Cell-Cell Interaction-Mediated Signaling in the Testis Induces Reproductive Dysfunction—Lesson from the Toxicant/Pharmaceutical Models
Abstract
1. Introduction
2. Unique Features of Cell Junctions in the Testis
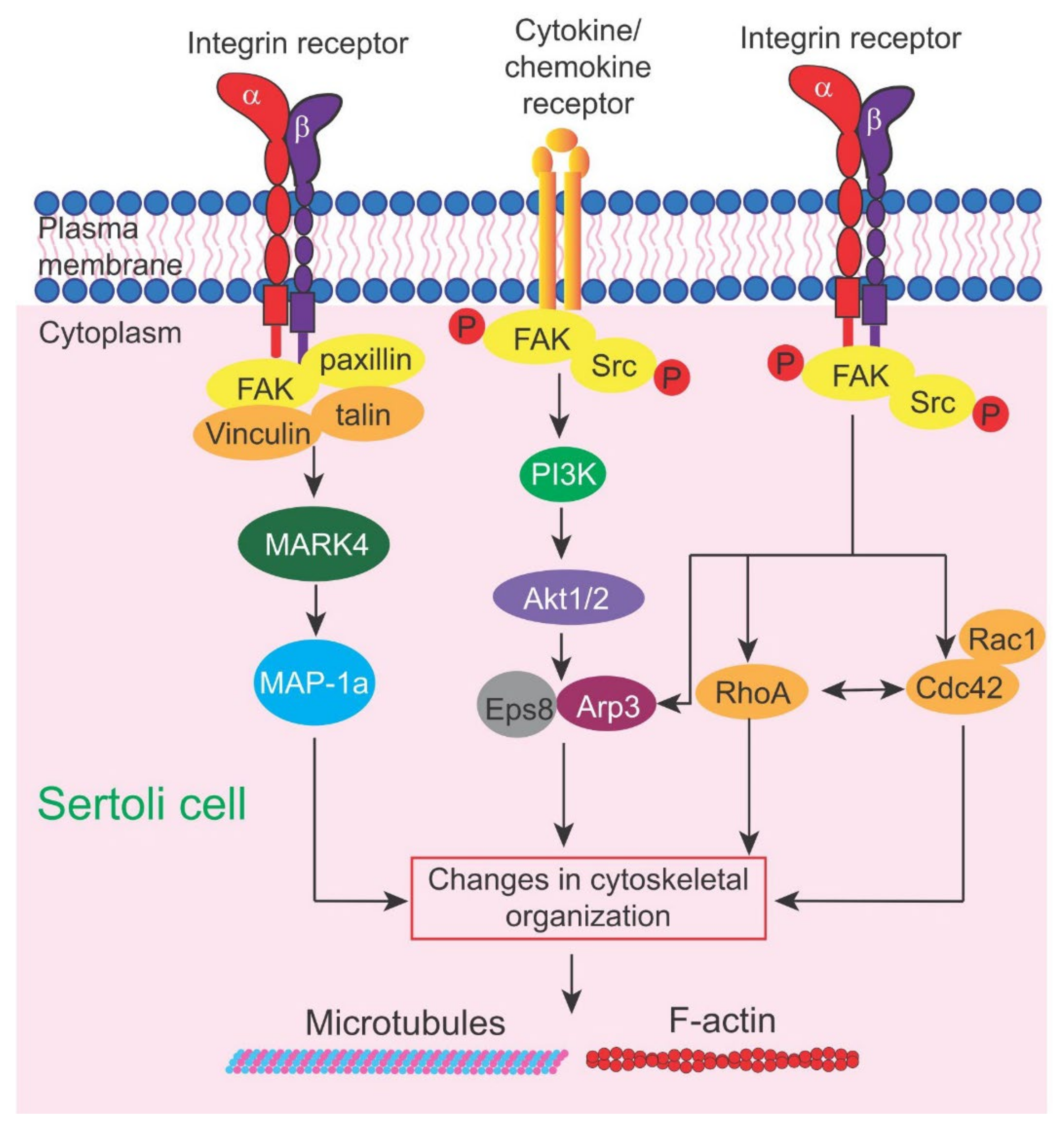
3. FAK (Focal Adhesion Kinase) and Small GTPase Cdc42
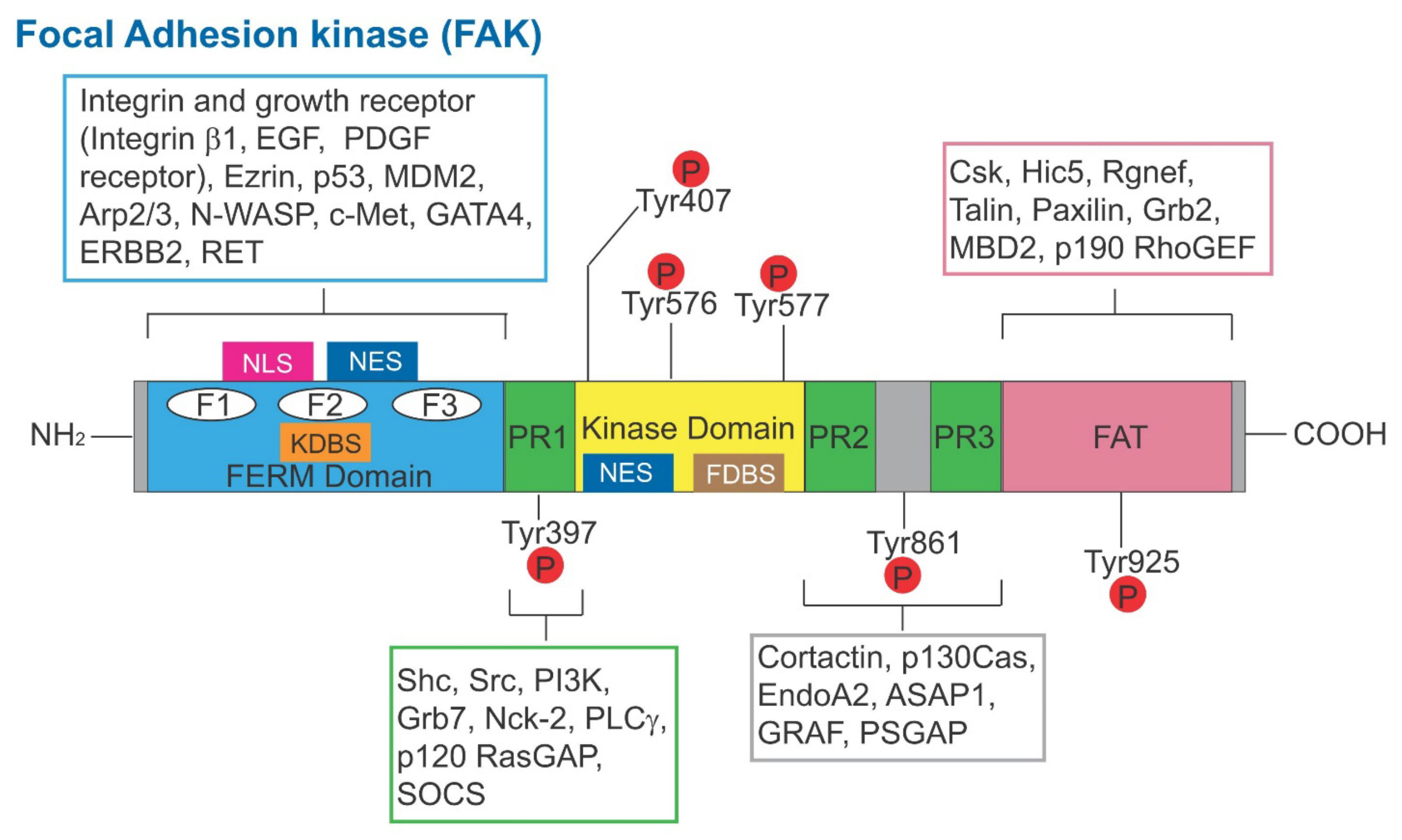
3.1. Focal Adhesion Kinase (FAK)
3.1.1. p-FAK-Y397
3.1.2. p-FAK-Y407
3.1.3. Potential Therapeutic Use of p-FAK-Y407E for Management of Toxicant-Induced Male Infertility
3.1.4. Additional Remarks—Possible Involvement of Akt1/2 Activation
3.2. Small GTPase Cdc42
4. Concluding Remarks and Future Perspectives
| Toxicant | Species | Tissue/Cell | Doses/Route | Observed Effects | Reference |
|---|---|---|---|---|---|
| Cadmium Chloride (CdCl2) | Rat | Testis | 3 mg/kg b.w., i.p. | Loss of occludin at the BTB in the epithelium | [32] |
| Rat | Testis | 3 mg/kg b.w., i.p. | Changes in spatial distribution of MAPs (MAP1a and CAMSAP2) in the seminiferous epithelium | [87] | |
| Rat | Testis | 3 mg/kg b.w., i.p. | CdCl2-induced BTB disruption, an increase in TGF-β2 and TGF-β3 (but not TGF-β1) and p-p38 -MAPK, a down-regulation of occludin and ZO-1 | [78] | |
| Rat | Testis | 3 mg/kg b.w., i.p. | Down-regulates the expression of efflux (e.g., P-glycoprotein, Mrp1, Abcg1) and influx (e.g., Oatp3, Slc15a1, Scl39a8) drug transporters | [141] | |
| Mouse | Testis | 2 mg/kg b.w., i.p. | Induces germ cell apoptosis in testes | [142] | |
| Rat | Testis | 2 mg/kg b.w., i.p. | Reduces body weight and testes weight, increases malondialdehyde content, reduces superoxide dismutase, glutathione peroxidase, catalase, and glutathione contents | [143] | |
| Rat | Testis | 3 mg/kg b.w., i.p. | Induces epithelial damage (e.g., edema), disorganization of collagen fibers, microvascular damage | [144] | |
| Rat | Sertoli Cell | 3 μM | Perturbs TJ barrier, induces occludin endocytosis in parallel with FAK and ZO-1 | [38] | |
| Rat | Sertoli Cell | 5–10 μM | Perturbs TJ assembly dose-dependently without any apparent cytotoxicity | [40] | |
| Rat | Sertoli Cell | 0.1–5 μM | Perturbs Sertoli cell TJ barrier dose dependently | [40] | |
| Human | Human Sertoli cell | 0.5–20 μM | Induces truncation actin filaments via disruptive distribution of Eps8 and Arp3 | [86] | |
| Perfluoro-octanesulfonate (PFOS) | Rat | Sertoli Cell | 10–20 μM | Induces Sertoli cell TJ barrier disruption mediated by a reduced expression of p-FAK-Tyr407 and Cx43, F-actin disorganization and impaired GJ intercellular communication, mislocalization of proteins at the cell-cell interface | [41] |
| Rat | Sertoli Cell | 10, 20, 50 μM | Induces Sertoli cell injury by perturbing TJ barrier, disorganization of actin cytoskeleton due to mis-localization of Arp3 and palladin, mis-distribution of BTB-associated proteins, downregulation of p-Akt1-S473 and p-Akt2-S474. | [84] | |
| Rat | Sertoli Cell | 20–40 μM | Induces Sertoli cell injury through truncation of actin filaments and MTs, which can be rescued by overexpressing p-FAK-Y407E mutant | [84] | |
| Rat | Sertoli Cell | 20 μM | Perturbs Sertoli cell TJ barrier, causing disruption of actin filaments in cell cytosol, perturbing the localization of cell junction proteins, reducing expression of GJ protein Cx43 | [145] | |
| Rat | Sertoli Cell/Gonocyte Cocultures | 0, 1, 10, 50, and 100 μM | Reduces cell viability, induces reactive oxygen species (ROS) production dose-dependently and disrupts organization of vimentin and actin filaments | [146] | |
| Mouse | Testis Sertoli Cell | 0.25–50 mg/kg/day (oral gavage) 10–30 μM | Reduces sperm count, induces Sertoli cell injury via an increase in vacuolization in Sertoli cells in seminiferous epithelium, disruptive changes in BTB ultrastructure leading to disassembly based on studies in vivo; perturbs Sertoli TJ barrier function, induces mis-distribution of BTB-associated proteins at the cell-cell interface, and increases expression of activated p38-MAPK and Erk1/2 | [37] |
