The Splicing Variant TFIIIA-7ZF of Viroid-Modulated Transcription Factor IIIA Causes Physiological Irregularities in Transgenic Tobacco and Transient Somatic Depression of “Degradome” Characteristic for Developing Pollen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation Conditions, Plant Transformation and Sampling
2.2. Viroid Inoculation, Detection and Quantification
2.3. Quantification of mRNA Levels of Genes Potentially Connected to Viroid Degradation and Markers of Senescence
2.4. Other Methods
3. Results and Discussion
3.1. The Ectopic Expression of TFIIIA-7ZF, the Splicing Variant of Viroid-Modulated TFIIIA, Causes Morphological Irregularities in Transgenic N. tabacum
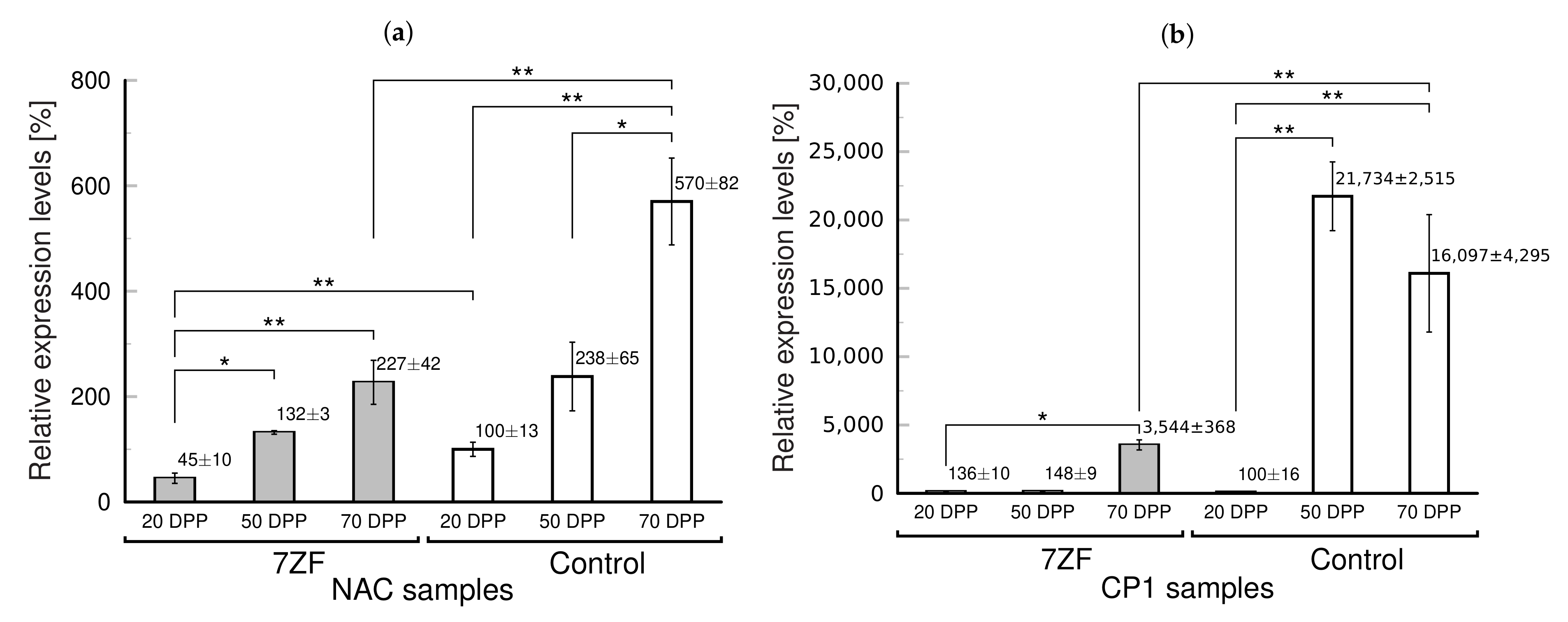
3.2. Delayed Aging and Senescence in TFIIIA-7ZF Transgenotes of Tobacco
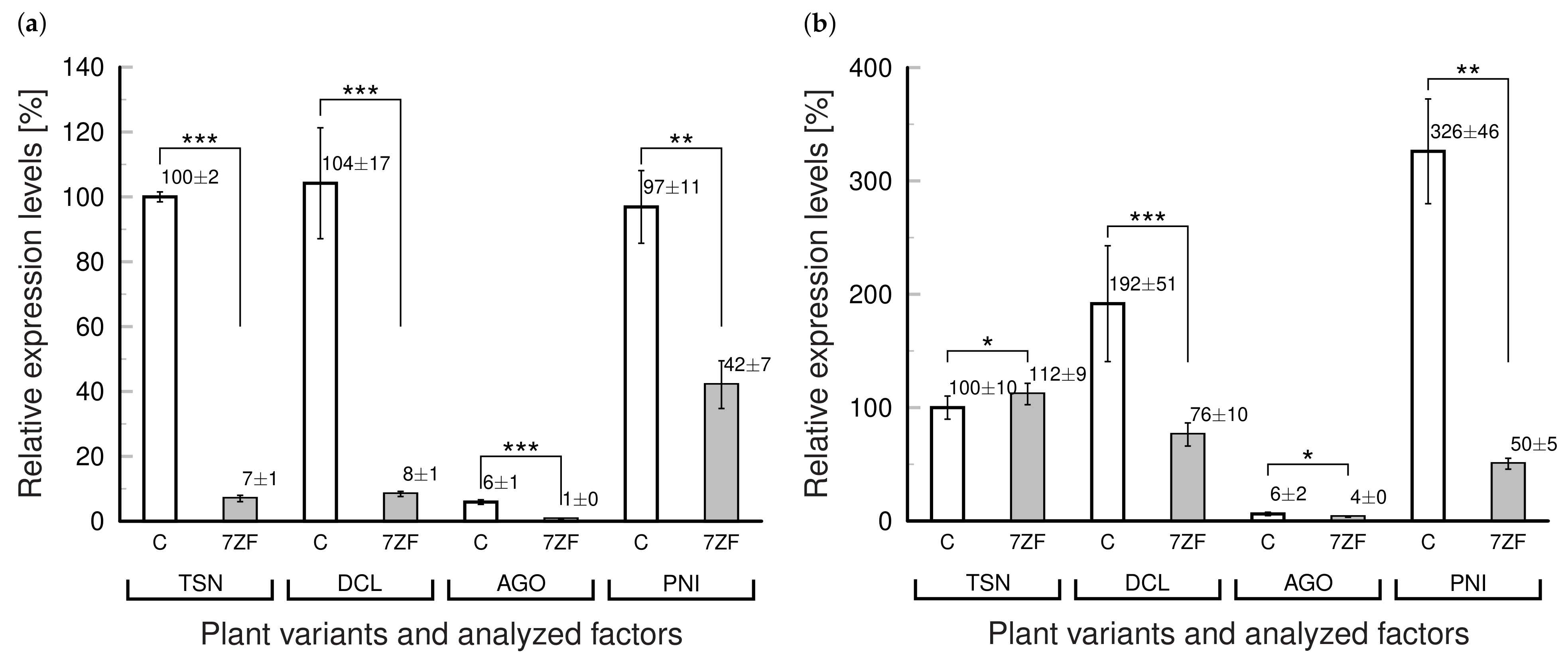
3.3. Transient Somatic Depression of Selected “Degradome” Due to NbTFIIIA-7ZF Overexpression
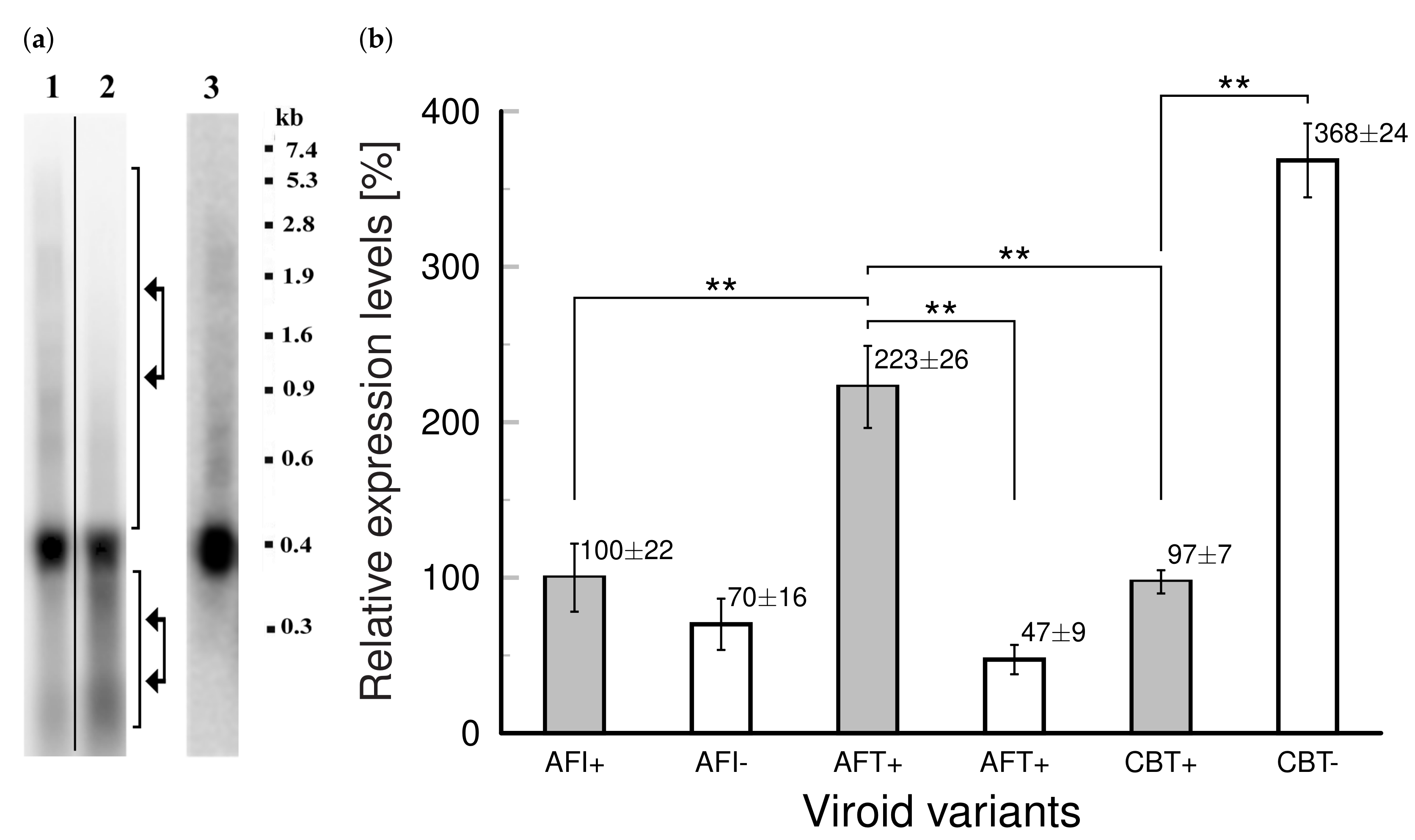
3.4. The “Degradation Complex” upon Viroid Infection in Tobacco Transformed with Viroid cDNAs and Potential Role of TFIIIA-7ZF in Viroid Adaptation and Pathogenesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shastry, B. Transcription factor IIIA (TFIIIA) in the second decade. J. Cell Sci. 1996, 109, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Engelke, D.; Ng, S.; Shastry, B.; Roeder, R. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell 1980, 19, 717–728. [Google Scholar] [CrossRef]
- Pelham, H.; Brown, D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc. Natl. Acad. Sci. USA 1980, 77, 4170–4174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Bannach, O.; Chen, H.; Teune, J.H.; Schmitz, A.; Steger, G.; Xiong, L.; Barbazuk, W. Alternative splicing of anciently exonized 5S rRNA regulates plant transcription factor TFIIIA. Genome Res. 2009, 19, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Hammond, M.; Wachter, A.; Breaker, R. A plant 5S ribosomal RNA mimic regulates alternative splicing of