Hereditary Ovarian Carcinoma: Cancer Pathogenesis Looking beyond BRCA1 and BRCA2
Abstract
1. Introduction
2. Pathogenesis in Hereditary Ovarian Cancer: Insights from Risk-Reducing Surgery Specimens
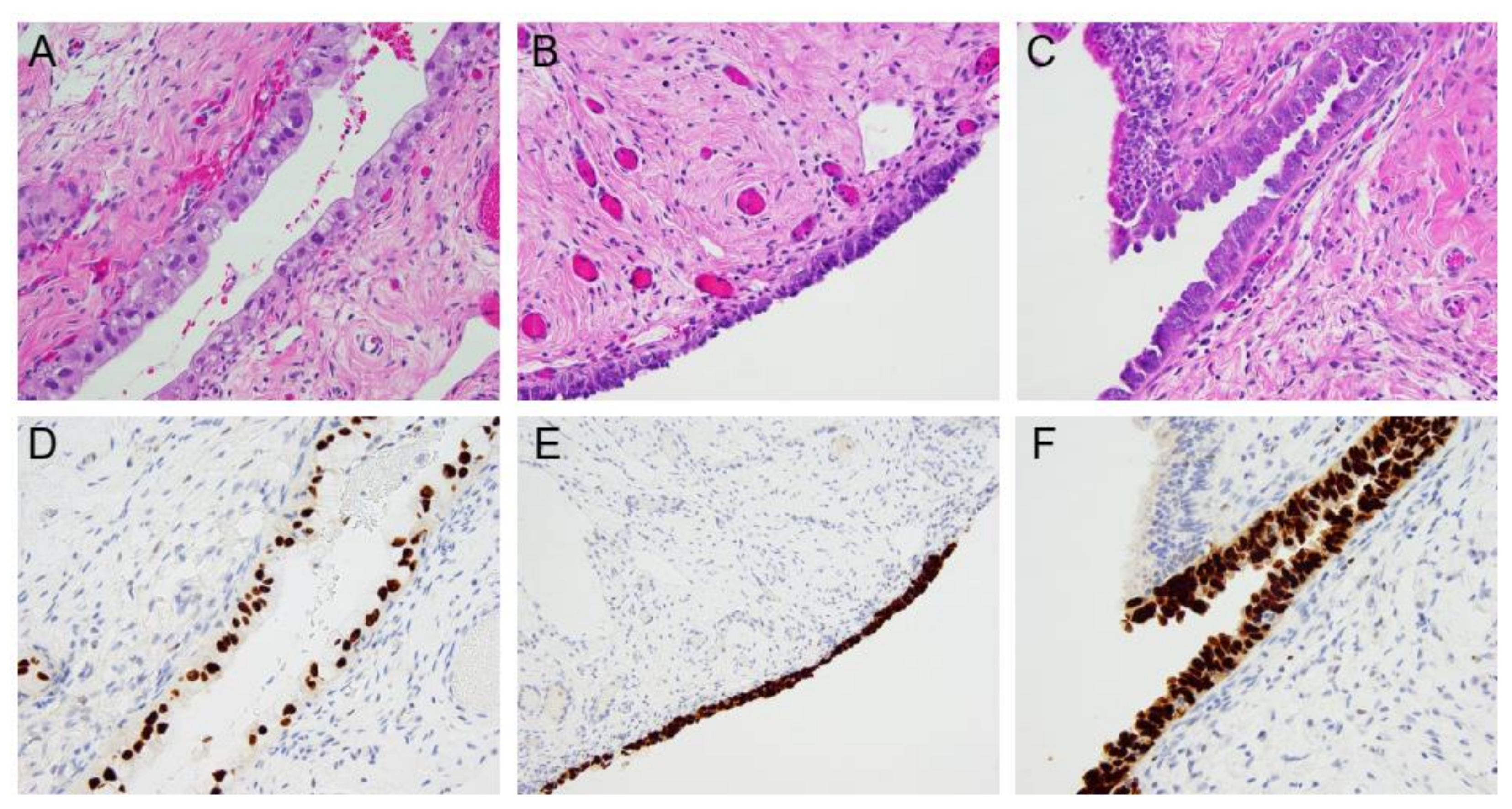
3. Types of Preneoplastic Fallopian Tube Lesions and Significance
4. Incidence of Preneoplastic Lesions and Occult Carcinoma in HOC Mutation Carriers
5. Clinical Management of Isolated Pre-Invasive Lesions in Hereditary Ovarian Cancer Mutation Carriers
6. Incidence of Ovarian Carcinoma with Rare HOC Susceptibility Mutations
6.1. BARD1
6.2. PALB2
6.3. ATM
6.4. NBN
6.5. MRE11
6.6. RAD50
7. Strategies for Risk-Reduction in HOC Mutation Carriers
8. Ongoing Clinical Trials
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, T.; Casadei, S.; Lee, M.K.; Pennil, C.C.; Nord, A.S.; Thornton, A.M.; Roeb, W.; Agnew, K.J.; Stray, S.M.; Wickramanayake, A.; et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. USA. 2011, 108, 18032–18037. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.M.; Selle, F.; Hilpert, F.; Reuss, A.; Savarese, A.; Vergote, I.; Witteveen, P.; Bamias, A.; Scotto, N.; Mitchell, L.; et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: Analysis by chemotherapy cohort of the randomized phase III aurelia trial. J. Clin. Oncol. 2015, 33, 3836–3838. [Google Scholar] [CrossRef]
- Penson, R.T.; Valencia, R.V.; Cibula, D.; Colombo, N.; Leath, C.A., III.; Bidziński, M.; Kim, J.W.; Nam, J.H.; Madry, R.; Hernández, C.; et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): A randomized phase III trial. J. Clin. Oncol. 2020, 38, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. SOLO1 Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. New Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Halverson, J.L.; Martinez-Donate, A.P.; Palta, M.; Leal, T.; Lubner, S.; Walsh, M.C.; Schaaf Strickland, J.; Smith, P.D.; Trentham-Dietz, A. Health Literacy and Health-Related Quality of Life Among a Population-Based Sample of Cancer Patients. J. Health Commun. 2015, 20, 1320–1329. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. New Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Pilarski, R.; Berry, M.P.; Jude, S.; Buys, S.S.; Friedman, S.; Garber, J.E.; Hutton, M.L.; Kauff, N.D.; Khan, S.; Lurie, R.H.; et al. FORCE: Facing Our Risk of Cancer Empowered NCCN Guidelines Version 3.2019 Genetic/Familial High-Risk Assessment: Breast and Ovarian; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2019. [Google Scholar]
- Konstantinopoulos, P.A.; Norquist, B.; Lacchetti, C.; Armstrong, D.; Grisham, R.N.; Goodfellow, P.J.; Kohn, E.C.; Levine, D.A.; Liu, J.F.; Lu, K.H.; et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J. Clin. Oncol. 2020, 38, 1222–1245. [Google Scholar] [CrossRef]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women with Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef]
- Schoolmeester, J.K.; Moyer, A.M.; Goodenberger, M.L.; Keeney, G.L.; Carter, J.M.; Bakkum-Gamez, J.N. Pathologic findings in breast, fallopian tube, and ovary specimens in non-BRCA hereditary breast and/or ovarian cancer syndromes: A study of 18 patients with deleterious germline mutations in RAD51C, BARD1, BRIP1, PALB2, MUTYH, or CHEK2. Hum. Pathol. 2017, 70, 14–26. [Google Scholar] [CrossRef]
