Neutrophil Extracellular Traps Do Not Induce Injury and Inflammation in Well-Differentiated RSV-Infected Airway Epithelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. HAE Cultures
2.2. Immunostaining of HAE Cultures
2.3. Neutrophil Isolation and Stimulation
2.4. NET Visualization
2.5. RSV Infection
2.6. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) for RSV Infection
2.7. HAE Culture Exposure to NETs or Unstimulated Neutrophil Supernatant
2.8. LDH Cytotoxicity Assay
2.9. Scanning Electron Microscopy of Cell Cultures
2.10. Measurement of Cytokine Release
2.11. mRNA-Related Gene Expression (NanoString)
3. Statistical Analysis
Analysis of NanoString Data
4. Results
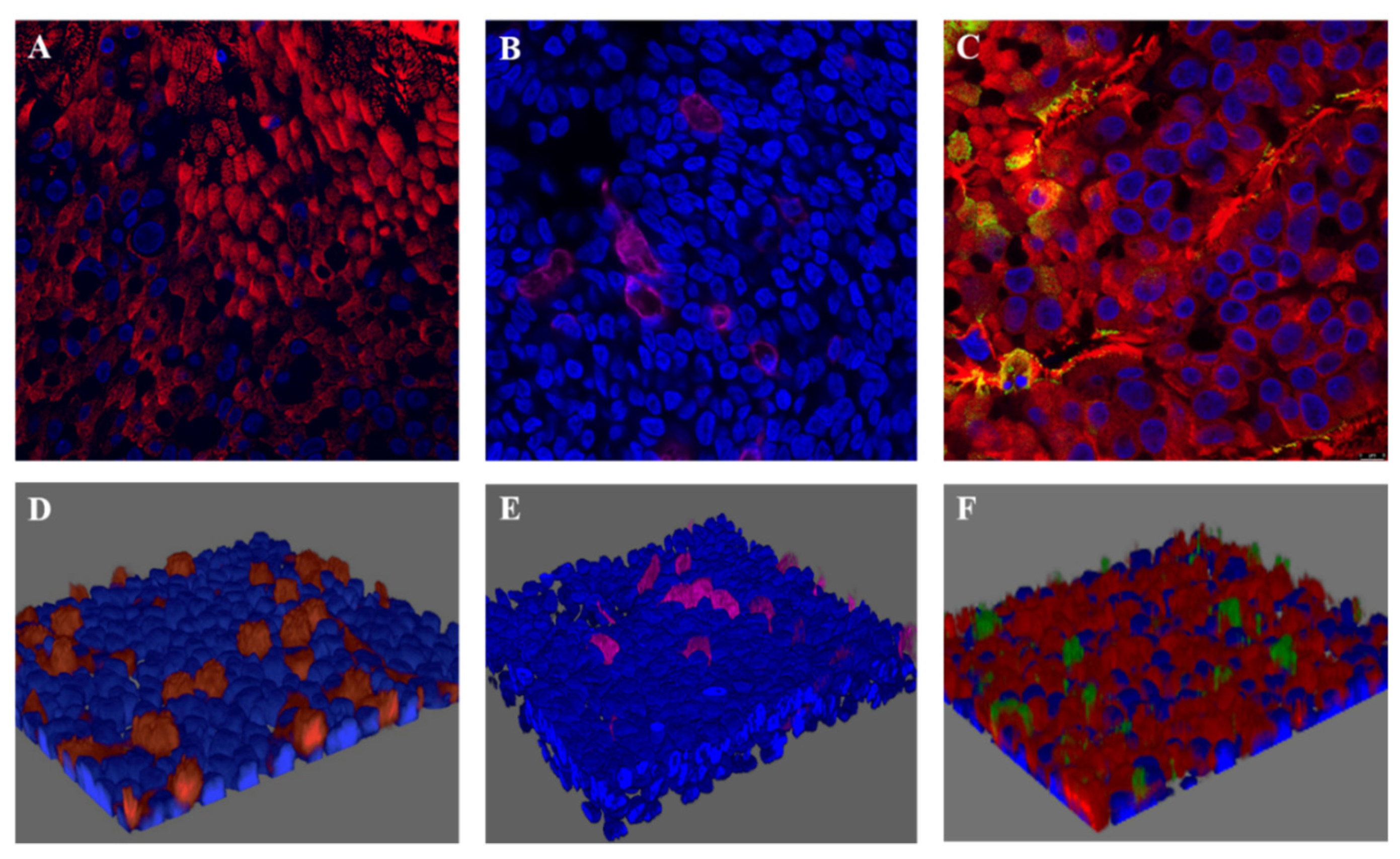
4.1. RSV-A Successfully Infected Ciliated Cells in HAE Cultures
4.2. NET Production
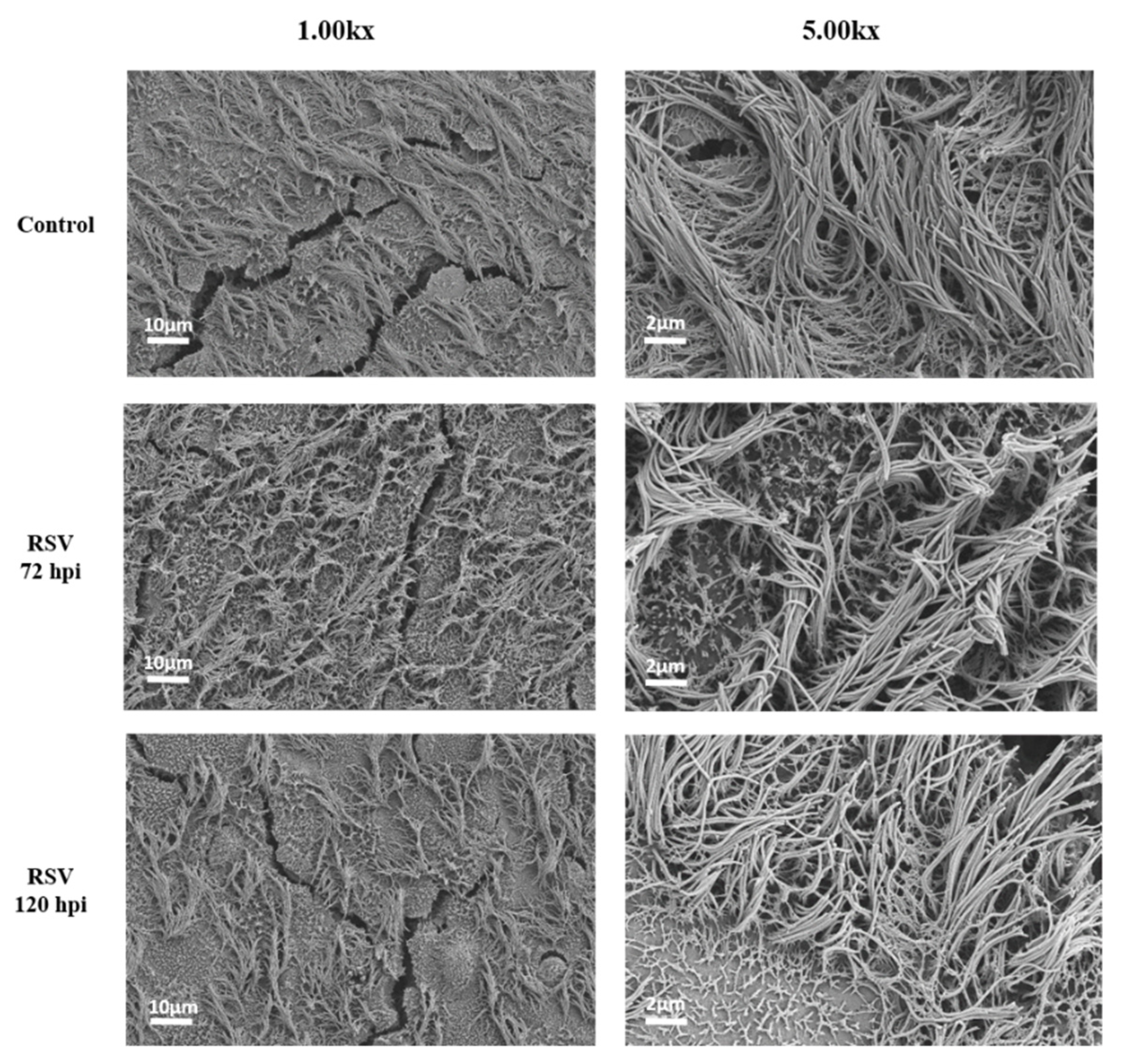
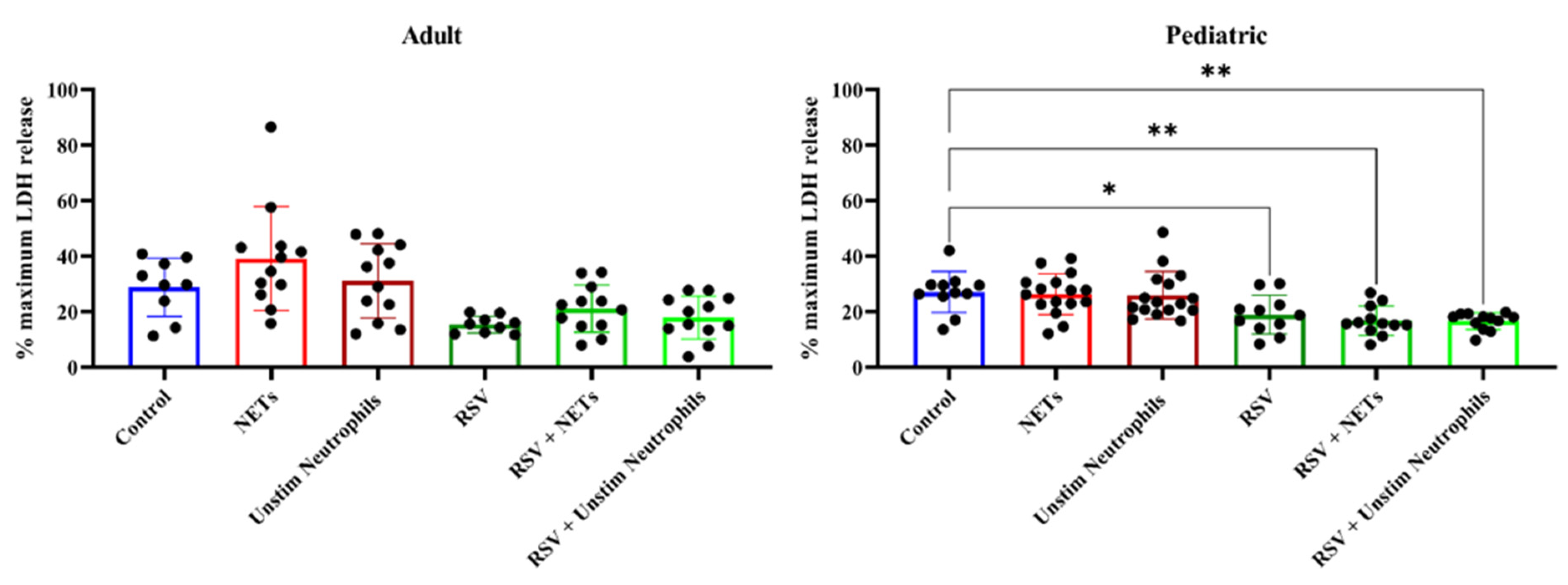
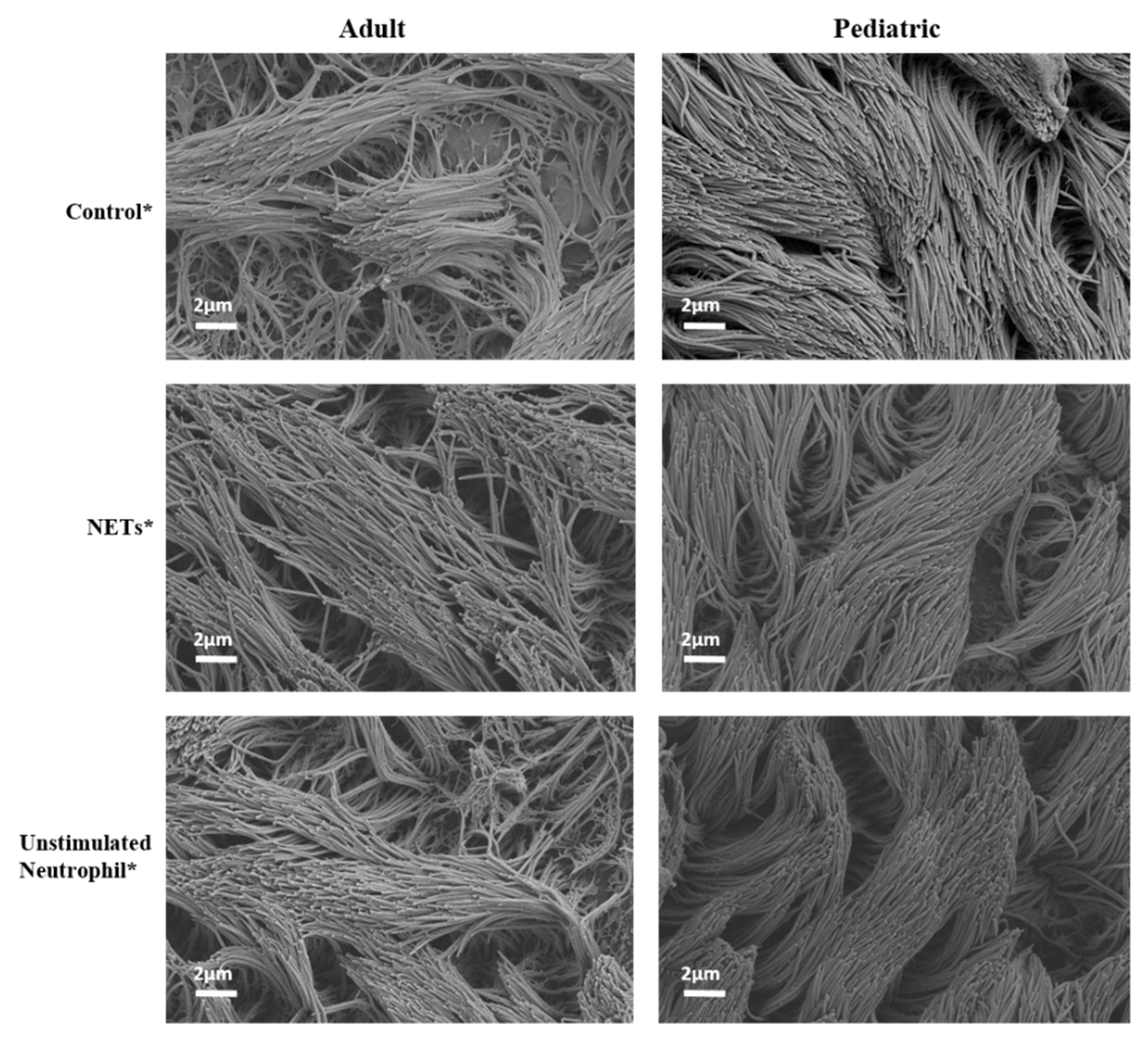
4.3. NETs Did Not Induce Injury to (RSV-Infected) HAE Cultures
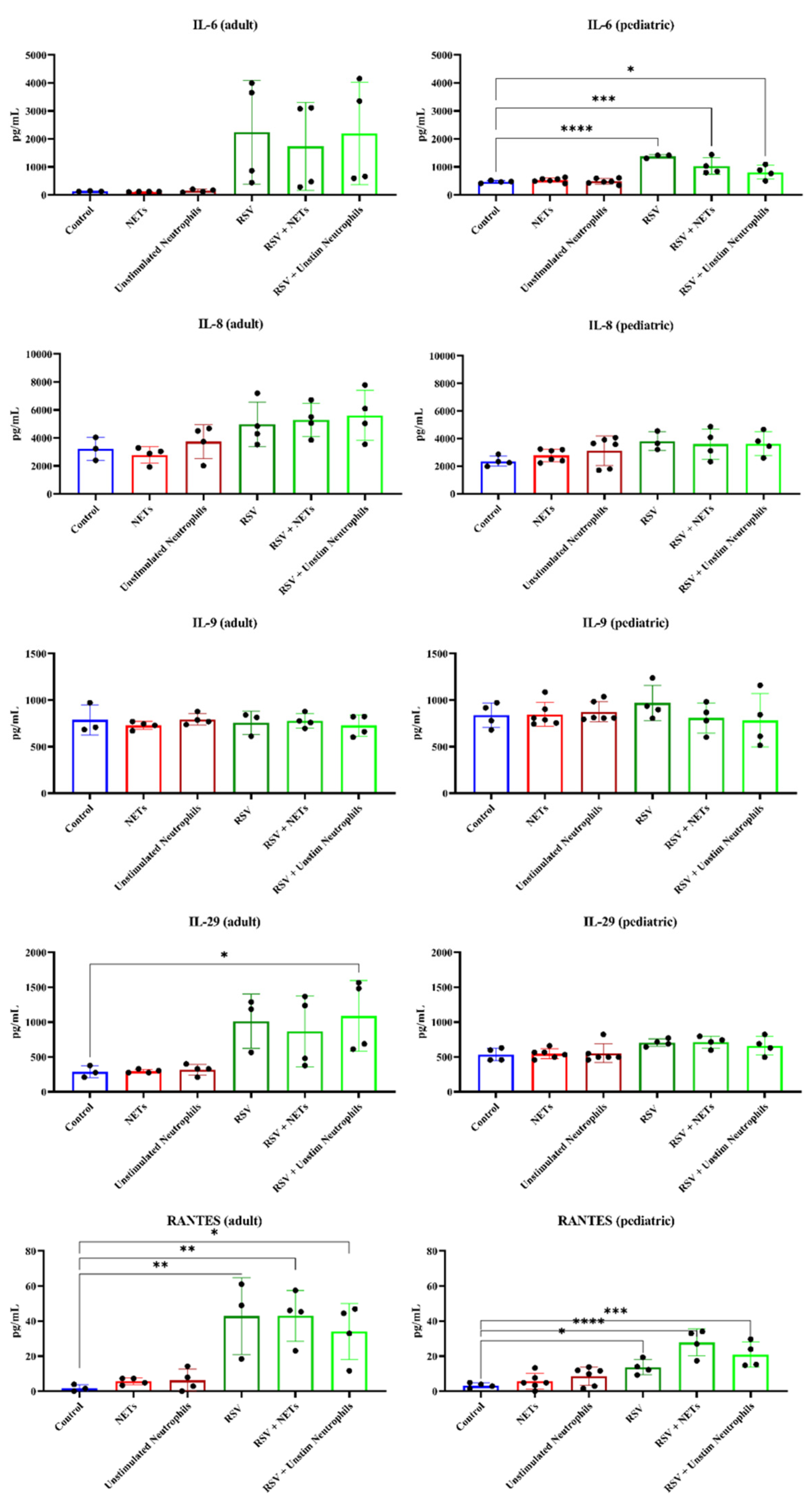
4.4. RSV Infection, but Not NETs, Caused Increased Pro-Inflammatory Cytokine Release from HAE Cultures
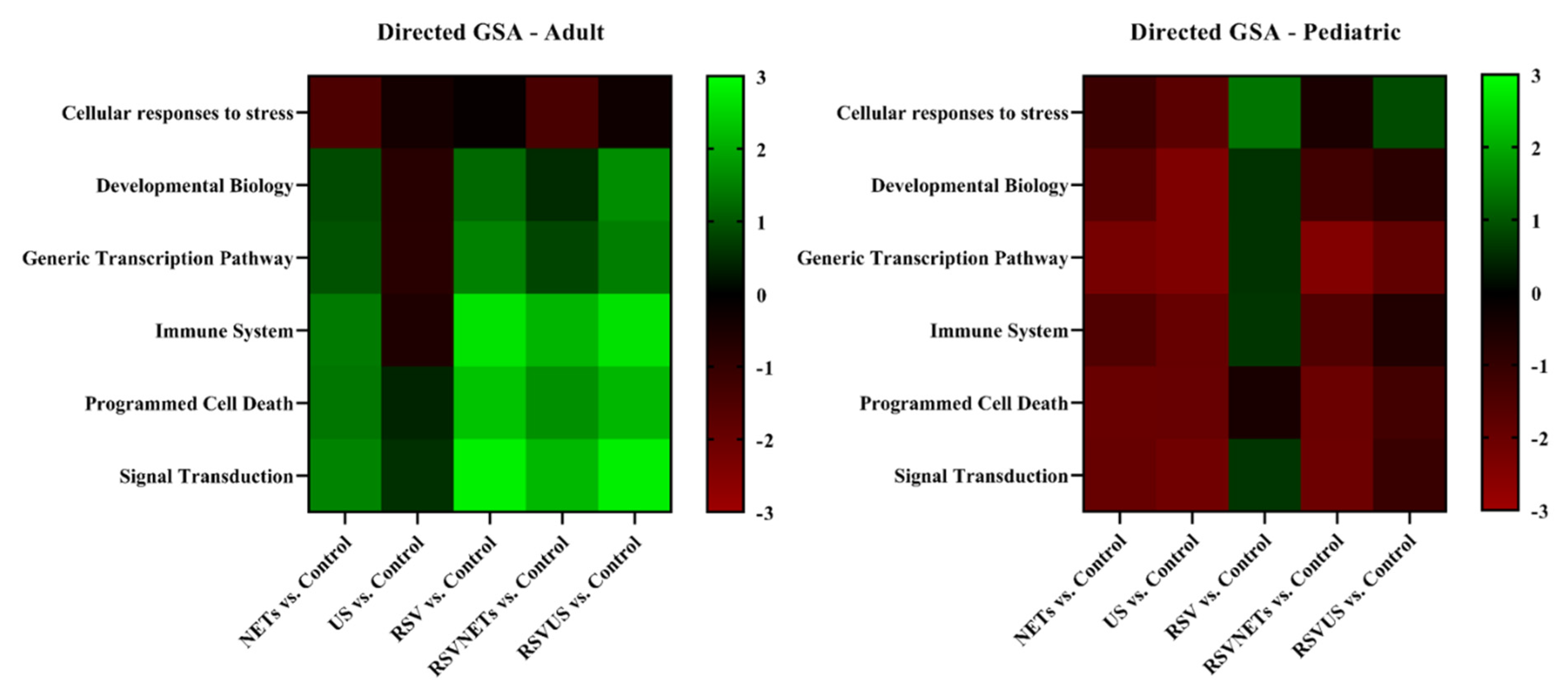
4.5. Age-Related Differential Gene Expression to RSV Infection in HAE Cultures
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Pickles, R.J.; DeVincenzo, J.P. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J. Pathol. 2015, 235, 266–276. [Google Scholar] [CrossRef]