transcription factor IIIA pre-mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layat, E.; Cotterell, S.; Vaillant, I.; Yukawa, Y.; Tutois, S.; Tourmente, S. Transcript levels, alternative splicing and proteolytic cleavage of TFIIIA control 5S rRNA accumulation during Arabidopsis thaliana development. Plant J. 2012, 71, 35–44. [Google Scholar] [CrossRef]
- Dissanayaka Mudiyanselage, S.; Qu, J.; Tian, N.; Jiang, J.; Wang, Y. Potato spindle tuber viroid RNA-templated transcription: Factors and regulation. Viruses 2018, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Navarro, B.; Flores, R.; Di Serio, F. Advances in viroid-host interactions. Annu. Rev. Virol. 2021, 8, 305–325. [Google Scholar] [CrossRef]
- Venkataraman, S.; Badar, U.; Shoeb, E.; Hashim, G.; AbouHaidar, M.; Hefferon, K. An inside look into biological miniatures: Molecular mechanisms of viroids. Int. J. Mol. Sci. 2021, 22, 2795. [Google Scholar] [CrossRef]
- Hadidi, A.; Randles, J.; Flores, R.; Palukaitis, P. (Eds.) Viroids and Satellites; Academic Press, Elsevier: Cambridge, MA, USA, 2017. [Google Scholar]
- Matoušek, J.; Steinbachová, L.; Drábková, L.; Kocábek, T.; Potěšil, D.; Mishra, A.; Honys, D.; Steger, G. Elimination of viroids from tobacco pollen involves a decrease in propagation rate and an increase of the degradation processes. Int. J. Mol. Sci. 2020, 21, 3029. [Google Scholar] [CrossRef] [PubMed]
- Steinbachová, L.; Matoušek, J.; Steger, G.; Matoušková, H.; Radišek, S.; Honys, D. Transformation of seed non-transmissible hop viroids in Nicotiana benthamiana causes distortions in male gametophyte development. Plants 2021, 10, 2398. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, J.; Ji, S.; Wallace, A.; Wu, J.; Li, Y.; Gopalan, V.; Ding, B. A land plant-specific transcription factor directly enhances transcription of a pathogenic noncoding RNA template by DNA-dependent RNA polymerase II. Plant Cell 2016, 28, 1094–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Smith, H.; Ren, D.; Dissanayaka Mudiyanselage, S.; Dawe, A.; Wang, L.; Wang, Y. Potato spindle tuber viroid modulates its replication through a direct interaction with a splicing regulator. J. Virol. 2018, 92, e01004-18. [Google Scholar] [CrossRef] [Green Version]