- Potapova, A.; Hoffman, A.M.; Godwin, A.K.; Al-Saleem, T.; Cairns, P. Promoter hypermethylation of the PALB2 susceptibility gene in inherited and sporadic breast and ovarian cancer. Cancer Res. 2008, 68, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Pylkäs, K.; Erkko, H.; Nikkilä, J.; Sólyom, S.; Winqvist, R. Analysis of large deletions in BRCA1, BRCA2 and PALB2 genes in Finnish breast and ovarian cancer families. BMC Cancer 2008, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Thorstenson, Y.R.; Roxas, A.; Kroiss, R.; Jenkins, M.A.; Kristine, M.Y.; Bachrich, T.; Muhr, D.; Wayne, T.L.; Chu, G.; Davis, R.W.; et al. Contributions of ATM mutations to familial breast and ovarian cancer. Cancer Res. 2003, 63, 3325–3333. [Google Scholar] [PubMed]
- Meindl, A.; Hellebrand, H.; Wiek, C.; Erven, V.; Wappenschmidt, B.; Niederacher, D.; Freund, M.; Lichtner, P.; Hartmann, L.; Schaal, H.; et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat. Genet. 2010, 42, 410–414. [Google Scholar] [CrossRef]
- Loveday, C.; Turnbull, C.; Ramsay, E.; Hughes, D.; Ruark, E.; Frankum, J.R.; Bowden, G.; Kalmyrzaev, B.; Warren-Perry, M.; Snape, K.; et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat. Genet. 2011, 43, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horváth, J.; Kast, K.; Niederacher, D.; Turnbull, C.; et al. Ovarian and Breast Cancer Risks Associated with Pathogenic Variants in RAD51C and RAD51D. J. Natl. Cancer Inst. 2020, 112, 1242–1250. [Google Scholar] [CrossRef]
- Moyer, C.L.; Ivanovich, J.; Gillespie, J.L.; Doberstein, R.; Radke, M.R.; Richardson, M.E.; Kaufmann, S.H.; Swisher, E.M.; Goodfellow, P.J. Rare BRIP1 missense alleles confer risk for ovarian and breast cancer. Cancer Res. 2020, 80, 857–867. [Google Scholar] [CrossRef]
- Rafnar, T.; Gudbjartsson, D.F.; Sulem, P.; Jonasdottir, A.; Sigurdsson, A.; Jonasdottir, A.; Besenbacher, S.; Lundin, P.; Stacey, S.N.; Gudmundsson, J.; et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat. Genet. 2011, 43, 1104–1107. [Google Scholar] [CrossRef]
- Møller, P. The Prospective Lynch Syndrome Database reports enable evidence-based personal precision health care. Hered. Cancer Clin. Pract. 2020, 18, 6. [Google Scholar] [CrossRef]
- Falconer, H.; Yin, L.; Grönberg, H.; Altman, D. Ovarian cancer risk after salpingectomy: A nationwide population-based study. J. Natl. Cancer Inst. 2015, 107, dju410. [Google Scholar] [CrossRef]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Kurman, R.J.; Vang, R.; Sehdev, A.S.; Han, G.; Soslow, R.; Wang, T.L.; Shih, I.M. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma-evidence supporting the clonal relationship of the two lesions. J. Pathol. 2012, 226, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Perets, R.; Wyant, G.A.; Muto, K.W.; Bijron, J.G.; Poole, B.B.; Chin, K.T.; Chen, J.Y.H.; Ohman, A.W.; Stepule, C.D.; Kwak, S.; et al. Transformation of the Fallopian Tube Secretory Epithelium Leads to High-Grade Serous Ovarian Cancer in Brca; Tp53; Pten Models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef]
- Visvanathan, K.; Vang, R.; Shaw, P.; Gross, A.; Soslow, R.; Parkash, V.; Shih, I.M.; Kurman, R.J. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: A reproducibility study. Am. J. Surg. Pathol. 2011, 35, 1766–1775. [Google Scholar] [CrossRef]
- Vang, R.; Shih, I.M.; Kurman, R.J. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology 2013, 62, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Karst, A.M.; Levanon, K.; Drapkin, R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. USA 2011, 108, 7547–7552. [Google Scholar] [CrossRef]
- Piek, J.M.; Van Diest, P.J.; Zweemer, R.P.; Jansen, J.W.; Poort-Keesom, R.J.; Menko, F.H.; Gille, J.J.; Jongsma, A.P.; Pals, G.; Kenemans, P.; et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol. 2011, 195, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Kurman, R.J.; Sehdev, A.S.; Shih, I.M. Ki-67 labeling index as an adjunct in the diagnosis of serous tubal intraepithelial carcinoma. Int. J. Gynecol. Pathol. 2012, 31, 416–422. [Google Scholar] [CrossRef]