- Everard, M.L.; Swarbrick, A.; Wrightham, M.; McIntyre, J.; Dunkley, C.; James, P.D.; Sewell, H.F.; Milner, A.D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch. Dis. Child. 1994, 71, 428–432. [Google Scholar] [CrossRef] [Green Version]
- McNamara, P.S.; Ritson, P.; Selby, A.; Hart, C.A.; Smyth, R.L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child. 2003, 88, 922–926. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, E.; van Woensel, J.B.; Bem, R.A. Apoptosis in pneumovirus infection. Viruses 2013, 5, 406–422. [Google Scholar] [CrossRef] [Green Version]
- Smyth, R.L.; Brearey, S.P. Bronchiolitis. Encycl. Respir. Med. 2006, 268–275. [Google Scholar] [CrossRef]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef]
- Deng, Y.; Herbert, J.A.; Robinson, E.; Ren, L.; Smyth, R.L.; Smith, C.M. Neutrophil-Airway Epithelial Interactions Result in Increased Epithelial Damage and Viral Clearance during Respiratory Syncytial Virus Infection. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [Green Version]
- Cortjens, B.; de Boer, O.J.; de Jong, R.; Antonis, A.F.; Sabogal Piñeros, Y.S.; Lutter, R.; van Woensel, J.B.; Bem, R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016, 238, 401–411. [Google Scholar] [CrossRef]
- Sebina, I.; Phipps, S. The Contribution of Neutrophils to the Pathogenesis of RSV Bronchiolitis. Viruses 2020, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; de Jong, R.; Bonsing, J.G.; van Woensel, J.B.M.; Antonis, A.F.G.; Bem, R.A. Local dornase alfa treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax 2018, 73, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef]
