Lipopolysaccharide-Induced Strain-Specific Differences in Neuroinflammation and MHC-I Pathway Regulation in the Brains of Bl6 and 129Sv Mice
Abstract
:1. Introduction
2. Materials and Methods
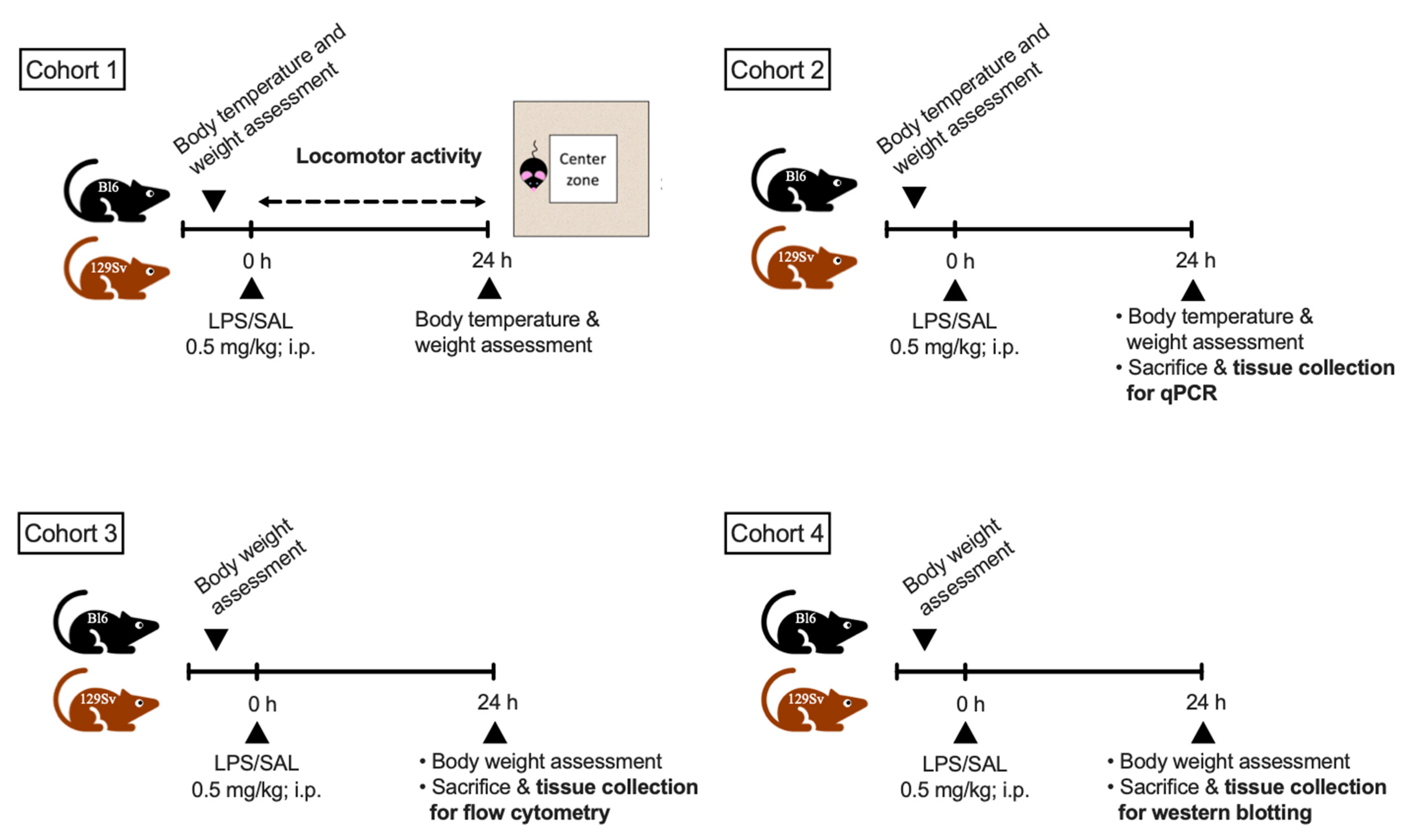
2.1. Animals and Their Distribution into Experimental Groups
2.2. LPS Treatment
2.3. Bodyweight and Temperature Determination
2.4. Locomotor Activity
2.5. Total RNA Isolation cDNA Synthesis and Real-Time qPCR
2.6. Flow Cytometry
2.7. Protein Extraction and Western Blotting
2.8. Statistical Analysis
2.9. Ethics
3. Results
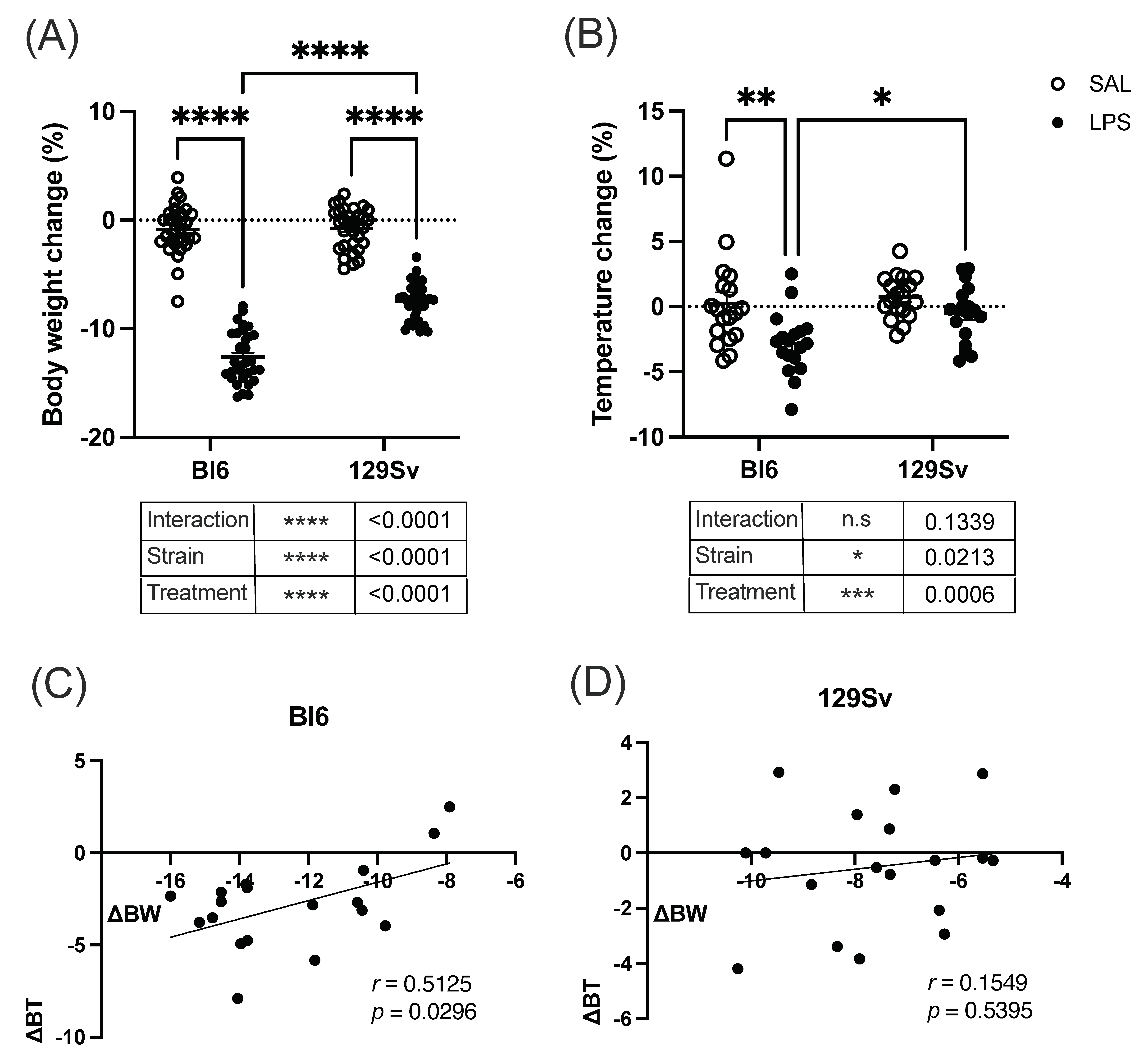
3.1. LPS-Implicated Body Temperature and Weight Changes
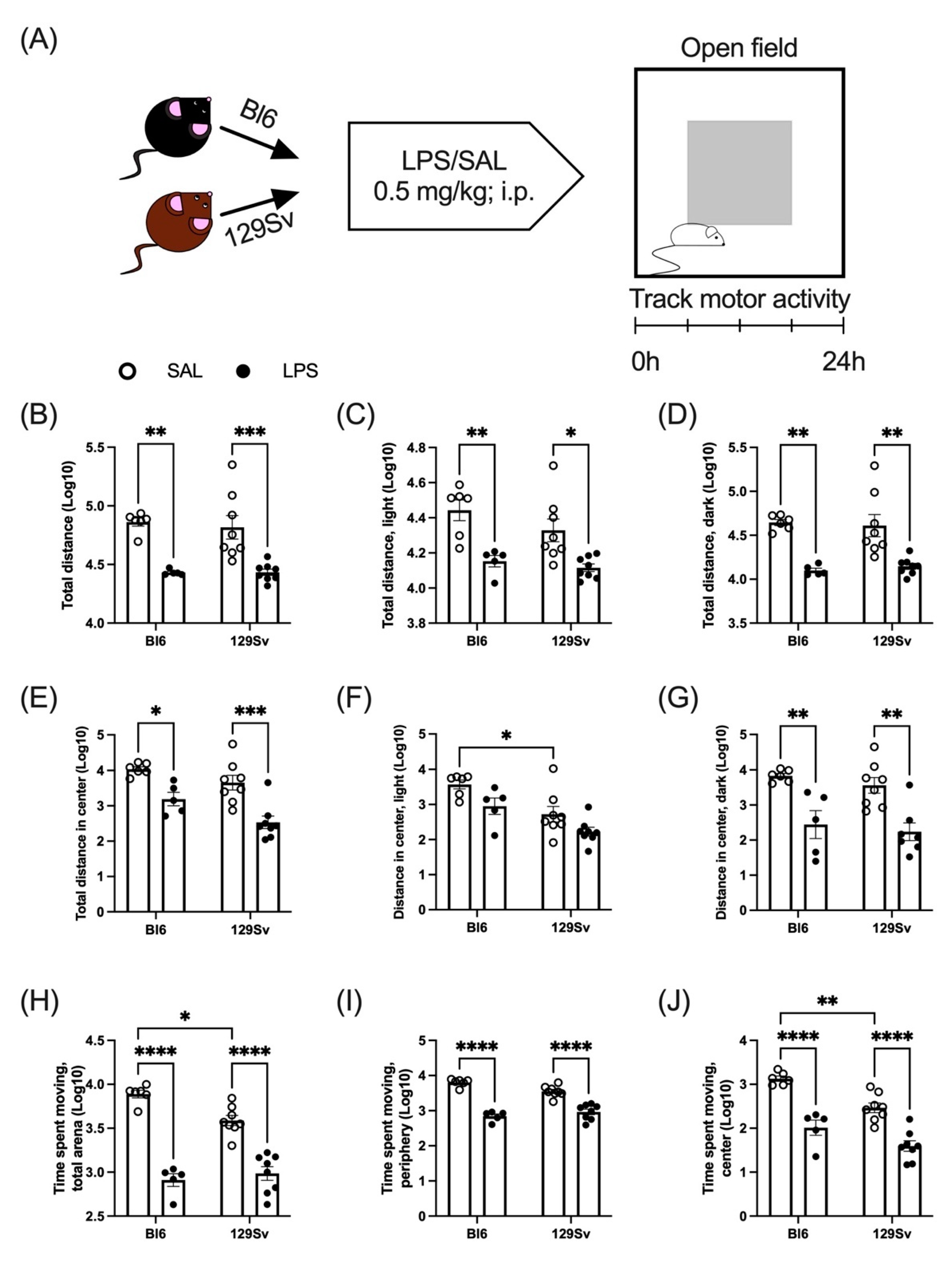
3.2. LPS-Induced Depression of Locomotor Activity
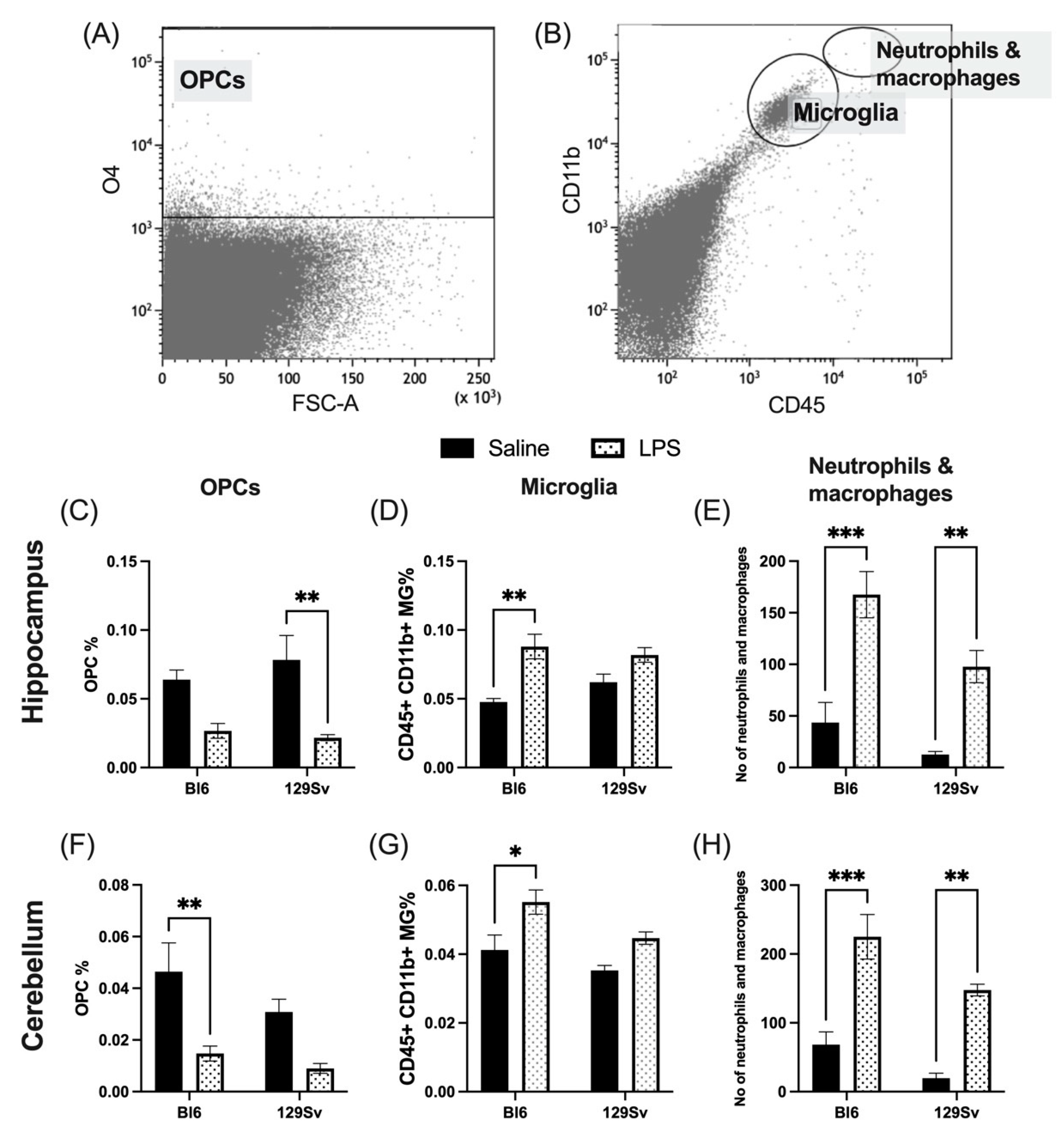
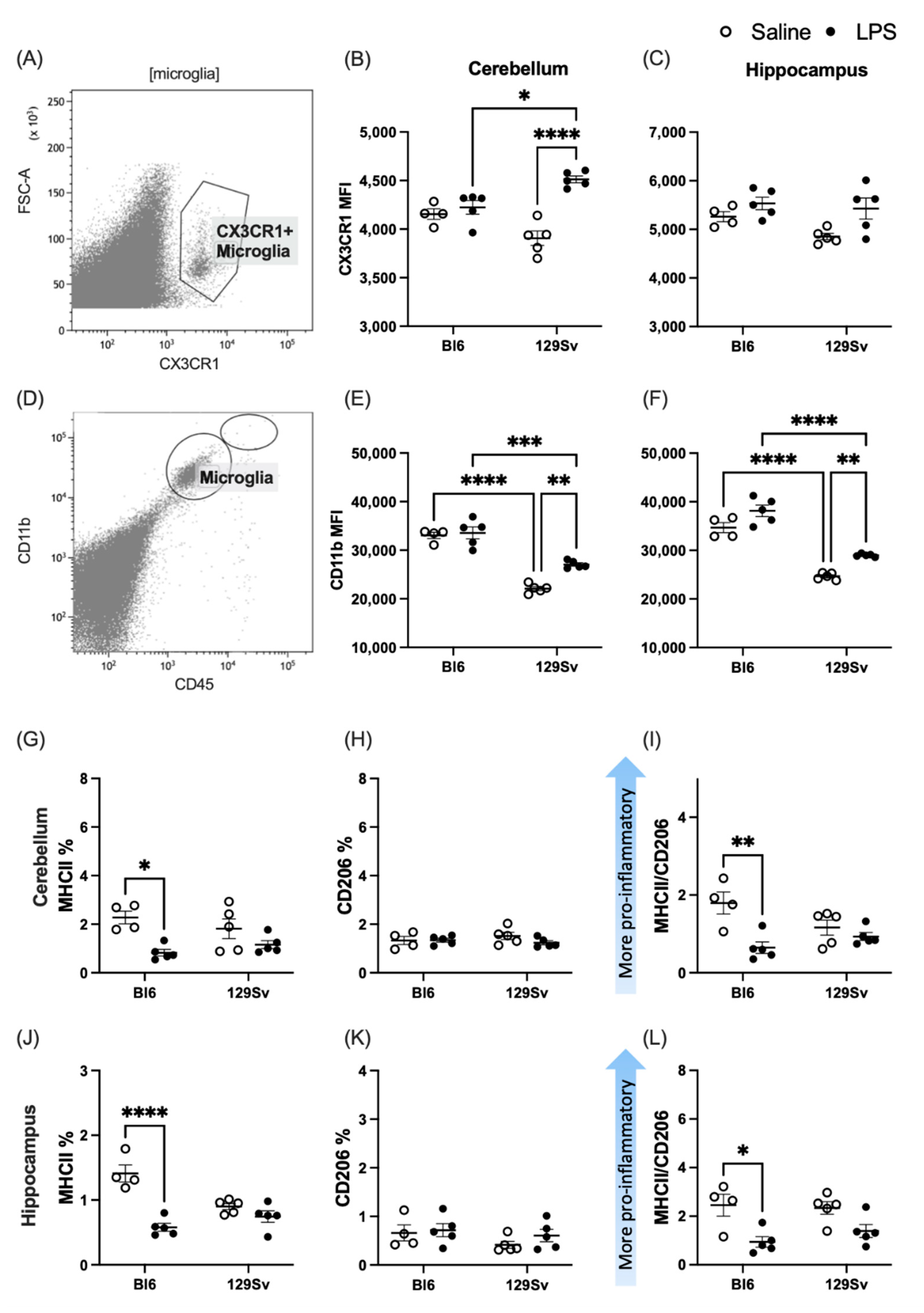
3.3. Microglial Profile and Subpopulations
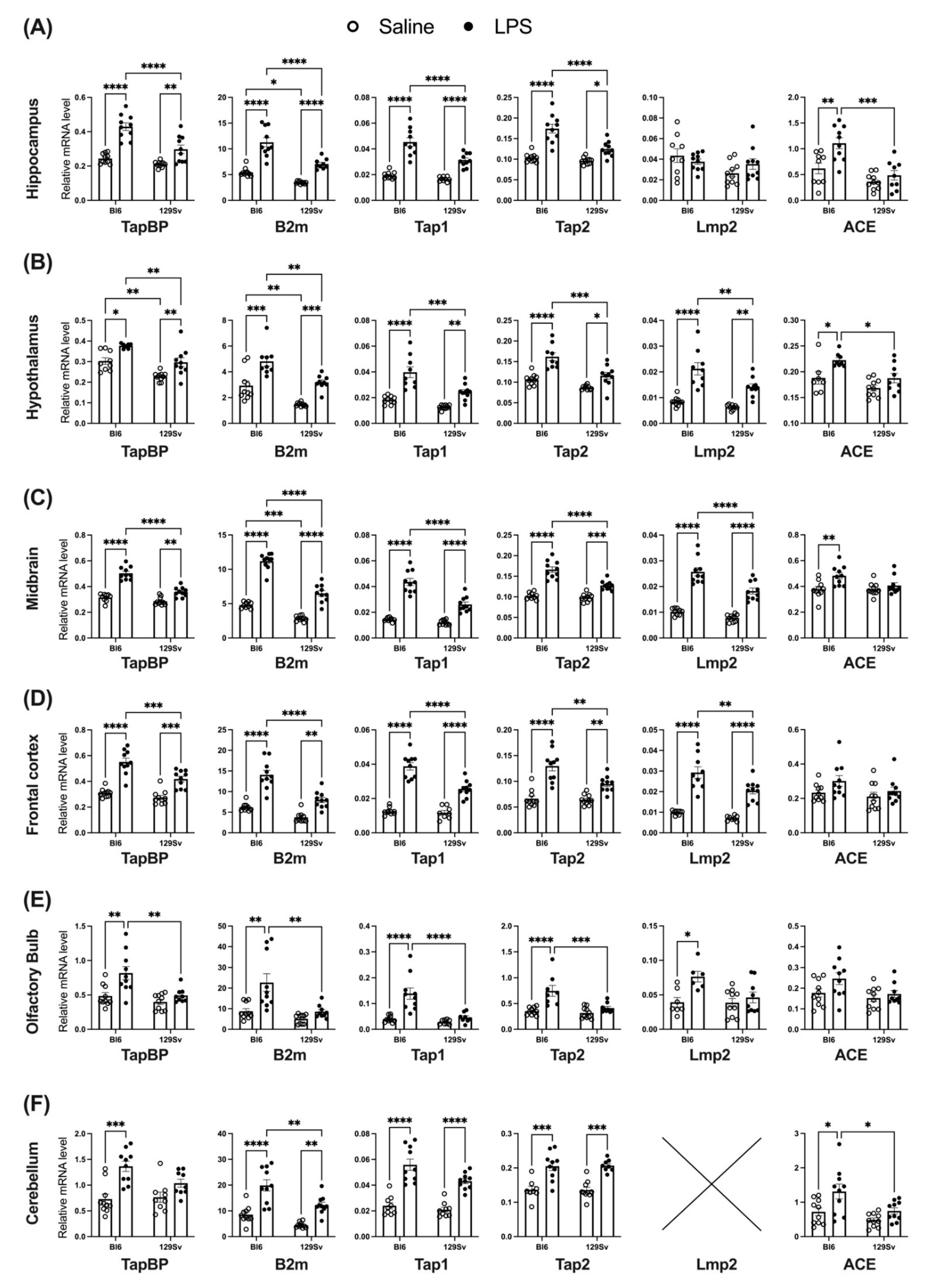
3.4. Impact of LPS on mRNA Levels of Genes Related to the MHC-I Pathway
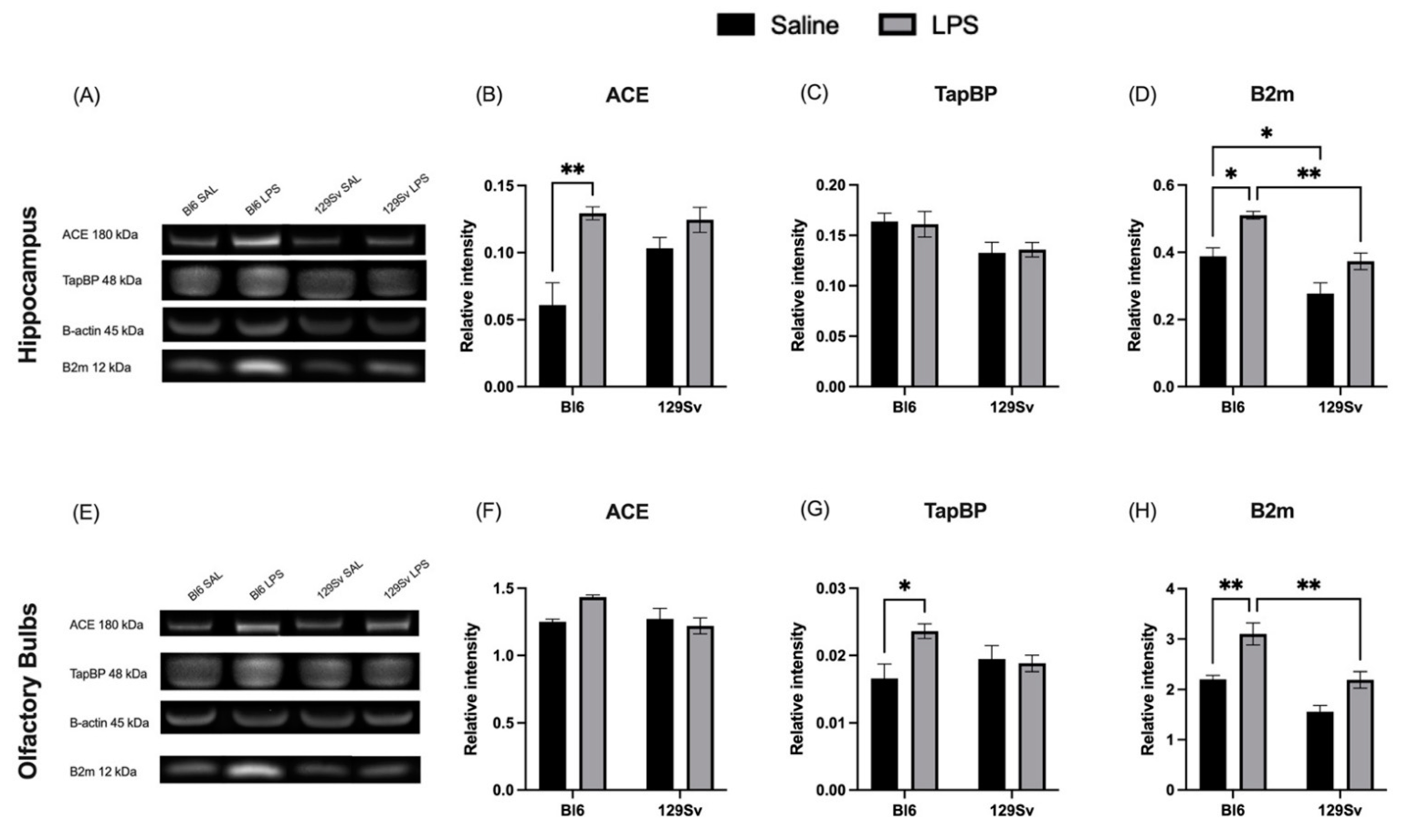
3.5. Impact of LPS on Selected MHC-I-Pathway Protein Levels
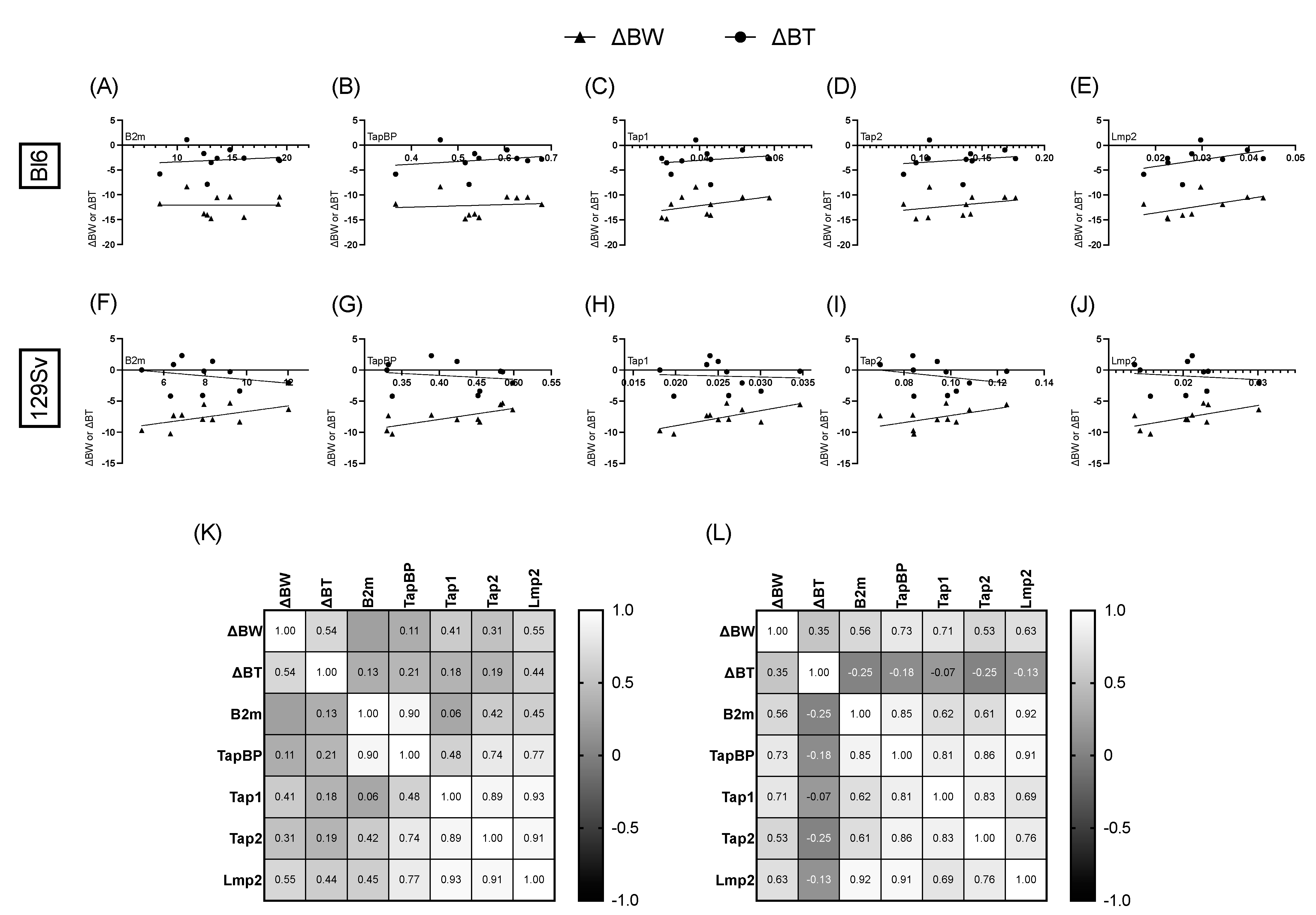
3.6. Association between Bodyweight, Temperature Change, and Relative mRNA Expression of MHC-I Pathway Components
4. Discussion
4.1. LPS-Induced Loss of Body Temperature and Weight and Depression of Locomotor Activity