Author Contributions
Funding
Conflicts of Interest
References
- Denz, F.A. Myoneural junctions and toxic agents. J. Pathol. Bacteriol. 1951, 63, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, J.H.; Killos, P.J.; Harrison, C.S. Effects of puffer poison on neuromuscular transmission. J. Pharmacol. Exp. Ther. 1961, 133, 98–105. [Google Scholar] [PubMed]
- Bowman, W.C.; Rand, M.J. Actions of triethylcholine on neuromuscular transmission. Br. J. Pharmacol. Chemother. 1961, 17, 176–195. [Google Scholar] [CrossRef] [PubMed]
- Welsch, F.; Stedman, D.B. Inhibition of intercellular communication between normal human embryonal palatal mesenchyme cells by teratogenic glycol ethers. Environ. Heal. Perspect. 1984, 57, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Pogach, L.M.; Lee, Y.; Gould, S.; Giglio, W.; Meyenhofer, M.; Huang, H.F. Characterization of cis-platinum-induced Sertoli cell dysfunction in rodents. Toxicol. Appl. Pharmacol. 1989, 98, 350–361. [Google Scholar] [CrossRef]
- Murthy, R.C.; Saxena, D.K.; Gupta, S.K.; Chandra, S.V. Ultrastructural observations in testicular tissue of chromium-treated rats. Reprod. Toxicol. 1991, 5, 443–447. [Google Scholar] [CrossRef]
- Steinberger, A.; Klinefelter, G. Sensitivity of Sertoli and leydig cells to xenobiotics in in vitro models. Reprod. Toxicol. 1993, 7, 23–37. [Google Scholar] [CrossRef]
- Janecki, A.; Jakubowiak, A.; Steinberger, A. Effect of cadmium chloride on transepithelial electrical resistance of sertoli cell monolayers in two-compartment cultures—A new model for toxicological investigations of the “Blood-testis” barrier in vitro. Toxicol. Appl. Pharmacol. 1992, 112, 51–57. [Google Scholar] [CrossRef]
- Wong, E.; Yan, H.; Li, M.; Lie, P.; Mruk, D.; Cheng, C. Cell Junctions in the Testis as Targets for Toxicants. In Comprehensive Toxicology: Reproductive and Endocrine Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 11, pp. 167–188. [Google Scholar] [CrossRef]
- Cyr, D.G.; Dufresne, J.; Gregory, M. Cellular junctions in the epididymis, a critical parameter for understanding male reproductive toxicology. Reprod. Toxicol. 2018, 81, 207–219. [Google Scholar] [CrossRef]
- Scarano, W.R.; Pinho, C.F.; Pissinatti, L.; Gonçalves, B.F.; Mendes, L.O.; Campos, S.G. Cell junctions in the prostate: An overview about the effects of Endocrine Disrupting Chemicals (EDCS) in different experimental models. Reprod. Toxicol. 2018, 81, 147–154. [Google Scholar] [CrossRef]
- Hejmej, A.; Bilinska, B. The effects of flutamide on cell-cell junctions in the testis, epididymis, and prostate. Reprod. Toxicol. 2018, 81, 1–16. [Google Scholar] [CrossRef]
- Mao, B.; Mruk, D.; Lian, Q.; Ge, R.; Li, C.; Silvestrini, B.; Cheng, C.Y. Mechanistic Insights into PFOS-Mediated Sertoli Cell Injury. Trends Mol. Med. 2018, 24, 781–793. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Wong, E.W.P.; Lie, P.P.Y.; Mruk, D.D.; Xiao, X.; Li, M.W.M.; Lui, W.-Y.; Lee, W.M. Polarity proteins and actin regulatory proteins are unlikely partners that regulate cell adhesion in the seminiferous epithelium during spermatogenesis. Histol. Histopathol. 2011, 26, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y. Toxicants target cell junctions in the testis: Insights from the indazole-carboxylic acid model. Spermatogenesis 2014, 4, e981485. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis 2014, 4, e979106. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef] [PubMed]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef]
- Boekelheide, K. Mechanisms of Toxic Damage to Spermatogenesis. JNCI Monogr. 2005, 2005, 6–8. [Google Scholar] [CrossRef]
- Boekelheide, K.; Neely, M.; Sioussat, T.M. The sertoli cell cytoskeleton: A target for toxicant-induced germ cell loss. Toxicol. Appl. Pharmacol. 1989, 101, 373–389. [Google Scholar] [CrossRef]
- Lee, J.; Richburg, J.H.; Younkin, S.C.; Boekelheide, K. The fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997, 138, 2081–2088. [Google Scholar] [CrossRef]