- Sabbione, F.; Keitelman, I.A.; Iula, L.; Ferrero, M.; Giordano, M.N.; Baldi, P.; Rumbo, M.; Jancic, C.; Trevani, A.S. Neutrophil Extracellular Traps Stimulate Proinflammatory Responses in Human Airway Epithelial Cells. J. Innate Immun. 2017, 9, 387–402. [Google Scholar] [CrossRef]
- Diniz, L.F.A.; Matsuba, B.K.; Souza, P.S.S.; Lopes, B.R.P.; Kubo, L.H.; Oliveira, J.; Toledo, K.A. Effects of neutrophil extracellular traps during human respiratory syncytial virus infection in vitro. Braz J. Biol. 2021, 83, e248717. [Google Scholar] [CrossRef]
- Hudock, K.M.; Collins, M.S.; Imbrogno, M.; Snowball, J.; Kramer, E.L.; Brewington, J.J.; Gollomp, K.; McCarthy, C.; Ostmann, A.J.; Kopras, E.J.; et al. Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia. Am. J. Physiol.-Lung Cell Mol. Physiol. 2020, 319, L137–L147. [Google Scholar] [CrossRef]
- Tahamtan, A.; Besteman, S.; Samadizadeh, S.; Rastegar, M.; Bont, L.; Salimi, V. Neutrophils in respiratory syncytial virus infection: From harmful effects to therapeutic opportunities. Br. J. Pharmacol. 2021, 178, 515–530. [Google Scholar] [CrossRef]
- Geerdink, R.J.; Pillay, J.; Meyaard, L.; Bont, L. Neutrophils in respiratory syncytial virus infection: A target for asthma prevention. J. Allergy Clin. Immunol. 2015, 136, 838–847. [Google Scholar] [CrossRef]