4.2. Microglial Profile and Subpopulations
4.3. Impact of LPS on mRNA and Protein Levels of Genes Related to the MHC-I Pathway
4.4. Association between Bodyweight, Temperature Change, and Relative mRNA Expression of MHC-I Pathway Components
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abramov, U.; Puussaar, T.; Raud, S.; Kurrikoff, K.; Vasar, E. Behavioural differences between C57BL/6 and 129S6/SvEv strains are reinforced by environmental enrichment. Neurosci. Lett. 2008, 443, 223–227. [Google Scholar] [CrossRef]
- Võikar, V.; Koks, S.; Vasar, E.; Rauvala, H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 2001, 72, 271–281. [Google Scholar] [CrossRef]
- Heinla, I.; Leidmaa, E.; Visnapuu, T.; Philips, M.-A.; Vasar, E. Enrichment and individual housing reinforce the differences in aggressiveness and amphetamine response in 129S6/SvEv and C57BL/6 strains. Behav. Brain Res. 2014, 267, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Varul, J.; Eskla, K.-L.; Piirsalu, M.; Innos, J.; Philips, M.-A.; Visnapuu, T.; Plaas, M.; Vasar, E. Dopamine System, NMDA Receptor and EGF Family Expressions in Brain Structures of Bl6 and 129Sv Strains Displaying Different Behavioral Adaptation. Brain Sci. 2021, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.B.