- Richburg, J.H.; Johnson, K.; Schoenfeld, H.A.; Meistrich, M.L.; Dix, D.J. Defining the cellular and molecular mechanisms of toxicant action in the testis. Toxicol. Lett. 2002, 135, 167–183. [Google Scholar] [CrossRef]
- Richburg, J.H.; Nañez, A.; Williams, L.R.; Embree, M.E.; Boekelheide, K. Sensitivity of Testicular Germ Cells to Toxicant-Induced Apoptosis in gld Mice That Express a Nonfunctional Form of Fas Ligand. Endocrinology 2000, 141, 787–793. [Google Scholar] [CrossRef]
- Boekelheide, K.; Lee, J.; Shipp, E.B.; Richburg, J.; Li, G. Expression of Fas system-related genes in the testis during development and after toxicant exposure. Toxicol. Lett. 1998, 102–103, 503–508. [Google Scholar] [CrossRef]
- Li, L.; Wine, R.N.; Miller, D.S.; Reece, J.M.; Smith, M.; Chapin, R. Protection against Methoxyacetic-Acid-Induced Spermatocyte Apoptosis with Calcium Channel Blockers in Cultured Rat Seminiferous Tubules: Possible Mechanisms. Toxicol. Appl. Pharmacol. 1997, 144, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Benoff, S.; Jacob, A.; Hurley, I.R. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Updat. 2000, 6, 107–121. [Google Scholar] [CrossRef]
- Ghanayem, B.I.; Chapin, R.E. Calcium channel blockers protect against ethylene glycol monomethyl ether (2-methoxyethanol)-induced testicular toxicity. Exp. Mol. Pathol. 1990, 52, 279–290. [Google Scholar] [CrossRef]
- Liu, B.; Shen, L.-J.; Zhao, T.-X.; Sun, M.; Wang, J.-K.; Long, C.-L.; He, D.-W.; Lin, T.; Wu, S.-D.; Wei, G.-H. Automobile exhaust-derived PM2.5 induces blood-testis barrier damage through ROS-MAPK-Nrf2 pathway in sertoli cells of rats. Ecotoxicol. Environ. Saf. 2019, 189, 110053. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lu, Z.; Li, D.; Zhang, L.; Wang, Z.; Du, Q.; Huang, Y.; Zhao, X.; Tong, D. Melamine causes testicular toxicity by destroying blood-testis barrier in piglets. Toxicol. Lett. 2018, 296, 114–124. [Google Scholar] [CrossRef]
- Jia, X.; Xu, Y.; Wu, W.; Fan, Y.; Wang, G.; Zhang, T.; Su, W. Aroclor1254 disrupts the blood-testis barrier by promoting endocytosis and degradation of junction proteins via p38 MAPK pathway. Cell Death Dis. 2017, 8, e2823. [Google Scholar] [CrossRef]
- Lui, W.Y.; Lee, W.M.; Cheng, C.Y. Transforming growth factor-b3 regulates the dynamics of sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol. Reprod. 2003, 68, 1597–1612. [Google Scholar] [CrossRef]
- Lui, W.Y.; Wong, C.H.; Mruk, D.D.; Cheng, C.Y. Tgf-b3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (map) kinase pathway: An in vivo study. Endocrinology 2003, 144, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2011, 64, 16–64. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Vogl, A.W.; Vaid, K.S.; Guttman, J.A. The sertoli cell cytoskeleton. Adv. Exp. Med. Biol. 2008, 636, 186–211. [Google Scholar]
- Lui, W.-Y.; Lee, W.M.; Cheng, C.Y. Sertoli-Germ Cell Adherens Junction Dynamics in the Testis Are Regulated by RhoB GTPase via the ROCK/LIMK Signaling Pathway. Biol. Reprod. 2003, 68, 2189–2206. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Zhang, X.; Zhang, Y.; Gu, J.; Chen, M.; Zhang, Z.; Wang, X.; Wang, S.-L. Sertoli Cell Is a Potential Target for Perfluorooctane Sulfonate–Induced Reproductive Dysfunction in Male Mice. Toxicol. Sci. 2013, 135, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Siu, E.R.; Wong, E.W.P.; Mruk, D.D.; Sze, K.L.; Porto, C.S.; Cheng, C.Y. An Occludin-Focal Adhesion Kinase Protein Complex at the Blood-Testis Barrier: A Study Using the Cadmium Model. Endocrinology 2009, 150, 3336–3344. [Google Scholar] [CrossRef]
- Wong, C.-H.; Mruk, D.D.; Siu, M.K.Y.; Cheng, C.Y. Blood-Testis Barrier Dynamics Are Regulated by α2-Macroglobulin via the c-Jun N-Terminal Protein Kinase Pathway. Endocrinology 2005, 146, 1893–1908. [Google Scholar] [CrossRef][Green Version]
- Chung, N.P.Y.; Cheng, C.Y. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology 2001, 142, 1878–1888. [Google Scholar] [CrossRef]