- Villenave, R.; O’Donoghue, D.; Thavagnanam, S.; Touzelet, O.; Skibinski, G.; Heaney, L.G.; McKaigue, J.P.; Coyle, P.V.; Shields, M.D.; Power, U.F. Differential cytopathogenesis of respiratory syncytial virus prototypic and clinical isolates in primary pediatric bronchial epithelial cells. Virol. J. 2011, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villenave, R.; Thavagnanam, S.; Sarlang, S.; Parker, J.; Douglas, I.; Skibinski, G.; Heaney, L.G.; McKaigue, J.P.; Coyle, P.V.; Shields, M.D.; et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 5040–5045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Parra, G.; Dobrovolny, H.M. A quantitative assessment of dynamical differences of RSV infections in vitro and in vivo. Virology 2018, 523, 129–139. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef]
- Linssen, R.S.; Chai, G.; Ma, J.; Kummarapurugu, A.B.; van Woensel, J.B.M.; Bem, R.A.; Kaler, L.; Duncan, G.A.; Zhou, L.; Rubin, B.K.; et al. Neutrophil Extracellular Traps Increase Airway Mucus Viscoelasticity and Slow Mucus Particle Transit. Am. J. Respir. Cell Mol. Biol. 2021, 64, 69–78. [Google Scholar] [CrossRef]
- Beld, M.; Minnaar, R.; Weel, J.; Sol, C.; Damen, M.; van der Avoort, H.; Wertheim-van Dillen, P.; van Breda, A.; Boom, R. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J. Clin. Microbiol. 2004, 42, 3059–3064. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Herbert, J.A.; Smith, C.M.; Smyth, R.L. An in vitro transepithelial migration assay to evaluate the role of neutrophils in Respiratory Syncytial Virus (RSV) induced epithelial damage. Sci. Rep. 2018, 8, 6777. [Google Scholar] [CrossRef] [Green Version]
