Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Prevalence of Olfactory and Gustative Dysfunctions
3.2. Evolution of Olfactory and Gustative Dysfunctions
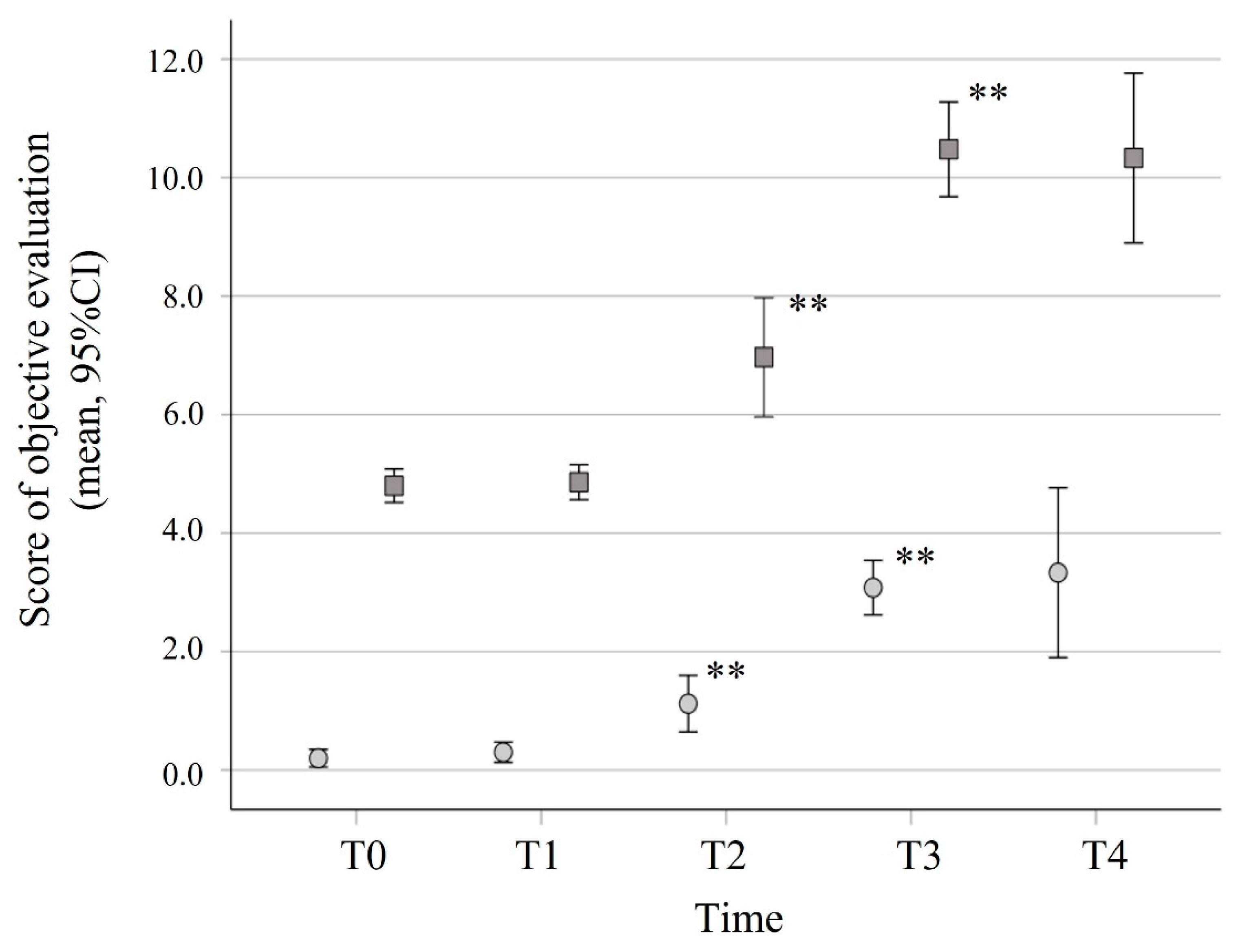
3.3. Objective Olfactory and Gustative Dysfunctions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 December 2021).
- Carbone, M.; Lednicky, J.; Xiao, S.Y.; Venditti, M.; Bucci, E. Coronavirus 2019 Infectious Disease Epidemic: Where We Are, What Can Be Done and Hope For. J. Thorac. Oncol. 2021, 16, 546–571. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Vaira, L.A.; De Riu, G.; Cammaroto, G.; Chekkoury-Idrissi, Y.; Circiu, M.; Distinguin, L.; Journe, F.; de Terwangne, C.; et al. Epidemiological, otolaryngological, olfactory and gustatory outcomes according to the severity of COVID-19: A study of 2579 patients. Eur. Arch. Otorhinolaryngol. 2021, 278, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, K.; Masaki, K.; Uwamino, Y.; Kabata, H.; Uchida, S.; Uno, S.; Asakura, T.; Funakoshi, T.; Kanzaki, S.; Ishii, M.; et al. Acute onset olfactory/taste disorders are associated with a high viral burden in mild or asymptomatic SARS-CoV-2 infections. Int. J. Infect. Dis. 2020, 99, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Avcı, H.; Karabulut, B.; Farasoglu, A.; Boldaz, E.; Evman, M. Relationship between anosmia and hospitalisation in patients with coronavirus disease 2019: An otolaryngological perspective. J. Laryngol. Otol. 2020, 134, 710–716. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020, 11, 2944–2961. [Google Scholar] [CrossRef]
- Boscutti, A.; Delvecchio, G.; Pigoni, A.; Cereda, G.; Ciappolino, V.; Bellani, M.; Fusar-Poli, P.; Brambilla, P. Olfactory and gustatory dysfunctions in SARS-CoV-2 infection: A systematic review. Brain Behav. Immun. Health 2021, 15, 100268. [Google Scholar] [CrossRef]
- Seok, J.; Shim, Y.J.; Rhee, C.S.; Kim, J.W. Correlation between olfactory severity ratings based on olfactory function test scores and self-reported severity rating of olfactory loss. Acta Otolaryngol. 2017, 137, 750–754. [Google Scholar] [CrossRef]
- Mariño-Sánchez, F.; Santamaría-Gadea, A.; de Los Santos, G.; Alobid, I.; Mullol, J. Psychophysical olfactory testing in COVID-19: Is smell function really impaired in nearly all patients? Int. Forum. Allergy Rhinol. 2020, 10, 951–952. [Google Scholar] [CrossRef]
- Bollettino Istituto Superiore di Sanità. Available online: https://www.iss.it/documents/20126/0/reportBollettino+varianti+fino+al+19+maggio+2021.pdf/1e7218cc-c084-a7af-0a4c-6573acb3eba9?t=1621944222307 (accessed on 5 February 2022).