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piirsalu, M.; Taalberg, E.; Lilleväli, K.; Tian, L.; Zilmer, M.; Vasar, E. Treatment with Lipopolysaccharide Induces Distinct Changes in Metabolite Profile and Body Weight in 129Sv and Bl6 Mouse Strains. Front. Pharmacol. 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Jänne, J.; Alhonen, L.; Keinänen, T.A.; Pietilä, M.; Uimari, A.; Pirinen, E.; Hyvönen, M.T.; Järvinen, A. Animal disease models generated by genetic engineering of polyamine metabolism. J. Cell. Mol. Med. 2005, 9, 865–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Kelso, M.L.; Hopkins, D.M.; Pauly, J.R.; Nixon, K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 2013, 54, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Campbell, L.; Zheng, B.; Fan, L.; Cai, Z.; Rhodes, P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience 2010, 166, 464–475. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Hughes, P.M.; Botham, M.S.; Frentzel, S.; Mir, A.; Perry, V.H. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia 2002, 37, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like Receptor-4 Mediates Lipopolysaccharide-induced Signal Transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [Green Version]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A.; Robinson, S.M. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Caraveo, A.; Sayd, A.; Maus, S.R.; Caso, J.R.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci. Rep. 2017, 7, 13113. [Google Scholar] [CrossRef] [Green Version]