- Wan, H.-T.; Mruk, D.D.; Wong, C.K.C.; Cheng, C.Y. Perfluorooctanesulfonate (PFOS) Perturbs Male Rat Sertoli Cell Blood-Testis Barrier Function by Affecting F-Actin Organization via p-FAK-Tyr407: An in Vitro Study. Endocrinology 2014, 155, 249–262. [Google Scholar] [CrossRef]
- Wan, H.T.; Mruk, D.D.; Wong, C.K.C.; Cheng, C.Y. Targeting testis-specific proteins to inhibit spermatogenesis—Lesion from endocrine disrupting chemicals. Expert Opin. Ther. Targets 2013, 17, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H.; Cheng, C.Y. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: A review of recent data. Dev. Biol. 2005, 286, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.W.; Cheng, C.Y. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol. Sci. 2011, 32, 290–299. [Google Scholar] [CrossRef]
- Li, M.W.M.; Mruk, D.D.; Cheng, C.Y. Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 2009, 15, 159–168. [Google Scholar] [CrossRef]
- Ramos-Treviño, J.; Bassol-Mayagoitia, S.; Hernández-Ibarra, J.A.; Ruiz-Flores, P.; Nava-Hernández, M.P. Toxic Effect of Cadmium, Lead, and Arsenic on the Sertoli Cell: Mechanisms of Damage Involved. DNA Cell Biol. 2018, 37, 600–608. [Google Scholar] [CrossRef]
- Frame, M.C.; Patel, H.; Serrels, B.; Lietha, D.; Eck, M.J. The FERM domain: Organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 2010, 11, 802–814. [Google Scholar] [CrossRef]
- Baumann, K. FAK or talin: Who goes first? Nat. Rev. Mol. Cell Biol. 2012, 13, 139. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lie, P.P.Y.; Wong, E.W.P.; Mruk, D.D. Focal adhesion kinase and actin regulatory/binding proteins that modulate f-actin organization at the tissue barrier. Lession from the testis. Tissue Barriers 2013, 1, e24252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dym, M. Basement membrane regulation of sertoli cells. Endocr. Rev. 1994, 15, 102–115. [Google Scholar]
- Siu, M.K.; Cheng, C.Y. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays 2004, 26, 978–992. [Google Scholar] [CrossRef]
- Siu, M.K.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Adhering junction dynamics in the testis are regulated by an interplay of b1-integrin and focal adhesion complex (fac)-associated proteins. Endocrinology 2003, 144, 2141–2163. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, A.; Robertson, D.M.; O’Donnell, L. A complex containing a6b1-integrin and phosphorylated focal adhesion kinase between sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 2006, 190, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Lie, P.P.Y.; Mruk, D.D.; Mok, K.W.; Su, L.; Lee, W.M.; Cheng, C.Y. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc. Natl. Acad. Sci. USA 2012, 109, 12562–12567. [Google Scholar] [CrossRef] [PubMed]
- Vogl, A.W.; Pfeiffer, D.C.; Mulholland, D.; Kimel, G.; Guttman, J. Unique and Multifunctional Adhesion Junctions in the Testis. Ectoplasmic Specializations. Arch. Histol. Cytol. 2000, 63, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.W.P.; Mruk, D.D.; Cheng, C.Y. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem. Biophys. Acta 2008, 1778, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, S.; Wang, L.; Bu, T.; Wu, S.; Zhou, L.; Shi, J.; Wu, D.; Sun, F.; Cheng, C.Y. Role of laminin and collagen chains in human spermatogenesis—Insights from studies in rodents and scRNA-Seq transcriptome profiling. Semin. Cell Dev. Biol. 2021, 121, 125–132. [Google Scholar] [CrossRef]
- Li, M.W.M.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Disruption of the blood-testis barrier integrity by bisphenol a in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009, 41, 2302–2314. [Google Scholar] [CrossRef]
- Yao, P.-L.; Lin, Y.-C.; Richburg, J.H. Mono-(2-Ethylhexyl) Phthalate-Induced Disruption of Junctional Complexes in the Seminiferous Epithelium of the Rodent Testis Is Mediated by MMP2. Biol. Reprod. 2010, 82, 516–527. [Google Scholar] [CrossRef]