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Doty, R.L.; Marcus, A.; Lee, W.W. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 1996, 106, 353–356. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- El Rassi, E.; Mace, J.C.; Steele, T.O.; Alt, J.A.; Soler, Z.M.; Fu, R.; Smith, T.L. Sensitivity analysis and diagnostic accuracy of the Brief Smell Identification Test in patients with chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2016, 6, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Bramerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafar, A.; Lasso, A.; Shorr, R.; Hutton, B.; Kilty, S. Olfactory recovery following infection with COVID-19: A systematic review. PLoS ONE 2021, 16, e0259321. [Google Scholar] [CrossRef] [PubMed]
- Ugurlu, B.N.; Akdogan, O.; Yilmaz, Y.A.; Yapar, D.; Aktar Ugurlu, G.; Yerlikaya, H.S.; Aslan Felek, S. Quantitative evaluation and progress of olfactory dysfunction in COVID-19. Eur. Arch. Otorhinolaryngol. 2021, 278, 2363–2369. [Google Scholar] [CrossRef]
- Bertlich, M.; Stihl, C.; Lusebrink, E.; Hellmuth, J.C.; Scherer, C.; Freytag, S.; Spiegel, J.L.; Stoycheva, I.; Canis, M.; Weiss, B.G.; et al. The course of subjective and objective chemosensory dysfunction in hospitalized patients with COVID-19: A 6-month follow-up. Eur. Arch. Otorhinolaryngol. 2021, 278, 4855–4861. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Neri, G.; D’Ardes, D.; De Luca, C.; Marinari, S.; Porreca, E.; Cipollone, F.; Vecchiet, J.; Falcicchia, C.; Panichi, V.; et al. Smell and Taste in Severe COVID-19: Self-Reported vs. Testing. Front. Med. 2020, 7, 589409. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Xia, X.; Yang, Y.; Zhou, C. Effect of gender on odor identification at different life stages: A meta-analysis. Rhinology 2019, 57, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, X. Initiation of the age-related decline of odor identification in humans: A meta-analysis. Ageing Res. Rev. 2017, 40, 45–50. [Google Scholar] [CrossRef]
- Hayden, S.; Teeling, E.C. The molecular biology of vertebrate olfaction. Anat. Rec. 2014, 297, 2216–2226. [Google Scholar] [CrossRef]
- Villar, P.S.; Vergara, C.; Bacigalupo, J. Energy sources that fuel metabolic processes in protruding finger-like organelles. FEBS J. 2021, 288, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Torabi, A.; Mohammadbagheri, E.; Akbari Dilmaghani, N.; Bayat, A.H.; Fathi, M.; Vakili, K.; Alizadeh, R.; Rezaeimirghaed, O.; Hajiesmaeili, M.; Ramezani, M.; et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem. Neurosci. 2020, 11, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Schirinzi, A.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Comite, M.; Ciavarella, D.; et al. Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chem. Neurosci. 2020, 11, 2774–2781. [Google Scholar] [CrossRef]
- Kirschenbaum, D.; Imbach, L.L.; Ulrich, S.; Rushing, E.J.; Keller, E.; Reimann, R.R.; Frauenknecht, K.B.M.; Lichtblau, M.; Witt, M.; Hummel, T.; et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet 2020, 396, 166. [Google Scholar] [CrossRef]