- Folkins, A.K.; Jarboe, E.A.; Saleemuddin, A.; Lee, Y.; Callahan, M.J.; Drapkin, R.; Garber, J.E.; Muto, M.G.; Tworoger, S.; Crum, C.P. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol. Oncol. 2008, 109, 168–173. [Google Scholar] [CrossRef]
- Lee, Y.; Miron, A.; Drapkin, R.; Nucci, M.R.; Medeiros, F.; Saleemuddin, A.; Garber, J.; Birch, C.; Mou, H.; Gordon, R.W.; et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 2007, 211, 26–35. [Google Scholar] [CrossRef]
- Powell, C.B.; Chen, L.M.; McLennan, J.; Crawford, B.; Zaloudek, C.; Rabban, J.T.; Moore, D.H.; Ziegler, J. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: Experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int. J. Gynecol. Cancer 2011, 21, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Mingels, M.J.; Roelofsen, T.; van der Laak, J.A.; de Hullu, J.A.; van Ham, M.A.; Massuger, L.F.; Bulten, J.; Bol, M. Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecol. Oncol. 2012, 127, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.J.; Crum, C.P.; Medeiros, F.; Kindelberger, D.W.; Elvin, J.A.; Garber, J.E.; Feltmate, C.M.; Berkowitz, R.S.; Muto, M.G. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007, 25, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Shaw, P.; Rosen, B.; Murphy, J.; Narod, S.A.; Colgan, T.J. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol. Oncol. 2006, 100, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Wang, P.; Lin, S.F.; Zhang, M.; Song, Q.; Chu, T.; Wang, B.G.; Kurman, R.J.; Vang, R.; Kinzler, K.; et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J. Pathol. 2019, 248, 41. [Google Scholar] [CrossRef]
- Shih, I.M.; Wang, Y.; Wang, T.L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef]
- Tone, A.A.; Begley, H.; Sharma, M.; Murphy, J.; Rosen, B.; Brown, T.J.; Shaw, P.A. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin. Cancer Res. 2008, 14, 4067–4078. [Google Scholar] [CrossRef]
- George, S.H.; Greenaway, J.; Milea, A.; Clary, V.; Shaw, S.; Sharma, M.; Virtanen, C.; Shaw, P.A. Identification of abrogated pathways in fallopian tube epithelium from BRCA1 mutation carriers. J. Pathol. 2011, 225, 106–117. [Google Scholar] [CrossRef]
- Sowamber, R.; Chehade, R.; Bitar, M.; Dodds, L.V.; Milea, A.; Slomovitz, B.; Shaw, P.A.; George, S.H. CCAAT/enhancer binding protein delta (C/EBPδ) demonstrates a dichotomous role in tumour initiation and promotion of epithelial carcinoma. EBioMedicine 2019, 44, 261–274. [Google Scholar] [CrossRef]
- Staff, S.; Tolonen, T.; Laasanen, S.L.; Mecklin, J.P.; Isola, J.; Mäenpää, J. Quantitative analysis of γ-H2AX and p53 nuclear expression levels in ovarian and fallopian tube epithelium from risk-reducing salpingo-oophorectomies in BRCA1 and BRCA2 mutation carriers. Int. J. Gynecol. Pathol. 2014, 33, 309–316. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef]
- Trabert, B.; Tworoger, S.S.; O’Brien, K.M.; Townsend, M.K.; Fortner, R.T.; Iversen, E.S.; Hartge, P.; White, E.; Amiano, P.; Arslan, A.A.; et al. The Risk of Ovarian Cancer Increases with an Increase in the Lifetime Number of Ovulatory Cycles: An Analysis from the Ovarian Cancer Cohort Consortium (OC3). Cancer Res. 2020, 80, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef] [PubMed]
- Tone, A.A.; Virtanen, C.; Shaw, P.; Brown, T.J. Prolonged postovulatory proinflammatory signaling in the fallopian tube epithelium may be mediated through a BRCA1/DAB2 axis. Clin. Cancer Res. 2012, 18, 4334–4344. [Google Scholar] [CrossRef]