- Matoušek, J.; Kozlová, P.; Orctová, L.; Schmitz, A.; Pešina, K.; Bannach, O.; Diermann, D.; Steger, G.; Riesner, D. Accumulation of viroid-specific small RNAs and increase of nucleolytic activities linked to viroid-caused pathogenesis. Biol. Chem. 2007, 388, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tupý, J.; Süss, J.; Hrabětová, E.; Říhova, L. Developmental changes in gene expression during pollen differentiation and maturation in Nicotiana tabacum L. Biol. Plant. 1983, 25, 231. [Google Scholar] [CrossRef]
- Jim Haseloff, J.; Siemering, K.; Prasher, D.; Hodge, S. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 1997, 94, 2122–2127. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, M.; Voinnet, O.; Baulcombe, D. Initiation and maintenance of virus-induced gene silencing. Plant. Cell 1998, 10, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Horsch, R.; Fry, J.; Hoffmann, N.; Wallroth, M.; Eichholtz, D.; Rogers, S.; Fraley, R. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef]
- Matoušek, J.; Siglová, K.; Jakše, J.; Radišek, S.; Brass, J.; Tsushima, T.; Guček, T.; Duraisamy, G.; Sano, T.; Steger, G. Propagation and some physiological effects of Citrus bark cracking viroid and Apple fruit crinkle viroid in multiple infected hop (Humulus lupulus L.). J. Plant Physiol. 2017, 213, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Matoušek, J.; Schröder, A.; Trěná, L.; Reimers, M.; Baumstark, T.; Dědič, P.; Vlasak, J.; Becker, I.; Kreuzaler, F.; Fladung, M.; et al. Inhibition of viroid infection by antisense RNA expression in transgenic plants. Biol. Chem. Hoppe-Seyler 1994, 375, 765–777. [Google Scholar] [CrossRef]
- Palukaitis, M.; Cotts, S.; Zaitlin, M. Detection and identification of viroids and viral nucleic acids by “dot-blot” hybridization. Acta Hortic. 1985, 164, 109–118. [Google Scholar] [CrossRef]