- Aragao, M.; Leal, M.C.; Cartaxo Filho, O.Q.; Fonseca, T.M.; Valenca, M.M. Anosmia in COVID-19 Associated with Injury to the Olfactory Bulbs Evident on MRI. AJNR Am. J. Neuroradiol. 2020, 41, 1703–1706. [Google Scholar] [CrossRef]
- Morbini, P.; Benazzo, M.; Verga, L.; Pagella, F.G.; Mojoli, F.; Bruno, R.; Marena, C. Ultrastructural Evidence of Direct Viral Damage to the Olfactory Complex in Patients Testing Positive for SARS-CoV-2. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949.e15. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Bryche, B.; St Albin, A.; Murri, S.; Lacôte, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef]
- Bilinska, K.; Butowt, R. Anosmia in COVID-19: A Bumpy Road to Establishing a Cellular Mechanism. ACS Chem. Neurosci. 2020, 11, 2152–2155. [Google Scholar] [CrossRef]
- Romoli, M.; Jelcic, I.; Bernard-Valnet, R.; García Azorín, D.; Mancinelli, L.; Akhvlediani, T.; Monaco, S.; Taba, P.; Sellner, J. A systematic review of neurological manifestations of SARS-CoV-2 infection: The devil is hidden in the details. Eur. J. Neurol. 2020, 27, 1712–1726. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2020, 24, 168–175. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021, 20, 753–761. [Google Scholar] [CrossRef]
- Negoias, S.; Meves, B.; Zang, Y.; Haehner, A.; Hummel, T. Characteristics of Olfactory Disorder with and without Reported Flavor Loss. Laryngoscope 2020, 130, 2869–2873. [Google Scholar] [CrossRef]
- Rozin, P. “Taste-smell confusions” and the duality of the olfactory sense. Percept. Psychophys. 1982, 31, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Lozada-Nur, F.; Chainani-Wu, N.; Fortuna, G.; Sroussi, H. Dysgeusia in COVID-19: Possible Mechanisms and Implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 344–346. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, R. Role of saliva in the maintenance of taste sensitivity. Crit. Rev. Oral Biol. Med. 2000, 11, 216–229. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Res 2021, 10, 40. [Google Scholar] [CrossRef]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int. Forum. Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, L.; Salzo, A.E.; Angarano, G.; Palmieri, V.O.; Portincasa, P.; Saracino, A.; Gelardi, M.; Dibattista, M.; Quaranta, N. Gaining Back What Is Lost: Recovering the Sense of Smell in Mild to Moderate Patients after COVID-19. Chem. Senses 2020, 45, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020, 42, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Hintschich, C.A.; Niv, M.Y.; Hummel, T. The taste of the pandemic-contemporary review on the current state of research on gustation in coronavirus disease 2019 (COVID-19). Int. Forum. Allergy Rhinol. 2021, 12, 210–216. [Google Scholar] [CrossRef]