- Press, J.Z.; Wurz, K.; Norquist, B.M.; Lee, M.K.; Pennil, C.; Garcia, R.; Welcsh, P.; Goff, B.A.; Swisher, E.M. Identification of a Preneoplastic Gene Expression Profile in Tubal Epithelium of BRCA1 Mutation Carriers. Neoplasia 2010, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Bijron, J.G.; Van Der Groep, P.; Van Dorst, E.B.; Seeber, L.M.; Sie-Go, D.M.; Verheijen, R.H.; Van Diest, P.J. Promoter hypermethylation patterns in fallopian tube epithelium of BRCA1 and BRCA2 germ line mutation carriers. Endocr. Relat. Cancer 2012, 19, 69–81. [Google Scholar] [CrossRef][Green Version]
- Bartlett, T.E.; Chindera, K.; McDermott, J.; Breeze, C.E.; Cooke, W.R.; Jones, A.; Reisel, D.; Karegodar, S.T.; Arora, R.; Beck, S.; et al. Epigenetic reprogramming of fallopian tube fimbriae in BRCA mutation carriers defines early ovarian cancer evolution. Nat. Commun. 2016, 7, 11620. [Google Scholar] [CrossRef]
- Dickson, K.A.; Cole, A.J.; Gill, A.J.; Clarkson, A.; Gard, G.B.; Chou, A.; Kennedy, C.J.; Henderson, B.R.; Fereday, S.; Traficante, N.; et al. The RING finger domain E3 ubiquitin ligases BRCA1 and the RNF20/RNF40 complex in global loss of the chromatin mark histone H2B monoubiquitination (H2Bub1) in cell line models and primary high-grade serous ovarian cancer. Hum. Mol. Genet. 2016, 25, 5460–5471. [Google Scholar] [CrossRef]
- Hooda, J.; Novak, M.; Salomon, M.P.; Matsuba, C.; Ramos, R.I.; MacDuffie, E.; Song, M.; Hirsch, M.S.; Lester, J.; Parkash, V.; et al. Early Loss of Histone H2B Monoubiquitylation Alters Chromatin Accessibility and Activates Key Immune Pathways That Facilitate Progression of Ovarian Cancer. Cancer Res. 2019, 79, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.A.; Li, N.; Rowley, S.M.; Cheasley, D.; Zethoven, M.; McInerny, S.; Gorringe, K.L.; James, P.A.; Campbell, I.G. Molecular analysis of PALB2 -associated breast cancers. J. Pathol. 2018, 245, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Liu, L.; Xie, N.; Lu, J.; Liu, Z.; Tang, Y.; Wang, Y.; Yang, J.; Ouyang, Q. Germline PALB2 Mutations in Cancers and Its Distinction from Somatic PALB2 Mutations in Breast Cancers. Front. Genet. 2020, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Garcia-Soto, A.; Silva-Smith, R.; Pinto, A.; George, S.H.L. Primary Peritoneal Carcinoma in a BRCA1/2-negative, PALB2-positive patient. Gynecol. Oncol. Rep. 2016, 17, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Rush, S.K.; Swisher, E.M.; Garcia, R.L.; Pennington, K.P.; Agnew, K.J.; Kilgore, M.R.; Norquist, B.M. Pathologic findings and clinical outcomes in women undergoing risk-reducing surgery to prevent ovarian and fallopian tube carcinoma: A large prospective single institution experience. Gynecol. Oncol. 2020, 157, 514–520. [Google Scholar] [CrossRef]
- Chen, E.Y.; Mehra, K.; Mehrad, M.; Ning, G.; Miron, A.; Mutter, G.L.; Monte, N.; Quade, B.J.; McKeon, F.D.; Yassin, Y.; et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J. Pathol. 2010, 222, 110–116. [Google Scholar] [CrossRef]
- Bachert, S.E.; McDowell, A.; Piecoro, D.; Branch, L.B. Serous tubal intraepithelial carcinoma: A concise review for the practicing pathologist and clinician. Diagnostics 2020, 10, 102. [Google Scholar] [CrossRef]
- Jarboe, E.; Folkins, A.; Nucci, M.R.; Kindelberger, D.; Drapkin, R.; Miron, A.; Lee, Y.; Crum, C.P. Serous carcinogenesis in the fallopian tube: A descriptive classification. Int. J. Gynecol. Pathol. 2008, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Lheureux, S.; Shaw, P.A.; Karakasis, K.; Oza, A.M. Cancer precursor lesions in the BRCA population at the time of prophylactic salpingo-oophorectomy: Accuracy of assessment and potential surrogate marker for prevention. Gynecol. Oncol. 2015, 138, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Visvanathan, K.; Gross, A.; Maambo, E.; Gupta, M.; Kuhn, E.; Li, R.F.; Ronnett, B.M.; Seidman, J.D.; Yemelyanova, A.; et al. Validation of an Algorithm for the Diagnosis of Serous Tubal Intraepithelial Carcinoma. Int. J. Gynecol. Pathol. 2012, 31, 243. [Google Scholar] [CrossRef]
