeneity: Part and parcel in cardiac autonomic innervation and function. Front. Physiol. 2021, 12, 665298. [Google Scholar] [CrossRef]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef] [PubMed]
- Maggo, S.; Ashton, J.C. Effect of cannabinoid receptor agonists on isolated rat atria. J. Cardiovasc. Pharmacol. 2018, 72, 191–194. [Google Scholar] [CrossRef]
- Molderings, G.J.; Likungu, J.; Göthert, M. Presynaptic cannabinoid and imidazoline receptors in the human heart and their potential relationship. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 360, 157–164. [Google Scholar] [CrossRef]
- Nahas, G.; Trouve, R. Effects and interactions of natural cannabinoids on the isolated heart. Proc. Soc. Exp. Biol. Med. 1985, 180, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Smiley, K.A.; Karler, R.; Turkanis, S.A. Effects of cannabinoids on the perfused rat heart. Res. Commun. Chem. Pathol. Pharmacol. 1976, 14, 659–675. [Google Scholar] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Ermakov, S.Y.; Barzakh, E.I.; Lasukova, O.V.; Crawford, D.; Ghadessy, R.; Serebrov, V.Y. Negative chronotropic effect of cannabinoids and their water-soluble emulsion is related to activation of cardiac CB1 receptors. Bull. Exp. Biol. Med. 2006, 142, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Lasukova, O.V.; Pertwee, R.G. Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull. Exp. Biol. Med. 2005, 139, 558–561. [Google Scholar] [CrossRef]
- Maslov, L.N.; Lasukova, O.V.; Krylatov, A.V.; Uzhachenko, R.V.; Pertwee, R. Selective cannabinoid receptor agonist HU-210 decreases pump function of isolated perfused heart: Role of cAMP and cGMP. Bull. Exp. Biol. Med. 2004, 138, 550–553. [Google Scholar] [CrossRef]
- Ford, W.R.; Honan, S.A.; White, R.; Hiley, C.R. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br. J. Pharmacol. 2002, 135, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Abesser, M.; Karcher, J.; Laser, M.; Kunos, G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J. Cardiovasc. Pharmacol. 2005, 46, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, C.; Juárez-Oropeza, M.A.; Mascher, D.; Pavón, N.; Regla, I.; Paredes-Carbajal, M.C. Effects of oleamide on the vasomotor responses in the rat. Cannabis Cannabinoid Res. 2020, 5, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.M.; Gottschalk, C.; Schlicker, E.; Kathmann, M. Identification of a presynaptic cannabinoid CB1 receptor in the guinea-pig atrium and sequencing of the guinea-pig CB1 receptor. J. Physiol. Pharmacol. 2008, 59, 3–15. [Google Scholar]
- Ishac, E.J.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef]
- Weresa, J.; Pędzińska-Betiuk, A.; Kossakowski, R.; Malinowska, B. Cannabinoid CB1 and CB2 receptors antagonists AM251 and AM630 differentially modulate the chronotropic and inotropic effects of isoprenaline in isolated rat atria. Pharmacol. Rep. 2019, 71, 82–89. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Del Zar, C.F.; Borda, E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: Endogenous signal transduction pathways. Biochem. Pharmacol. 2005, 69, 1705–1713. [Google Scholar] [CrossRef]
- Li, D.M.; Ng, C.K. Effects of delta 1- and delta 6-tetrahydrocannabinol on the adenylate cyclase activity in ventricular tissue of the rat heart. Clin. Exp. Pharmacol. Physiol. 1984, 11, 81–85. [Google Scholar] [CrossRef]
- Formukong, E.A.; Evans, A.T.; Evans, F.J. The inhibitory effects of cannabinoids, the active constituents of Cannabis sativa L. on human and rabbit platelet aggregation. J. Pharm. Pharmacol. 1989, 41, 705–709. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Menichelli, A.; Del Principe, D.; Agrò, A.F. Anandamide activates human platelets through a pathway independent of the arachidonate cascade. FEBS Lett. 1999, 447, 277–282. [Google Scholar] [CrossRef]