- D’Ascanio, L.; Pandolfini, M.; Cingolani, C.; Latini, G.; Gradoni, P.; Capalbo, M.; Frausini, G.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol. Head Neck Surg. 2021, 164, 82–86. [Google Scholar] [CrossRef]
- Huart, C.; Philpott, C.; Konstantinidis, I.; Altundag, A.; Whitcroft, K.L.; Trecca, E.M.C.; Cassano, M.; Rombaux, P.; Hummel, T. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology 2020, 58, 623–625. [Google Scholar] [CrossRef]
- Niklassen, A.S.; Draf, J.; Huart, C.; Hintschich, C.; Bocksberger, S.; Trecca, E.M.C.; Klimek, L.; Le Bon, S.D.; Altundag, A.; Hummel, T. COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre study on Smell and Taste. Laryngoscope 2021, 131, 1095–1100. [Google Scholar] [CrossRef]
- Addison, A.B.; Wong, B.; Ahmed, T.; Macchi, A.; Konstantinidis, I.; Huart, C.; Frasnelli, J.; Fjaeldstad, A.W.; Ramakrishnan, V.R.; Rombaux, P.; et al. Clinical Olfactory Working Group consensus statement on the treatment of postinfectious olfactory dysfunction. J. Allergy Clin. Immunol. 2021, 147, 1704–1719. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H.; Kim, B.G.; Kang, J.M.; Cho, J.H.; Park, Y.J.; Kim, S.W. Prognosis of Olfactory Dysfunction according to Etiology and Timing of Treatment. Otolaryngol. Head Neck Surg. 2017, 156, 371–377. [Google Scholar] [CrossRef]
- Whitcroft, K.L.; Hummel, T. Clinical Diagnosis and Current Management Strategies for Olfactory Dysfunction: A Review. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 846–853. [Google Scholar] [CrossRef]
- Le Bon, S.D.; Konopnicki, D.; Pisarski, N.; Prunier, L.; Lechien, J.R.; Horoi, M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur. Arch. Otorhinolaryngol. 2021, 278, 3113–3117. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Rissom, K.; Reden, J.; Hahner, A.; Weidenbecher, M.; Huttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Abdelalim, A.A.; Mohamady, A.A.; Elsayed, R.A.; Elawady, M.A.; Ghallab, A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial. Am. J. Otolaryngol. 2021, 42, 102884. [Google Scholar] [CrossRef] [PubMed]




| Total Population | Group A | Group B | p Values | |
|---|---|---|---|---|
| No. of patients | 162 | 44 | 118 | |
| Age (years) | 57.0 (48.8–63.0) | 57.0 (51.0–64.0) | 56.0 (46.8–63.0) | 0.239 |
| Sex (female) | 80 (49.4%) | 27 (61.4%) | 55 (46.6%) | 0.113 |
| BMI | 26.9 (24.8–29.5) | 27.1 (25.1–30.1) | 26.7 (24.7–29.3) | 0.292 |
| Current smoker (n, %) | 33 (20.4%) | 10 (22.7%) | 23 (19.5%) | 0.665 |
| Infectious diseases (n, %) | 0 | 0 | 0 | - |
| Autoimmune diseases (n, %) | 22 (13.6%) | 12 (27.3%) | 10 (8.5%) | 0.004 |
| Hypertension (n, %) | 45 (27.8%) | 16 (36.4%) | 29 (24.6%) | 0.168 |
| Respiratory insufficiency (n, %) | 17 (10.5%) | 7 (15.9%) | 10 (8.5%) | 0.246 |
| Heart problems (n, %) | 61 (37.7) | 18 (40.9%) | 43 (36.4%) | 0.716 |
| Kidney insufficiency (n, %) | 6 (3.7%) | 4 (9.1%) | 2 (1.7%) | 0.047 |
| Allergies (n, %) | 10 (6.2%) | 1 (2.3%) | 9 (7.6%) | 0.108 |