- Yao, P.L.; Lin, Y.C.; Richburg, J.H. Tnfa-mediated disruption of spermatogenesis in response to sertoli cell injury in rodents is partially regulated by mmp2. Biol. Reprod. 2009, 80, 581–589. [Google Scholar] [CrossRef]
- Hew, K.-W.; Heath, G.L.; Jiwa, A.H.; Welsh, M.J. Cadmium in Vivo Causes Disruption of Tight Junction-Associated Microfilaments in Rat Sertoli Cells. Biol. Reprod. 1993, 49, 840–849. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol. Metab. 2004, 15, 439–447. [Google Scholar] [CrossRef]
- Wolski, K.M.; Perrault, C.; Tran-Son-Tay, R.; Cameron, D.F. Strength Measurement of the Sertoli-Spermatid Junctional Complex. J. Androl. 2005, 26, 354–359. [Google Scholar] [CrossRef]
- Setchell, B.P. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 212–233. [Google Scholar]
- Pelletier, R.M. The blood-testis barrier: The junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011, 46, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Stanton, P.G. Regulation of the blood-testis barrier. Semin. Cell Dev. Biol. 2016, 59, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Cheng, C.Y. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr. Topics Dev. Biol. 2005, 71, 263–296. [Google Scholar]
- Wolski, K.M.; Mruk, D.D.; Cameron, D.F. The Sertoli-Spermatid Junctional Complex Adhesion Strength Is Affected In Vitro by Adjudin. J. Androl. 2006, 27, 790–794. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.; Silvestrini, B.; Bonanomi, M.; Wong, C.-H.; Siu, M.K.; Lee, N.P.; Lui, W.Y.; Mo, M.-Y. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception 2005, 72, 251–261. [Google Scholar] [CrossRef]
- Mok, K.W.; Mruk, D.D.; Lie, P.P.Y.; Lui, W.Y.; Cheng, C.Y. Adjudin, a potential male contraceptive, exerts its effects locally in the seminifeorus epithelium of mammalian testes. Reproduction 2011, 141, 571–580. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Wong, E.W.P.; Lie, P.P.Y.; Li, M.W.M.; Su, L.; Siu, E.R.; Yan, H.H.N.; Mannu, J.; Mathur, P.P.; Bonanomi, M.; et al. Environmental toxicants and male reproductive function. Spermatogenesis 2011, 1, 2–13. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis 2011, 1, 137–146. [Google Scholar] [CrossRef][Green Version]
- Hew, K.; Ericson, W.; Welsh, M. A Single Low Cadmium Dose Causes Failure of Spermiation in the Rat. Toxicol. Appl. Pharmacol. 1993, 121, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.; Kowalik, A.; Gallardi, R.; Egeler, O.; Clubb, B. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in sertoli cells. J. Androl. 2000, 21, 625–635. [Google Scholar]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-G.; Ahmed, K.; Zaidi, S.F.; Muhammad, J.S. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat. Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Wong, C.-H.; Mruk, D.D.; Lui, W.Y.; Cheng, C.Y. Regulation of blood-testis barrier dynamics: An in vivo study. J. Cell Sci. 2004, 117, 783–798. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.-O.; Boffetta, P.; Cole, P.; Starr, T.B.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 2014, 44, 1–81. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.-O.; Boffetta, P.; Wedner, H.J.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit. Rev. Toxicol. 2016, 46, 279–331. [Google Scholar] [CrossRef]
- Tsuda, S. Differential toxicity between perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). J. Toxicol. Sci. 2016, 41, SP27–SP36. [Google Scholar] [CrossRef]