- Pfaffl, M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Chao, J.; Zhang, Z.; Wang, W.; Guo, Y. NAC family transcription factors in tobacco and their potential role in regulating leaf senescence. Front. Plant. Sci. 2018, 9, 1900. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoušek, J.; Kocábek, T.; Patzak, J.; Füssy, Z.; Procházková, J.; Heyerick, A. Combinatorial analysis of lupulin gland transcription factors from R2R3Myb, bHLH and WDR families indicates a complex regulation of chs_H1 genes essential for prenylflavonoid biosynthesis in hop (Humulus lupulus L.). BMC Plant Biol. 2012, 12, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koval’, T.; Lipovová, P.; Podzimek, T.; Matoušek, J.; Dušková, J.; Skálová, T.; Štěpánková, A.; Hašek, J.; Dohnálek, J. Crystallization of recombinant bifunctional nuclease TBN1 from tomato. Acta Cryst. D 2013, 69, 1192. [Google Scholar] [CrossRef]
- Podzimek, T.; Matoušek, J.; Lipovová, P.; Poučková, P.; Spiwok, V.; Santrůček, J. Biochemical properties of three plant nucleases with anticancer potential. Plant. Sci. 2011, 180, 343–351. [Google Scholar] [CrossRef]
- Ito, J.; Fukuda, H. ZEN1 is a key enzyme in the degradation of nuclear DNA during programmed cell death of tracheary elements. Plant Cell 2002, 14, 3201–3211. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Amador, M.; Abler, M.; De Rocher, E.; Thompson, D.; van Hoof, A.; LeBrasseur, N.; Lers, A.; Green, P. Identification of BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. Plant. Physiol. 2000, 122, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Farage-Barhom, S.; Burd, S.; Sonego, L.; Mett, A.; Belausov, E.; Gidoni, D.; Lers, A. Localization of the Arabidopsis senescence- and cell death-associated BFN1 nuclease: From the ER to fragmented nuclei. Mol. Plant. 2011, 4, 1062–1073. [Google Scholar] [CrossRef] [Green Version]