- Mastronardi, C.A.; Yu, W.H.; Srivastava, V.K.; Dees, W.L.; McCann, S.M. Lipopolysaccharide-induced leptin release is neurally controlled. Proc. Natl. Acad. Sci. USA 2001, 98, 14720–14725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarraf, P.; Frederich, R.C.; Turner, E.M.; Ma, G.; Jaskowiak, N.T.; Rivet, I.D.J.; Flier, J.S.; Lowell, B.B.; Fraker, D.L.; Alexander, H.R. Multiple Cytokines and Acute Inflammation Raise Mouse Leptin Levels: Potential Role in Inflammatory Anorexia. J. Exp. Med. 1997, 185, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganeshan, K.; Nikkanen, J.; Man, K.; Leong, Y.A.; Sogawa, Y.; Maschek, J.A.; Van Ry, T.; Chagwedera, D.N.; Cox, J.E.; Chawla, A. Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell 2019, 177, 399–413.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, K.I.; Ida, K.K.; Davies, A.L.; Tachtsidis, I.; Papkovsky, D.B.; Dyson, A.; Singer, M.; Duchen, M.R.; Smith, K. Hypothermia protects brain mitochondrial function from hypoxemia in a murine model of sepsis. J. Cereb. Blood Flow Metab. 2016, 36, 1955–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Luan, H.H.; Medzhitov, R. An evolutionary perspective on immunometabolism. Science 2019, 363, eaar3932. [Google Scholar] [CrossRef] [PubMed]