- De Angelis, V.; Koekman, A.C.; Weeterings, C.; Roest, M.; de Groot, P.G.; Herczenik, E.; Maas, C. Endocannabinoids control platelet activation and limit aggregate formation under flow. PLoS ONE 2014, 9, e108282. [Google Scholar] [CrossRef] [PubMed]
- Signorello, M.G.; Giacobbe, E.; Leoncini, G. Activation by 2-arachidonoylglycerol of platelet p38MAPK/cPLA2 pathway. J. Cell Biochem. 2011, 112, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Keown, O.P.; Winterburn, T.J.; Wainwright, C.L.; Macrury, S.M.; Neilson, I.; Barrett, F.; Leslie, S.J.; Megson, I.L. 2-arachidonyl glycerol activates platelets via conversion to arachidonic acid and not by direct activation of cannabinoid receptors. Br. J. Clin. Pharmacol. 2010, 70, 180–188. [Google Scholar] [CrossRef]
- Baldassarri, S.; Bertoni, A.; Bagarotti, A.; Sarasso, C.; Zanfa, M.; Catani, M.V.; Avigliano, L.; Maccarrone, M.; Torti, M.; Sinigaglia, F. The endocannabinoid 2-arachidonoylglycerol activates human platelets through non-CB1/CB2 receptors. J. Thromb. Haemost. 2008, 6, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S.A.; Khandoga, A.L.; Siess, W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets 2014, 25, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, S.; Geraghty, D.; Ahuja, K.; Adams, M. Inhibition of platelet aggregation by vanilloid-like agents is not mediated by transient receptor potential vanilloid-1 channels or cannabinoid receptors. Clin. Exp. Pharmacol. Physiol. 2016, 43, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Braud, S.; Bon, C.; Touqui, L.; Mounier, C. Activation of rabbit blood platelets by anandamide through its cleavage into arachidonic acid. FEBS Lett. 2000, 471, 12–16. [Google Scholar] [CrossRef]
- Chiu, P.; Karler, R.; Craven, C.; Olsen, D.M.; Turkanis, S.A. The influence of delta9-tetrahydrocannabinol, cannabinol and cannabidiol on tissue oxygen consumption. Res. Commun. Chem. Pathol. Pharmacol. 1975, 12, 267–286. [Google Scholar]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; Rodríguez De Fonseca, F.; et al. Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef]
- Whyte, D.A.; Al-Hammadi, S.; Balhaj, G.; Brown, O.M.; Penefsky, H.S.; Souid, A.K. Cannabinoids inhibit cellular respiration of human oral cancer cells. Pharmacology 2010, 85, 328–335. [Google Scholar] [CrossRef]
- Athanasiou, A.; Clarke, A.B.; Turner, A.E.; Kumaran, N.M.; Vakilpour, S.; Smith, P.A.; Bagiokou, D.; Bradshaw, T.D.; Westwell, A.D.; Fang, L.; et al. Cannabinoid receptor agonists are mitochondrial inhibitors: A unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 2007, 364, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Niknejad, M.; Minouei., M.; Mojarad Aylar, E. Analysis of toxicity effects of delta-9-tetrahydrocannabinol on isolated rat heart mitochondria. Toxicol. Mech. Methods 2022, 32, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lee, D.I.; Roy Chowdhury, S.; Lu, P.; Kamboj, A.; Anderson, C.M.; Fernyhough, P.; Anderson, H.D. Activation of cannabinoid receptors attenuates endothelin-1-induced mitochondrial dysfunction in rat ventricular myocytes. J. Cardiovasc. Pharmacol. 2020, 75, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.D.; Turner, S.R.; Cooper, G.J.; Tattersall, J.E. Effects of seven drugs of abuse on action potential repolarisation in sheep cardiac Purkinje fibres. Eur. J. Pharmacol. 2005, 511, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.Y.; Zhou, J.J.; Wei, Y.; Li, Q.; Yang, J.; Zhang, Y. Inhibitory effects of endocannabinoid on the action potential of pacemaker cells in sinoatrial nodes of rabbits. Sheng Li Xue Bao 2013, 651, 29–34. [Google Scholar]
- Li, Q.; Ma, H.J.; Zhang, H.; Qi, Z.; Guan, Y.; Zhang, Y. Electrophysiological effects of anandamide on rat myocardium. Br. J. Pharmacol. 2009, 158, 2022–2029. [Google Scholar] [CrossRef]
- Li, Q.; Ma, H.J.; Song, S.L.; Shi, M.; Ma, H.; Li, D.P.; Zhang, Y. Effects of anandamide on potassium channels in rat ventricular myocytes: A suppression of I(to) and augmentation of K(ATP) channels. Am. J. Physiol. Cell Physiol. 2012, 302, C924–C930. [Google Scholar] [CrossRef]