- Matoušek, J.; Tupý, J. Developmental changes in nuclease and other phosphohydrolase activities in anthers of Nicotiana tabacum L. J. Plant Physiol. 1987, 129, 351–362. [Google Scholar] [CrossRef]
- Matoušek, J.; Orctová, L.; Škopek, J.; Pešina, K.; Steger, G. Elimination of hop latent viroid upon developmental activation of pollen nucleases. Biol. Chem. 2008, 389, 905–918. [Google Scholar] [CrossRef]
- Matoušek, J.; Tupý, J. The release of nucleases from tobacco pollen. Plant Sci. Lett. 1983, 30, 83–89. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.; Perreault, J. Current overview on viroid-host interactions. Wiley Interdiscip. Rev. RNA 2020, 11, e1570. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Navarro, B.; Delgado, S.; Serra, P.; Di Serio, F. Viroid pathogenesis: A critical appraisal of the role of RNA silencing in triggering the initial molecular lesion. FEMS Microbiol. Rev. 2020, 44, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Minoia, S.; Navarro, B.; Delgado, S.; Di Serio, F.; Flores, R. Viroid RNA turnover: Characterization of the subgenomic RNAs of potato spindle tuber viroid accumulating in infected tissues provides insights into decay pathways operating in vivo. Nucleic Acids Res. 2015, 43, 2313–2325. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Beltran, E.; Moschou, P.; Smertenko, A.; Bozhkov, P. Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell 2015, 27, 926–943. [Google Scholar] [CrossRef] [Green Version]
- Nohales, M.Á.; Flores, R.; Darós, J. Viroid RNA redirects host DNA ligase 1 to act as an RNA ligase. Proc. Natl. Acad. Sci. USA 2012, 109, 13805–13810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoušek, J.; Piernikarczyk, R.; Týcová, A.; Duraisamy, G.; Kocábek, T.; Steger, G. Expression of SANT/HTH Myb mRNA, a plant morphogenesis-regulating transcription factor, changes due to viroid infection. J. Plant Physiol. 2015, 183, 85–94. [Google Scholar] [CrossRef]