| Comorbidities (n, %) | 89 (54.9%) | 28 (63.6%) | 61 (51.7%) | 0.215 |
| Total Population | Group A | Group B | p Values | |
|---|---|---|---|---|
| n | 162 | 44 | 118 | |
| Symptomatic (n, %) | 149 (92.0) | 39 (88.6) | 110 (93.2) | 0.547 |
| Onset of COVID-19 symptoms (days) | 2.1 (1.9–2.4) | 2.5 (1.8–3.2) | 2.0 (1.7–2.3) | 0.230 |
| SpO2 (%) | 95 (93.8–97) | 95 (94–97) | 95 (93–97) | 0.447 |
| Fever (n, %) | 104 (64.2) | 27 (61.4) | 77 (65.3) | 0.713 |
| Asthenia (n, %) | 77 (47.5) | 28 (63.6) | 49 (41.5) | 0.014 * |
| Respiratory symptoms (n, %) | 55 (34.0) | 16 (36.4) | 39 (33.1) | 0.712 |
| Myalgia (n, %) | 33 (20.4 | 8 (18.2) | 25 (21.2) | 0.827 |
| Gastrointestinal symptoms (n, %) | 13 (8:0) | 1 (2.3) | 12 (10.2) | 0.189 |
| Headache (n, %) | 27 (16.7) | 12 (27.3) | 15 (12.7) | 0.034 * |
| Group A | Group B | p Values | |
|---|---|---|---|
| Anosmia (n, %) | 44 (27.2%) | 118 (72.8%) | <0.001 * |
| VAS score | 8.02 (7.70–8.35) | 2.63 (2.27–2.98) | |
| Ageusia (n, %) | 39 (88.6%) | 1 (0.8%) | <0.001 * |
| VAS score | 7.43 (7.03–7.83) | 2.47 (2.11–2.83) | |
| Nasal obstruction (n, %) | 8 (18.2%) | 5 (4.2%) | 0.007 * |
| VAS score | 4.91 (4.19–5.63) | 2.32 (1.94–2.70) | |
| Rhinorrea (n, %) | 0 (0%) | 3 (2.5%) | 0.286 |
| VAS score | 3.86 (3.35–4.38) | 1.77 (1.42–2.12) | |
| Postnasal drip (n, %) | 0 (0%) | 4 (3.4%) | 0.216 |
| VAS score | 3.50 (3.00–4.00) | 1.57 (1.11–1.92) | |
| Sneezes (n, %) | 1 (2.3%) | 1 (0.8%) | 0.465 |
| VAS score | 3.18 (2.61–3.75) | 1.72 (1.40–2.04) | |
| Headache (n, %) | 11 (25%) | 1 (0.8%) | <0.001 * |
| VAS score | 4.66 (3.86–5.45) | 2.04 (1.67–2.41) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciofalo, A.; Cavaliere, C.; Masieri, S.; Di Chicco, A.; Fatuzzo, I.; Lo Re, F.; Baroncelli, S.; Begvarfaj, E.; Adduci, A.; Mezzaroma, I.; et al. Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19. Cells 2022, 11, 788. https://doi.org/10.3390/cells11050788
Ciofalo A, Cavaliere C, Masieri S, Di Chicco A, Fatuzzo I, Lo Re F, Baroncelli S, Begvarfaj E, Adduci A, Mezzaroma I, et al. Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19. Cells. 2022; 11(5):788. https://doi.org/10.3390/cells11050788
Chicago/Turabian StyleCiofalo, Andrea, Carlo Cavaliere, Simonetta Masieri, Alessandra Di Chicco, Irene Fatuzzo, Federica Lo Re, Silvia Baroncelli, Elona Begvarfaj, Andrea Adduci, Ivano Mezzaroma, and et al. 2022. "Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19" Cells 11, no. 5: 788. https://doi.org/10.3390/cells11050788
APA StyleCiofalo, A., Cavaliere, C., Masieri, S., Di Chicco, A., Fatuzzo, I., Lo Re, F., Baroncelli, S., Begvarfaj, E., Adduci, A., Mezzaroma, I., Mastroianni, C. M., de Vincentiis, M., Greco, A., Zamai, L., & Artico, M. (2022). Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19. Cells, 11(5), 788. https://doi.org/10.3390/cells11050788