- Li, Q.; Cui, N.; Du, Y.; Ma, H.; Zhang, Y. Anandamide reduces intracellular Ca2+ concentration through suppression of Na+/Ca2+ exchanger current in rat cardiac myocytes. PLoS ONE 2013, 8, e63386. [Google Scholar] [CrossRef]
- Merve, A.O.; Sobiecka, P.; Remeškevičius, V.; Taylor, L.; Saskoy, L.; Lawton, S.; Jones, B.P.; Elwakeel, A.; Mackenzie, F.E.; Polycarpou, E.; et al. Metabolites of cannabis induce cardiac toxicity and morphological alterations in cardiac myocytes. Int. J. Mol. Sci. 2022, 23, 1401. [Google Scholar] [CrossRef]
- Murata, T.; Noritake, K.; Aki, T.; Uemura, K. Possible roles of AMPK and macropinocytosis in the defense responses against Δ9-THC toxicity on HL-1 cardiomyocytes. Toxicol. Rep. 2021, 8, 980–987. [Google Scholar] [CrossRef]
- Perper, J.A.; Van Thiel, D.H. Cardiovascular complications of cocaine abuse. Recent Dev. Alcohol. 1992, 10, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Isoardi, K.Z.; Ayles, S.F.; Harris, K.; Finch, C.J.; Page, C.B. Methamphetamine presentations to an emergency department: Management and complications. Emerg. Med. Australas. 2019, 31, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.P.; Snyder, S.H.; Mechoulam, R. Cannabinoids: Influence on neurotransmitter uptake in rat brain synaptosomes. J. Pharmacol. Exp. Ther. 1975, 194, 74–81. [Google Scholar]
- Ilayan, E.; Feliszek, M.; Malinowska, B.; Schlicker, E. Do cannabinoids exhibit a tyramine-like effect? Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Bönisch, H.; Fink, K.B.; Malinowska, B.; Molderings, G.J.; Schlicker, E. Serotonin and beyond—A tribute to Manfred Göthert (1939-2019). Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1829–1867. [Google Scholar] [CrossRef] [PubMed]
- Clyburn, C.; Sepe, J.J.; Habecker, B.A. What gets on the nerves of cardiac patients? Pathophysiological changes in cardiac innervation. J. Physiol. 2022, 600, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamamoto, M.; Zou, S.; Shimizu, S.; Higashi, Y.; Saito, M. Stimulation of brain cannabinoid CB1 receptors can ameliorate hypertension in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1254–1262. [Google Scholar] [CrossRef]
- Pfitzer, T.; Niederhoffer, N.; Szabo, B. Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. Br. J. Pharmacol. 2004, 142, 943–952. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res. 2011, 1414, 1–9. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J. Pharmacol. Exp. Ther. 2012, 341, 579–586. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, Z.; Zhao, T.; Dai, S.X.; Liu, J.M.; Jia, B.Z.; Zhang, Y.; Li, Q. Role of cannabinoid receptor type 1 in rostral ventrolateral medulla in high-fat diet-induced hypertension in rats. J. Hypertens. 2018, 36, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Penumarti, A.; Abdel-Rahman, A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014, 349, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Padley, J.R.; Li, Q.; Pilowsky, P.M.; Goodchild, A.K. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol. 2003, 140, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Dean, C. Cannabinoid and GABA modulation of sympathetic nerve activity and blood pressure in the dorsal periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1765–R1772. [Google Scholar] [CrossRef]
- Dean, C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R771–R779. [Google Scholar] [CrossRef]
- Dean, C.; Hillard, C.J.; Seagard, J.L.; Hopp, F.A.; Hogan, Q.H. Components of the cannabinoid system in the dorsal periaqueductal gray are related to resting heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R254–R262. [Google Scholar] [CrossRef]
- Grzęda, E.; Schlicker, E.; Luczaj, W.; Harasim, E.; Baranowska-Kuczko, M.; Malinowska, B. Bi-directional CB1 receptor-mediated cardiovascular effects of cannabinoids in anaesthetized rats: Role of the paraventricular nucleus. J. Physiol. Pharmacol. 2015, 66, 343–353. [Google Scholar]