- Fragki, S.; Dirven, H.; Fletcher, T.; Grasl-Kraupp, B.; Gützkow, K.B.; Hoogenboom, R.; Kersten, S.; Lindeman, B.; Louisse, J.; Peijnenburg, A.; et al. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: What do we know and what not? Crit. Rev. Toxicol. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, H.; Xiao, X.; Lui, W.-Y.; Lee, W.M.; Mruk, D.D.; Cheng, C.Y. Perfluorooctanesulfonate (PFOS)-induced Sertoli cell injury through a disruption of F-actin and microtubule organization is mediated by Akt1/2. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Mruk, D.D.; Xiao, X.; John, C.M.; Turek, P.J.; Lui, W.Y.; Lee, W.M.; Silvestrini, B.; Cheng, C.Y. Rescue of PFOS-induced human Sertoli cell injury by overexpressing a p-FAK-Y407E phosphomimetic mutant. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Xiao, X.; Mruk, D.D.; Tang, E.I.; Wong, C.K.C.; Lee, W.M.; John, C.M.; Turek, P.J.; Silvestrini, B.; Cheng, C.Y. Environmental toxicants perturb human Serotli cell adhesive function via changes in f-actin organization medicated by actin regulatory proteins. Hum. Reprod. 2014, 29, 1279–1291. [Google Scholar] [CrossRef]
- Wang, L.; Yan, M.; Li, H.; Wu, S.; Ge, R.; Wong, C.K.C.; Silvestrini, B.; Sun, F.; Cheng, C.Y. The Non-hormonal Male Contraceptive Adjudin Exerts its Effects via MAPs and Signaling Proteins mTORC1/rpS6 and FAK-Y. Endocrinology 2020, 162. [Google Scholar] [CrossRef]
- Su, W.; Cheng, C.Y. Cdc42 is involved in NC1 peptide–regulated BTB dynamics through actin and microtubule cytoskeletal reorganization. FASEB J. 2019, 33, 14461–14478. [Google Scholar] [CrossRef]
- Wong, E.W.P.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Regulation of blood-testis barrier dynamics by tgf-b3 is a cdc42-dependent protein trafficking event. Proc. Natl. Acad. Sci. USA 2010, 107, 11399–11404. [Google Scholar] [CrossRef]
- Hicks-Berthet, J.; Varelas, X. Integrin-FAK-CDC42-PP1A signaling gnaws at YAP/TAZ activity to control incisor stem cells. BioEssays 2017, 39, 1700116. [Google Scholar] [CrossRef]
- A Wozniak, M.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta Bioenerg. 2004, 1692, 103–119. [Google Scholar] [CrossRef]
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev. 2009, 20, 329–338. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Nicholls, P.K.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.-T.; Mruk, D.D.; Li, S.Y.T.; Mok, K.-W.; Lee, W.M.; Wong, C.K.C.; Cheng, C.Y. p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am. J. Physiol. Metab. 2013, 305, E687–E699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lie, P.P.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009, 23, 2555–2567. [Google Scholar] [CrossRef]
- Lie, P.P.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytoskeletal dynamics and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1581–1592. [Google Scholar] [CrossRef]
- Inagaki, M.; Irie, K.; Ishizaki, H.; Tanaka-Okamoto, M.; Miyoshi, J.; Takai, Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells 2006, 11, 1125–1132. [Google Scholar] [CrossRef]
- Ozaki-Kuroda, K.; Nakanishi, H.; Ohta, H.; Tanaka, H.; Kurihara, H.; Mueller, S.; Irie, K.; Ikeda, W.; Sakai, T.; Wimmer, E.; et al. Nectin Couples Cell-Cell Adhesion and the Actin Scaffold at Heterotypic Testicular Junctions. Curr. Biol. 2002, 12, 1145–1150. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian microRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Linsen, S.E.; de Wit, E.; de Bruijn, E.; Cuppen, E. Small RNA expression and strain specificity in the rat. BMC Genom. 2010, 11, 249. [Google Scholar] [CrossRef]
- Chen, H.; Duo, Y.; Hu, B.; Wang, Z.; Zhang, F.; Tsai, H.; Zhang, J.; Zhou, L.; Wang, L.; Wang, X.; et al. PICT-1 triggers a pro-death autophagy through inhibiting rRNA transcription and AKT/mTOR/p70S6K signaling pathway. Oncotarget 2016, 7, 78747–78763. [Google Scholar] [CrossRef]
- Di Fiore, P.P. and G. Scita. Eps8 in the midst of gtpases. Int. J. Biochem. Cell Biol. 2002, 34, 1178–1183. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: Novel insights from studies on eps8 and arp3. Biochem. J. 2011, 435, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Lee, M.; Higgs, H.N. Multiple roles for actin in secretory and endocytic pathways. Curr. Biol. 2021, 31, R603–R618. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.I.; Mok, K.-W.; Lee, W.M.; Cheng, C.Y. EB1 regulates tubulin and actin cytoskeletal networks at the sertoli cell blood-testis barrier in male rats: An in vitro study. Endocrinology 2014, 156, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Basson, M.D. Akt directly regulates focal adhesion kinase through association and serine phosphorylation: Implication for pressure-induced colon cancer metastasis. Am. J. Physiol. Physiol. 2011, 300, C657–C670. [Google Scholar] [CrossRef]