- Silwedel, C.; Förster, C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J. Neuroimmunol. 2006, 179, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Maes, F.; Nuttin, B.; Helsen, W.; Malfait, V.; Nagels, G.; Lavrysen, A.; Liu, X. Relationship between multiple sclerosis intention tremor severity and lesion load in the brainstem. NeuroReport 2005, 16, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; St. Louis, E.K. Management of Acute Cerebellar Stroke. Arch. Neurol. 2005, 62, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernandez-Klett, F.; Lin, G.; Sagar; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial brain region−dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.-Y.; Lin, Y.-T.; Chen, H.-H.; Ho, W.-Y.; Sun, G.-C.; Hsiao, M.; Lu, P.-J.; Cheng, P.-W.; Tseng, C.-J. CX3CR1-microglia mediates neuroinflammation and blood pressure regulation in the nucleus tractus solitarii of fructose-induced hypertensive rats. J. Neuroinflamm. 2020, 17, 185. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abellanas, M.A.; Zamarbide, M.; Basurco, L.; Luquin, E.; Garcia-Granero, M.; Clavero, P.; Martin-Uriz, P.S.; Vilas-Zornoza, A.; Mengual, E.; Hervas-Stubbs, S.; et al. Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J. Neuroinflamm. 2019, 16, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntjewerff, E.M.; Meesters, L.D.; van den Bogaart, G.; Revelo, N.H. Reverse Signaling by MHC-I Molecules in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 605958. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, X.; Bao, Y.; Zhu, X.; Han, C.; Zhang, P.; Zhang, X.; Li, W.; Cao, X. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat. Immunol. 2012, 13, 551–559. [Google Scholar] [CrossRef]
- Khan, Z.; Shen, X.Z.; Bernstein, E.A.; Giani, J.F.; Eriguchi, M.; Zhao, T.V.; Gonzalez-Villalobos, R.A.; Fuchs, S.; Liu, G.Y.; Bernstein, K.E. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 2017, 130, 328–339. [Google Scholar] [CrossRef]
- Raud, S.; Sütt, S.; Plaas, M.; Luuk, H.; Innos, J.; Philips, M.-A.; Kõks, S.; Vasar, E. Cat odor exposure induces distinct changes in the exploratory behavior and Wfs1 gene expression in C57Bl/6 and 129Sv mice. Neurosci. Lett. 2007, 426, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Kazanis, R.; Chinnery, H.R.; McMenamin, P.G. Regional and functional heterogeneity of antigen presenting cells in the mouse brain and meninges. Glia 2019, 67, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Soto, R.; Yandamuri, S.; Stone, C.; Dickey, L.; Gomes-Neto, J.C.; Pastuzyn, E.D.; Bell, R.; Petersen, C.; Buhrke, K.; et al. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. eLife 2019, 8, e47117. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence (5′-3′) | Product Length (bp) | |
|---|---|---|---|
| Hprt | Forward | 5′-GCAGTACAGCCCCAAAATGG-3′ | 85 bp |
| Reverse | 5′-AACAAAGTCTGGCCTGTATCCAA-3′ | ||
| β2m | Forward | 5′-TGGTCTTTCTGGTGCTTGTC-3′ | 107 bp |
| Reverse | 5′-TATGTTCGGCTTCCCATTCTCC-3′ | ||
| Tap1 | Forward | 5′-CTTGGATGATGCCACCAGTG-3′ | 99 bp |
| Reverse | 5′-AGAAGAACCGTCCGAGAAGC-3′ | ||
| Tap2 | Forward | 5′-GCGCCATCTTTTTCATGTGC-3′ | 143 bp |
| Reverse | 5′-AAGGTCTTGGCGCAACAAAG-3′ | ||
| Lmp2 | Forward | 5′-ATGGGAGGGATGCTAATTCGAC-3′ | 134 bp |
| Reverse | 5′-ATGGCATCTGTGGTGAAACG-3′ | ||
| Tapbp | Forward | 5′-CAGCTACCTCCAGTCACTGC-3′ | 124 bp |
| Reverse | 5′-GCCCTGAGAAGCCTGCCA-3′ | ||
| ACE | Forward | 5′-TCGCTACAACTTCGACTGGT-3′ | 163 bp |
| Reverse | 5′-GAACTGGAACTGCAGCACAA-3′ |
| Protein | Host | Dilution | Company | Catalog No |
|---|---|---|---|---|
| Beta 2 microglobulin (β2m) | Rabbit | 1:10,000 | Abcam | ab75853 |
| Tapasin (TapBP) | Rabbit | 1:1000 | Abcam | ab196764 |
| Angiotensin Converting Enzyme 1 (ACE) | Rabbit | 1:10,000 | Abcam | ab254222 |