- Grzęda, E.; Schlicker, E.; Toczek, M.; Zalewska, I.; Baranowska-Kuczko, M.; Malinowska, B. CB1 receptor activation in the rat paraventricular nucleus induces bi-directional cardiovascular effects via modification of glutamatergic and GABAergic neurotransmission. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 25–35. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Oliveira, L.A.; Benini, R.; Rodella, P.; Costa-Ferreira, W.; Crestani, C.C. Involvement of endocannabinoid neurotransmission in the bed nucleus of stria terminalis in cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2016, 173, 2833–2844. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Costa-Ferreira, W.; Oliveira, L.A.; Benini, R.; Crestani, C.C. Cannabinoid receptor type 1 in the bed nucleus of the stria terminalis modulates cardiovascular responses to stress via local N-methyl-D-aspartate receptor/neuronal nitric oxide synthase/soluble guanylate cyclase/protein kinase G signaling. J. Psychopharmacol. 2020, 34, 429–440. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Costa-Ferreira, W.; Mendonça, M.M.; Xavier, C.H.; Crestani, C.C. Lateral hypothalamus involvement in control of stress response by bed nucleus of the stria terminalis endocannabinoid neurotransmission in male rats. Sci. Rep. 2021, 11, 16133. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J.; Patel, S.; Hopp, F.A.; Dean, C.; Hillard, C.J.; Seagard, J.L. Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1570–H1576. [Google Scholar] [CrossRef] [PubMed]
- Durakoglugil, M.S.; Orer, H.S. Cannabinoid receptor activation in the nucleus tractus solitaries produces baroreflex-like responses in the rat. Int. J. Biomed. Sci. 2008, 4, 229–237. [Google Scholar] [PubMed]
- Seagard, J.L.; Dean, C.; Patel, S.; Rademacher, D.J.; Hopp, F.A.; Schmeling, W.T.; Hillard, C.J. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H992–H1000. [Google Scholar] [CrossRef]
- Lagatta, D.C.; Kuntze, L.B.; Ferreira-Junior, N.C.; Resstel, L.B.M. Medial prefrontal cortex TRPV1 and CB1 receptors modulate cardiac baroreflex activity by regulating the NMDA receptor/nitric oxide pathway. Pflugers Arch. 2018, 470, 1521–1542. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Ranganathan, M.; Braley, G.; Gueorguieva, R.; Zimolo, Z.; Cooper, T.; Perry, E.; Krystal, J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 2008, 33, 2505–2516. [Google Scholar] [CrossRef]
- El-Dahan, K.S.; Machtoub, D.; Massoud, G.; Nasser, S.A.; Hamam, B.; Kobeissy, F.; Zouein, F.A.; Eid, A.H. Cannabinoids and myocardial ischemia: Novel insights, updated mechanisms, and implications for myocardial infarction. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Furberg, C.D.; Psaty, B.M.; Meyer, J.V. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation 1995, 92, 1326–1331. [Google Scholar] [CrossRef]
- Dorey, A.; Scheerlinck, P.; Nguyen, H.; Albertson, T. Acute and chronic carbon monoxide toxicity from tobacco smoking. Mil. Med. 2020, 185, 61–67. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, J.; Toan, S.; Mui, D. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res. Rev. 2021, 66, 101250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm. Sin. B 2020, 10, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tan, Y.; Du, W.; Li, Y.; Toan, S.; Mui, D.; Tian, F.; Zhou, H. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021, 38, 101777. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Mui, D.; Toan, S.; Zhu, P.; Li, R.; Zhou, H. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol. Ther. Nucleic Acids 2020, 22, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Lochner, A.; Wang, H.H.; Wang, S.; Zhu, H.; Ren, J.; Zhou, H. Coronary microvascular injury in myocardial infarction: Perception and knowledge for mitochondrial quality control. Theranostics 2021, 11, 6766–6785. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).