- Wang, S.; Basson, M.D. Protein kinase B/AKT and focal adhesion kinase: Two close signaling partners in cancer. Anti-Cancer Agents Med. Chem. 2011, 11, 993–1002. [Google Scholar] [CrossRef]
- Zheng, Y.; Gierut, J.; Wang, Z.; Miao, J.; Asara, J.M.; Tyner, A.L. Protein tyrosine kinase 6 protects cells from anoikis by directly phosphorylating focal adhesion kinase and activating AKT. Oncogene 2012, 32, 4304–4312. [Google Scholar] [CrossRef]
- Jo, H.; Mondal, S.; Tan, D.; Nagata, E.; Takizawa, S.; Sharma, A.K.; Hou, Q.; Shanmugasundaram, K.; Prasad, A.; Tung, J.K.; et al. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. USA 2012, 109, 10581–10586. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef]
- Murphy, N.P.; Mott, H.R.; Owen, D. Progress in the therapeutic inhibition of Cdc42 signalling. Biochem. Soc. Trans. 2021, 49, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Combedazou, A.; Gayral, S.; Colombié, N.; Fougerat, A.; Laffargue, M.; Ramel, D. Small GTPases orchestrate cell-cell communication during collective cell movement. Small GTPases 2017, 11, 103–112. [Google Scholar] [CrossRef]
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701. [Google Scholar] [CrossRef]
- Takai, Y.; Sasaki, T.; Matozaki, T. Small gtp-binding proteins. Physiol. Rev. 2001, 81, 153–208. [Google Scholar] [CrossRef] [PubMed]
- Salloum, G.; Jaafar, L.; El-Sibai, M. Rho A and Rac1: Antagonists moving forward. Tissue Cell 2020, 65. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Díaz, C.; Baonza, G.; Martín-Belmonte, F. The vertebrate epithelial apical junctional complex: Dynamic interplay between Rho GTPase activity and cell polarization processes. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183398. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015, 36, 103–112. [Google Scholar] [CrossRef]
- Czuchra, A.; Wu, X.; Meyer, H.; Van Hengel, J.; Schroeder, T.; Geffers, R.; Rottner, K.; Brakebusch, C. Cdc42 Is Not Essential for Filopodium Formation, Directed Migration, Cell Polarization, and Mitosis in Fibroblastoid Cells. Mol. Biol. Cell 2005, 16, 4473–4484. [Google Scholar] [CrossRef]
- Myers, J.P.; Robles, E.; Ducharme-Smith, A.; Gomez, T.M. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J. Cell Sci. 2012, 125, 2918–2929. [Google Scholar] [CrossRef]
- Kumar, B.; Chandran, B. KSHV Entry and Trafficking in Target Cells—Hijacking of Cell Signal Pathways, Actin and Membrane Dynamics. Viruses 2016, 8, 305. [Google Scholar] [CrossRef]
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef]
- Chen, H.; Mruk, D.D.; Lee, W.M.; Cheng, Y. Regulation of spermatogenesis by a local functional axis in the testis: Role of the basement membrane–derived noncollagenous 1 domain peptide. FASEB J. 2017, 31, 3587–3607. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Wu, S.; Li, L.; Ge, R.; Cheng, C.Y. NC1-peptide regulates spermatogenesis through changes in cytoskeletal organization mediated by EB1. FASEB J. 2020, 34, 3105–3128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Wu, S.; Ge, R.; Cheng, C.Y. NC1-Peptide From Collagen α3 (IV) Chains in the Basement Membrane of Testes Regulates Spermatogenesis via p-FAK-Y. Endocrinology 2020, 161. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Takashima, S.; Kanatsu-Shinohara, M.; Yi, Z.; Shinohara, T. Cdc42 is required for male germline niche development in mice. Cell Rep. 2021, 36. [Google Scholar] [CrossRef]