| B-actin | Mouse | 1:10,000 | Santa Cruz Biotechnology | sc-47778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piirsalu, M.; Chithanathan, K.; Jayaram, M.; Visnapuu, T.; Lilleväli, K.; Zilmer, M.; Vasar, E. Lipopolysaccharide-Induced Strain-Specific Differences in Neuroinflammation and MHC-I Pathway Regulation in the Brains of Bl6 and 129Sv Mice. Cells 2022, 11, 1032. https://doi.org/10.3390/cells11061032
Piirsalu M, Chithanathan K, Jayaram M, Visnapuu T, Lilleväli K, Zilmer M, Vasar E. Lipopolysaccharide-Induced Strain-Specific Differences in Neuroinflammation and MHC-I Pathway Regulation in the Brains of Bl6 and 129Sv Mice. Cells. 2022; 11(6):1032. https://doi.org/10.3390/cells11061032
Chicago/Turabian StylePiirsalu, Maria, Keerthana Chithanathan, Mohan Jayaram, Tanel Visnapuu, Kersti Lilleväli, Mihkel Zilmer, and Eero Vasar. 2022. "Lipopolysaccharide-Induced Strain-Specific Differences in Neuroinflammation and MHC-I Pathway Regulation in the Brains of Bl6 and 129Sv Mice" Cells 11, no. 6: 1032. https://doi.org/10.3390/cells11061032
APA StylePiirsalu, M., Chithanathan, K., Jayaram, M., Visnapuu, T., Lilleväli, K., Zilmer, M., & Vasar, E. (2022). Lipopolysaccharide-Induced Strain-Specific Differences in Neuroinflammation and MHC-I Pathway Regulation in the Brains of Bl6 and 129Sv Mice. Cells, 11(6), 1032. https://doi.org/10.3390/cells11061032