- Soong, T.R.; Howitt, B.E.; Miron, A.; Horowitz, N.S.; Campbell, F.; Feltmate, C.M.; Muto, M.G.; Berkowitz, R.S.; Nucci, M.R.; Xian, W.; et al. Evidence for lineage continuity between early serous proliferations (ESPs) in the Fallopian tube and disseminated high-grade serous carcinomas. J. Pathol. 2018, 246, 344–351. [Google Scholar] [CrossRef]
- Soong, T.R.; Howitt, B.E.; Horowitz, N.; Nucci, M.R.; Crum, C.P. The fallopian tube, ‘precursor escape’ and narrowing the knowledge gap to the origins of high-grade serous carcinoma. Gynecol. Oncol. 2019, 152, 426–433. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef]
- Carlson, J.W.; Jarboe, E.A.; Kindelberger, D.; Nucci, M.R.; Hirsch, M.S.; Crum, C.P. Serous tubal intraepithelial carcinoma: Diagnostic reproducibility and its implications. Int. J. Gynecol. Pathol. 2010, 29, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.B. Risk reducing salpingo-oophorectomy for BRCA mutation carriers: Twenty years later. Gynecol. Oncol. 2014, 132, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, K.; Shaw, P.; May, B.J.; Bahadirli-Talbott, A.; Kaushiva, A.; Risch, H.; Narod, S.; Wang, T.L.; Parkash, V.; Vang, R.; et al. Fallopian Tube Lesions in Women at High Risk for Ovarian Cancer: A Multicenter Study. Cancer Prev. Res. 2018, 11, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Rudaitis, V.; Mikliusas, V.; Januska, G.; Jukna, P.; Mickys, U.; Janavicius, R. The incidence of occult ovarian neoplasia and cancer in BRCA1/2 mutation carriers after the bilateral prophylactic salpingo-oophorectomy (PBSO): A single-center prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 26–31. [Google Scholar] [CrossRef]
- Lu, K.H.; Nebgen, D.R.; Norquist, B.; Bowen, D.J.; Bakkum-Gamez, J.N.; Romero, I.; Roche, K.L.; Levine, D.A.; Soletsky, B.; Carter, J.; et al. WISP: A prospective, multi-center trial of salpingectomy with delayed oophorectomy versus risk reducing salpingo-oophorectomy in women at increased risk for hereditary ovarian cancer. Gynecol. Oncol. 2019, 154, 22. [Google Scholar] [CrossRef]
- George, S.H.L.; Garcia, R.; Slomovitz, B.M. Ovarian cancer: The fallopian tube as the site of origin and opportunities for prevention. Front. Oncol. 2016, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, S.; Frank, C.; Laprise, C.; Quaiattini, A.; Gotlieb, W.H. Occult Tubal Carcinoma After Risk-Reducing Salpingo-oophorectomy: A Systematic Review. Obstet. Gynecol. 2020, 135, 498–508. [Google Scholar] [CrossRef]
- Patrono, M.G.; Corzo, C.; Iniesta, M.; Ramirez, P.T. Management of Preinvasive Lesions. Clin. Obstet. Gynecol. 2017, 60, 771–779. [Google Scholar] [CrossRef]
- Patrono, M.G.; Iniesta, M.D.; Malpica, A.; Lu, K.H.; Fernandez, R.O.; Salvo, G.; Ramirez, P.T. Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): A comprehensive review. Gynecol. Oncol. 2015, 139, 568–572. [Google Scholar] [CrossRef]
- Wethington, S.L.; Park, K.J.; Soslow, R.A.; Kauff, N.D.; Brown, C.L.; Dao, F.; Otegbeye, E.; Sonoda, Y.; Abu-Rustum, N.R.; Barakat, R.R.; et al. Clinical Outcome of Isolated Serous Tubal Intraepithelial Carcinomas (STIC). Int. J. Gynecol. Cancer 2013, 23, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.; Fanale, D.; Incorvaia, L.; Cancelliere, D.; Fiorino, A.; Calò, V.; Dimino, A.; Filorizzo, C.; Corsini, L.R.; Brando, C.; et al. Impact of deleterious variants in other genes beyond BRCA1/2 detected in breast/ovarian and pancreatic cancer patients by NGS-based multi-gene panel testing: Looking over the hedge. ESMO Open 2021, 6, 100235. [Google Scholar] [CrossRef] [PubMed]