- Heinrich, A.; Bhandary, B.; Potter, S.J.; Ratner, N.; DeFalco, T. Cdc42 activity in Sertoli cells is essential for maintenance of spermatogenesis. Cell Rep. 2021, 37. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Wang, L.; Wu, S.; Lv, L.; Tahir, A.; Xiao, X.; Wong, C.K.C.; Sun, F.; Ge, R.; et al. Role of cell polarity and planar cell polarity (PCP) proteins in spermatogenesis. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mao, B.; Wu, S.; Lian, Q.; Ge, R.-S.; Silvestrini, B.; Cheng, C.Y. Regulation of spermatid polarity by the actin- and microtubule (MT)-based cytoskeletons. Semin. Cell Dev. Biol. 2018, 81, 88–96. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the properties of sperm protamine-like II protein after exposure of Mytilus galloprovincialis (Lamarck 1819) to sub-toxic doses of cadmium. Ecotoxicol. Environ. Saf. 2018, 169, 600–606. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; D’Andrea, S.; Cordeschi, G.; Totaro, M.; Parisi, A.; Di Emidio, G.; Tatone, C.; Francavilla, S.; Barbonetti, A. Pathophysiology of Mitochondrial Dysfunction in Human Spermatozoa: Focus on Energetic Metabolism, Oxidative Stress and Apoptosis. Antioxidants 2021, 10, 695. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef] [PubMed]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.-M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, Z.; Shan, G.; Chu, L.-T.; Huang, Z.; Moskovtsev, S.; Librach, C.; Jarvi, K.; Sun, Y. Advances in sperm analysis: Techniques, discoveries and applications. Nat. Rev. Urol. 2021, 18, 447–467. [Google Scholar] [CrossRef]
- Su, L.; Mruk, D.D.; Cheng, C.Y. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis 2012, 2, 285–293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, G.-X.; Zhu, H.-L.; Shi, X.-T.; Nan, Y.; Liu, W.-B.; Dai, L.-M.; Xiong, Y.-W.; Yi, S.-J.; Cao, X.-L.; Xu, D.-X.; et al. Autophagy in Sertoli cell protects against environmental cadmium-induced germ cell apoptosis in mouse testes. Environ. Pollut. 2020, 270, 116241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Wang, K.; Yang, Z.; Liu, Z. Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ. Sci. Pollut. Res. 2020, 27, 25278–25286. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M.; Legáth, J. Changes in the Peritubular Tissue of Rat Testis after Cadmium Treatment. Biol. Trace Element Res. 2009, 134, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mruk, L.D.; Chen, H.; Wong, C.K.C.; Lee, W.M.; Cheng, C.Y. Rescue of perfluorooctanesulfonate (PFOS)-mediated Sertoli cell injury by overexpression of gap junction protein connexin. Sci. Rep. 2016, 6, 29667. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, J.; Zhu, H.; Li, C.; Wu, Q. PFOS and PCB 153 have direct adverse effects on neonatal testis modeled using a coculture of primary gonocyte and Sertoli cells. Environ. Toxicol. 2011, 28, 322–331. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Bu, T.; Wu, X.; Gao, S.; Li, X.; De Jesus, A.B.; Wong, C.K.C.; Chen, H.; Chung, N.P.Y.; Sun, F.; et al. Cell-Cell Interaction-Mediated Signaling in the Testis Induces Reproductive Dysfunction—Lesson from the Toxicant/Pharmaceutical Models. Cells 2022, 11, 591. https://doi.org/10.3390/cells11040591
Wang L, Bu T, Wu X, Gao S, Li X, De Jesus AB, Wong CKC, Chen H, Chung NPY, Sun F, et al. Cell-Cell Interaction-Mediated Signaling in the Testis Induces Reproductive Dysfunction—Lesson from the Toxicant/Pharmaceutical Models. Cells. 2022; 11(4):591. https://doi.org/10.3390/cells11040591
Chicago/Turabian StyleWang, Lingling, Tiao Bu, Xiaolong Wu, Sheng Gao, Xinyao Li, Angela Bryanne De Jesus, Chris K. C. Wong, Hao Chen, Nancy P. Y. Chung, Fei Sun, and et al. 2022. "Cell-Cell Interaction-Mediated Signaling in the Testis Induces Reproductive Dysfunction—Lesson from the Toxicant/Pharmaceutical Models" Cells 11, no. 4: 591. https://doi.org/10.3390/cells11040591
APA StyleWang, L., Bu, T., Wu, X., Gao, S., Li, X., De Jesus, A. B., Wong, C. K. C., Chen, H., Chung, N. P. Y., Sun, F., & Cheng, C. Y. (2022). Cell-Cell Interaction-Mediated Signaling in the Testis Induces Reproductive Dysfunction—Lesson from the Toxicant/Pharmaceutical Models. Cells, 11(4), 591. https://doi.org/10.3390/cells11040591