- Guo-Parke, H.; Canning, P.; Douglas, I.; Villenave, R.; Heaney, L.G.; Coyle, P.V.; Lyons, J.D.; Shields, M.D.; Power, U.F. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am. J. Respir. Crit. Care Med. 2013, 188, 842–851. [Google Scholar] [CrossRef]
- Louahed, J.; Toda, M.; Jen, J.; Hamid, Q.; Renauld, J.C.; Levitt, R.C.; Nicolaides, N.C. Interleukin-9 upregulates mucus expression in the airways. Am. J. Respir. Cell Mol. Biol. 2000, 22, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.C.; Thavagnanam, S.; Skibinski, G.; Lyons, J.; Bell, J.; Heaney, L.G.; Shields, M.D. Chronic IL9 and IL-13 exposure leads to an altered differentiation of ciliated cells in a well-differentiated paediatric bronchial epithelial cell model. PLoS ONE 2013, 8, e61023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villenave, R.; Broadbent, L.; Douglas, I.; Lyons, J.D.; Coyle, P.V.; Teng, M.N.; Tripp, R.A.; Heaney, L.G.; Shields, M.D.; Power, U.F. Induction and Antagonism of Antiviral Responses in Respiratory Syncytial Virus-Infected Pediatric Airway Epithelium. J. Virol. 2015, 89, 12309–12318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukura, S.; Kokubu, F.; Kubo, H.; Tomita, T.; Tokunaga, H.; Kadokura, M.; Yamamoto, T.; Kuroiwa, Y.; Ohno, T.; Suzaki, H.; et al. Expression of RANTES by Normal Airway Epithelial Cells after