- Arvai, K.J.; Roberts, M.E.; Torene, R.I.; Susswein, L.R.; Marshall, M.L.; Zhang, Z.; Carter, N.J.; Yackowski, L.; Rinella, E.S.; Klein, R.T.; et al. Age-adjusted association of homologous recombination genes with ovarian cancer using clinical exomes as controls. Hered. Cancer Clin. Pract. 2019, 17, 19. [Google Scholar] [CrossRef]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women with Ovarian Cancer. J. Natl. Cancer Inst. 2015, 107, djv214. [Google Scholar] [CrossRef]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer risks associated with germline PALB2 pathogenic variants: An international study of 524 families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Lilyquist, J.; LaDuca, H.; Polley, E.; Davis, B.T.; Shimelis, H.; Hu, C.; Hart, S.N.; Dolinsky, J.S.; Couch, F.J.; Goldgar, D.E. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol. Oncol. 2017, 147, 375. [Google Scholar] [CrossRef]
- Lu, H.M.; Li, S.; Black, M.H.; Lee, S.; Hoiness, R.; Wu, S.; Mu, W.; Huether, R.; Chen, J.; Sridhar, S.; et al. Association of Breast and Ovarian Cancers with Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol. 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Suspitsin, E.N.; Sherina, N.Y.; Ponomariova, D.N.; Sokolenko, A.P.; Iyevleva, A.G.; Gorodnova, T.V.; Zaitseva, O.A.; Yatsuk, O.S.; Togo, A.V.; Tkachenko, N.N.; et al. High frequency of BRCA1, but not CHEK2 or NBS1 (NBN), founder mutations in Russian ovarian cancer patients. Hered. Cancer Clin. Pract. 2009, 7, 5. [Google Scholar] [CrossRef]
- Plisiecka-Hałasa, J.; Dansonka-Mieszkowska, A.; Rembiszewska, A.; Bidziński, M.; Steffen, J.; Kupryjańczyk, J. Nijmegen breakage syndrome gene (NBS1) alterations and its protein (nibrin) expression in human ovarian tumours. Ann. Hum. Genet. 2002, 66, 353–359. [Google Scholar] [CrossRef]
- Heikkinen, K.; Karppinen, S.M.; Soini, Y.; Mäkinen, M.; Winqvist, R. Mutation screening of Mre11 complex genes: Indication of RAD50 involvement in breast and ovarian cancer susceptibility. J. Med. Genet. 2003, 40, 131. [Google Scholar] [CrossRef]
- Li, W.; Shao, D.; Li, L.; Wu, M.; Ma, S.; Tan, X.; Zhong, S.; Guo, F.; Wang, Z.; Ye, M. Germline and somatic mutations of multi-gene panel in Chinese patients with epithelial ovarian cancer: A prospective cohort study. J. Ovarian Res. 2019, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.N.; Fraser, L.S.; Philpott, S.; Manchanda, R.; Burnell, M.; Badman, P.; Hadwin, R.; Rizzuto, I.; Benjamin, E.; Singh, N.; et al. Evidence of Stage Shift in Women Diagnosed with Ovarian Cancer During Phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J. Clin. Oncol. 2017, 35, 1411. [Google Scholar] [CrossRef]
- Lai, T.; Kessel, B.; Ahn, H.J.; Terada, K.Y. Ovarian cancer screening in menopausal females with a family history of breast or ovarian cancer. J. Gynecol. Oncol. 2016, 27, e41. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Palaia, I.; Perniola, G.; Musella, A.; Musio, D.; Muzii, L.; Tombolini, V.; Panici, P.B. Risk-reducing salpingo-oophorectomy: A meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Womens. Health 2014, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2020 featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Tung, N.; Domchek, S.M.; Stadler, Z.; Nathanson, K.L.; Couch, F.; Garber, J.E.; Offit, K.; Robson, M.E. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat. Rev. Clin. Oncol. 2016, 13, 581–588. [Google Scholar] [CrossRef]