- Eiras, M.; Nohales, M.; Kitajima, E.; Flores, R.; Daròs, J. Ribosomal protein L5 and transcription factor IIIA from Arabidopsis thaliana bind in vitro specifically Potato spindle tuber viroid RNA. Arch. Virol. 2010, 156, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Matoušek, J.; Kocábek, T.; Patzak, J.; Bříza, J.; Siglová, K.; Mishra, A.; Duraisamy, G.; Týcová, A.; Ono, E.; Krofta, K. The “putative” role of transcription factors from HlWRKY family in the regulation of the final steps of prenylflavonid and bitter acids biosynthesis in hop (Humulus lupulus L.). Plant Mol. Biol. 2016, 92, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Patzak, J.; Henychová, A.; Matoušek, J. Developmental regulation of lupulin gland-associated genes in aromatic and bitter hops (Humulus lupulus L.). BMC Plant Biol. 2021, 21, 534. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matoušek, J.; Steger, G. The Splicing Variant TFIIIA-7ZF of Viroid-Modulated Transcription Factor IIIA Causes Physiological Irregularities in Transgenic Tobacco and Transient Somatic Depression of “Degradome” Characteristic for Developing Pollen. Cells 2022, 11, 784. https://doi.org/10.3390/cells11050784
Matoušek J, Steger G. The Splicing Variant TFIIIA-7ZF of Viroid-Modulated Transcription Factor IIIA Causes Physiological Irregularities in Transgenic Tobacco and Transient Somatic Depression of “Degradome” Characteristic for Developing Pollen. Cells. 2022; 11(5):784. https://doi.org/10.3390/cells11050784
Chicago/Turabian StyleMatoušek, Jaroslav, and Gerhard Steger. 2022. "The Splicing Variant TFIIIA-7ZF of Viroid-Modulated Transcription Factor IIIA Causes Physiological Irregularities in Transgenic Tobacco and Transient Somatic Depression of “Degradome” Characteristic for Developing Pollen" Cells 11, no. 5: 784. https://doi.org/10.3390/cells11050784
APA StyleMatoušek, J., & Steger, G. (2022). The Splicing Variant TFIIIA-7ZF of Viroid-Modulated Transcription Factor IIIA Causes Physiological Irregularities in Transgenic Tobacco and Transient Somatic Depression of “Degradome” Characteristic for Developing Pollen. Cells, 11(5), 784. https://doi.org/10.3390/cells11050784