Influenza Virus A Infection. Am. J. Respir. Cell Mol. Biol. 1998, 18, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culley, F.J.; Pennycook, A.M.; Tregoning, J.S.; Dodd, J.S.; Walzl, G.; Wells, T.N.; Hussell, T.; Openshaw, P.J. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 2006, 80, 8151–8157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitz, R.; Wattier, R.; Phillips, P.; Solomon, A.; Lawler, J.; Lazar, I.; Weibel, C.; Kahn, J.S. Induction of IL-6 and CCL5 (RANTES) in human respiratory epithelial (A549) cells by clinical isolates of respiratory syncytial virus is strain specific. Virol. J. 2012, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Tomfohr, J.; Lu, J.; Kepler, T.B. Pathway level analysis of gene expression using singular value decomposition. BMC Bioinform. 2005, 6, 225. [Google Scholar] [CrossRef] [Green Version]
- Nasr, S.Z.; Strouse, P.J.; Soskolne, E.; Maxvold, N.J.; Garver, K.A.; Rubin, B.K.; Moler, F.W. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001, 120, 203–208. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Konig, M.F.; Andrade, F. A Critical Reappraisal of Neutrophil Extracellular Traps and NETosis Mimics Based on Differential Requirements for Protein Citrullination. Front. Immunol. 2016, 7, 461. [Google Scholar] [CrossRef] [Green Version]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Yu, X.; Lakerveld, A.J.; Imholz, S.; Hendriks, M.; Ten Brink, S.C.A.; Mulder, H.L.; de Haan, K.; Schepp, R.M.; Luytjes, W.; de Jong, M.D.; et al. Antibody and Local Cytokine Response to Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults. mSphere 2020, 5, e00577-20. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, Y.; González, L.; Noguera, L.; González, P.A.; Riedel, C.A.; Bertrand, P.; Bueno, S.M. Cytokines in the Respiratory Airway as Biomarkers of Severity and Prognosis for Respiratory Syncytial Virus Infection: An Update. Front. Immunol. 2019, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Oshansky, C.M.; Barber, J.P.; Crabtree, J.; Tripp, R.A. Respiratory syncytial virus F and G proteins induce interleukin 1alpha, CC, and CXC chemokine responses by normal human bronchoepithelial cells. J. Infect. Dis. 2010, 201, 1201–1207. [Google Scholar] [CrossRef] [Green Version]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Roulston, A.; Marcellus, R.C.; Branton, P.E. Viruses and apoptosis. Annu Rev. Microbiol. 1999, 53, 577–628. [Google Scholar] [CrossRef] [PubMed]
- White, S.R. Apoptosis and the airway epithelium. J. Allergy 2011, 2011, 948406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotelkin, A.; Prikhod’ko, E.A.; Cohen, J.I.; Collins, P.L.; Bukreyev, A. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 2003, 77, 9156–9172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, D.W. From bench to clinic with apoptosis-based therapeutic agents. Nature 2000, 407, 810–816. [Google Scholar] [CrossRef]
- Bem, R.A.; Bos, A.P.; Asperen, R.M.W.-V.; Bruijn, M.; Lutter, R.; Sprick, M.R.; Woensel, J.B.M.V. Potential Role of Soluble TRAIL in Epithelial Injury in Children with Severe RSV Infection. Am. J. Respir. Cell Mol. Biol. 2010, 42, 697–705. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linssen, R.S.N.; Sridhar, A.; Moreni, G.; van der Wel, N.N.; van Woensel, J.B.M.; Wolthers, K.C.; Bem, R.A. Neutrophil Extracellular Traps Do Not Induce Injury and Inflammation in Well-Differentiated RSV-Infected Airway Epithelium. Cells 2022, 11, 785. https://doi.org/10.3390/cells11050785
Linssen RSN, Sridhar A, Moreni G, van der Wel NN, van Woensel JBM, Wolthers KC, Bem RA. Neutrophil Extracellular Traps Do Not Induce Injury and Inflammation in Well-Differentiated RSV-Infected Airway Epithelium. Cells. 2022; 11(5):785. https://doi.org/10.3390/cells11050785
Chicago/Turabian StyleLinssen, Rosalie S. N., Adithya Sridhar, Giulia Moreni, Nicole N. van der Wel, Job B. M. van Woensel, Katja C. Wolthers, and Reinout A. Bem. 2022. "Neutrophil Extracellular Traps Do Not Induce Injury and Inflammation in Well-Differentiated RSV-Infected Airway Epithelium" Cells 11, no. 5: 785. https://doi.org/10.3390/cells11050785
APA StyleLinssen, R. S. N., Sridhar, A., Moreni, G., van der Wel, N. N., van Woensel, J. B. M., Wolthers, K. C., & Bem, R. A. (2022). Neutrophil Extracellular Traps Do Not Induce Injury and Inflammation in Well-Differentiated RSV-Infected Airway Epithelium. Cells, 11(5), 785. https://doi.org/10.3390/cells11050785