- Choi, Y.H.; Terry, M.B.; Daly, M.B.; MacInnis, R.J.; Hopper, J.L.; Colonna, S.; Buys, S.S.; Andrulis, I.L.; John, E.M.; Kurian, A.W.; et al. Association of Risk-Reducing Salpingo-Oophorectomy with Breast Cancer Risk in Women with BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2021, 7, 585–592. [Google Scholar] [CrossRef]
- Stjepanovic, N.; Villacampa, G.; Nead, K.T.; Torres-Esquius, S.; Melis, G.G.; Nathanson, K.L.; Teule, A.; Brunet, J.; Teresa, R.; Llort, G.; et al. Association of premenopausal risk-reducing salpingo-oophorectomy with breast cancer risk in BRCA1/2 mutation carriers: Maximising bias-reduction. Eur. J. Cancer 2020, 132, 53–60. [Google Scholar] [CrossRef]
- Iodice, S.; Barile, M.; Rotmensz, N.; Feroce, I.; Bonanni, B.; Radice, P.; Bernard, L.; Maisonneuve, P.; Gandini, S. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: A meta-analysis. Eur. J. Cancer 2010, 46, 2275–2284. [Google Scholar] [CrossRef]
- Huber, D.; Seitz, S.; Kast, K.; Emons, G.; Ortmann, O. Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: A systematic review. Arch. Gynecol. Obstet. 2020, 301, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Hopper, J.L.; Win, A.K.; Dowty, J.G.; Sung, H.K.; Ahn, C.; Kim, S.W.; Lee, M.H.; Lee, J.; Lee, J.W.; et al. Reproductive factors as risk modifiers of breast cancer in BRCA mutation carriers and high-risk non-carriers. Oncotarget 2017, 8, 102110–102118. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, L.H.; Olsson, H.; Phillips, K.A.; Terry, M.B.; Goldgar, D.E.; Kast, K.; Engel, C.; Mooij, T.M.; Adlard, J.; Barrowdale, D.; et al. Oral Contraceptive Use and Breast Cancer Risk: Retrospective and Prospective Analyses from a BRCA1 and BRCA2 Mutation Carrier Cohort Study. JNCI Cancer Spectr. 2018, 2, pky023. [Google Scholar] [CrossRef]
- Cibula, D.; Gompel, A.; Mueck, A.O.; La Vecchia, C.; Hannaford, P.C.; Skouby, S.O.; Zikan, M.; Dusek, L. Hormonal contraception and risk of cancer. Hum. Reprod. Update 2010, 16, 631–650. [Google Scholar] [CrossRef]
- Trabert, B.; Ness, R.B.; Lo-Ciganic, W.H.; Murphy, M.A.; Goode, E.L.; Poole, E.M.; Brinton, L.A.; Webb, P.M.; Nagle, C.M.; Jordan, S.J.; et al. Aspirin, Nonaspirin Nonsteroidal Anti-inflammatory Drug, and Acetaminophen Use and Risk of Invasive Epithelial Ovarian Cancer: A Pooled Analysis in the Ovarian Cancer Association Consortium. JNCI J. Natl. Cancer Inst. 2014, 106, djt431. [Google Scholar] [CrossRef] [PubMed]
- Baandrup, L.; Kjaer, S.K.; Olsen, J.H.; Dehlendorff, C.; Friis, S. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 787–792. [Google Scholar] [CrossRef]
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017, 24, 728–753. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Narod, S.A. Quality of life and health status after prophylactic salpingo-oophorectomy in women who carry a BRCA mutation: A review. Maturitas 2011, 70, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, E.; Narducci, F.; Hudry, D.; Bresson, L.; Charvolin, J.Y.; Ferron, G.; Guyon, F.; Fourchotte, V.; Lambaudie, E.; Baron, M.; et al. First results of a prospective national controlled study: Prophylactic Radical Fimbriectomy (NCT01608074), in women with a hereditary familial risk of breast/ovarian cancer—Tolerance and pathological findings. J. Clin. Oncol. 2018, 36, 5574. [Google Scholar] [CrossRef]
- Gaba, F.; Robbani, S.; Singh, N.; McCluggage, W.G.; Wilkinson, N.; Ganesan, R.; Bryson, G.; Rowlands, G.; Tyson, C.; Arora, R.; et al. Preventing Ovarian Cancer through early Excision of Tubes and late Ovarian Removal (PROTECTOR): Protocol for a prospective non-randomised multi-center trial. Int. J. Gynecol. Cancer 2021, 31, 286–291. [Google Scholar] [CrossRef] [PubMed]
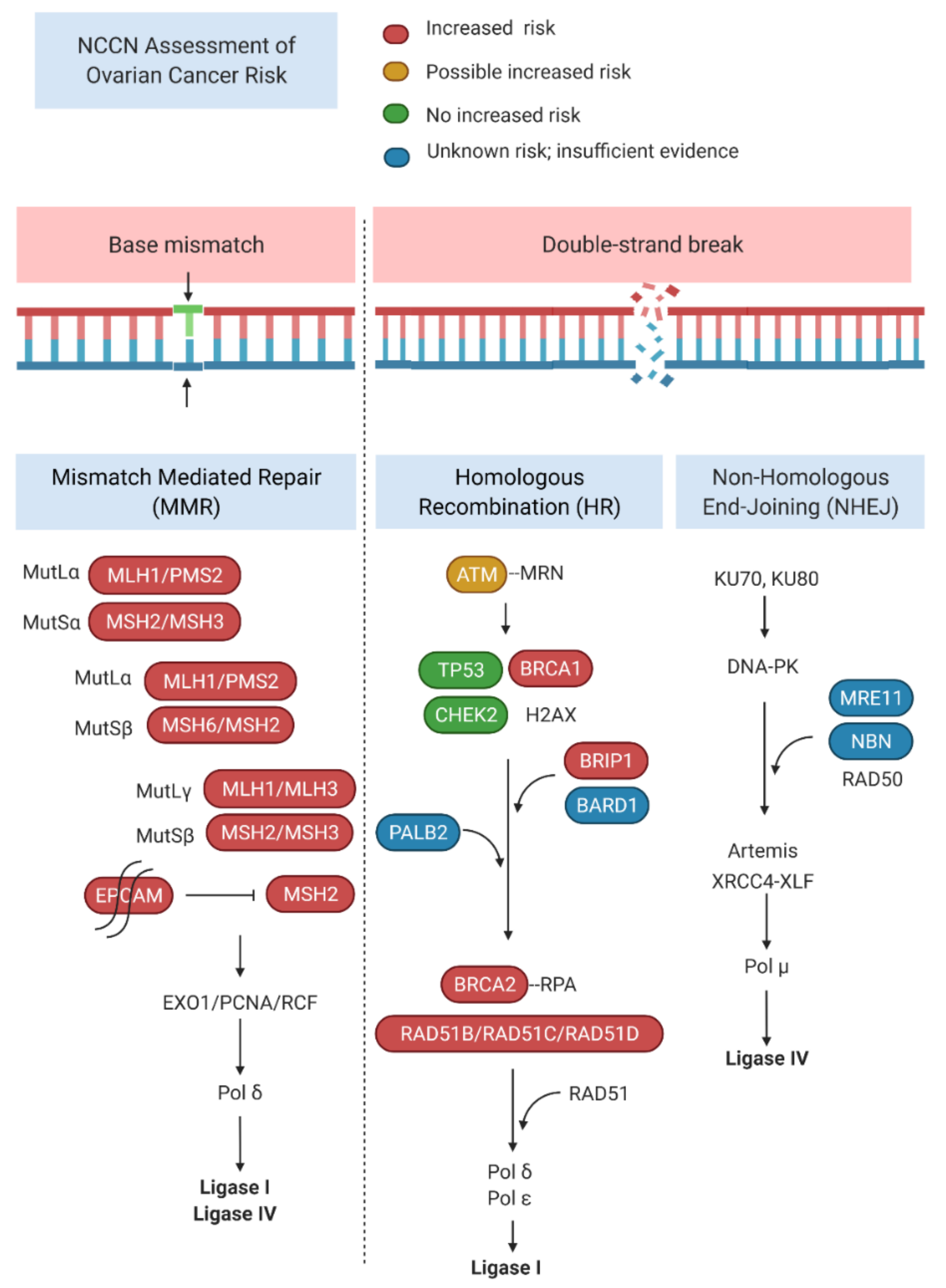
| Mutation | Incidence | Lesions | Reference |
| BARD1 | 0/4 0/1 | STIC or OC STIC or OC | 53 11 |
| BRIP1 | 0/9 0/2 | STIC or OC STIC or OC | 53 11 |
| PALB2 | 1/10 1/? 0/3 | STIC STIC STIC or OC | 53 69 11 |
| MLH1 | 0/1 | STIC or OC | 53 |
| MSH2 | 0/4 | STIC or OC | 53 |
| MSH6 | 0/8 | STIC or OC | 53 |
| PMS2 | 0/2 | STIC or OC | 53 |
| RAD51C | 0/2 0/4 | STIC or OC STIC or OC | 53 11 |
| RAD51D | 0/2 | STIC or OC | 53 |
| Gene | Ovarian Cancer Risk | Recommended Management |
| ATM | Possible increased risk | Insufficient evidence for RRBSO |
| BARD1 | Unknown risk or insufficient evidence | |
| BRCA1 | Increased risk | RRBSO at 35–40 years or completion of childbearing |
| BRCA2 | RRBSO at 40–45 years or completion of childbearing | Increased risk |
| BRIP1 | Increased risk | Consider RRBSO at 45–50 years |
| MSH2, MLH1, EPCAM | Increased risk | Consider RRBSO, timing individualized |
| MSH6, PMS2 | Increased risk | Insufficient evidence for RRBSO |
| NBN | Unknown risk or insufficient evidence | |
| PALB2 | Unknown risk or insufficient evidence | |
| RAD51C | Increased risk | Consider RRBSO at 45–50 years |
| RAD51D | Increased risk | Consider RRBSO at 45–50 years |
| STK11 | Increased risk of non-epithelial ovarian cancer |
| TUBA-WISP-II | SOROCk | Radical Fimbriectomy | PROTECTOR | WISP | STICS and STONEs | |
| Identifier | NCT04294927 | NCT04251052 | NCT01608074 | ISRCTN25173360 | NCT02760849 | NCT03480776 |
| Trial design | 2-arm non randomized | 2-arm non randomized | Single-arm | 3-arm non randomized | 2-arm non randomized | 2-arm randomized |
| Number of patients | 3000 (estimated) | 2262 (estimated) | 123 (actual) | 1000 (estimated) | 423 (actual) | 414 (estimated) |
| Treatment arms | 1. RRS + RRO 2. RRSO | 1. RRS 2. RRSO | 1. Bilateral radical fimbriectomy. | 1. RRS + RRO 2. RRSO 3. No surgery | 1. RRS + RRO 2. RRSO | 1. Daily aspirin 2. Placebo |
| Patient population | Premenopausal women aged 25–50 years old with BRCA1, BRCA2, RAD51C, RAD51D, or BRIP1 germline mutation. | Premenopausal women aged 35–50 years old with a pathogenic or likely pathogenic germline BRCA1 mutation. | Premenopausal women over 35 with a BRCA1/2 mutation or family history of breast/ovarian cancer. | Premenopausal women with increased genetic risk due to genetic mutation (BRCA1, BRCA2, RAD51C, RAD51D, BRIP1) or strong family history. | Premenopausal women aged 30–50 years old with a deleterious mutation in: BRCA1, BRCA2, BRIP1, PALB2, RAD51C, RAD51D, BARD1, MSH2, MSH6, MLH1, PMS2, or EPCAM. | Adult women with BRCA1/BRCA2 germline mutation planning risk-reducing surgery in 6 months to 2 years. |
| Primary outcome | High grade serous ovarian cancer incidence | Time to development of high grade serous carcinoma | Rate of pelvic cancer | Sexual function | Change in Female Sexual Function Index | Premalignant and malignant lesions found after RRBSO |
| Secondary outcomes | Incidence of pre-malignant findings in tubes/ovaries Perioperative mortality and morbidity Incidence of pelvic cancer Incidence of breast cancer Uptake of risk reducing oophorectomy | Health-related quality of life Sexual dysfunction Menopausal symptoms Cancer distress Estrogen deprivation symptoms Medical decision making Adverse events | Surgical morbidity Rate of occult lesions on fimbriectomy specimens Incidence of breast cancer and recurrence of breast cancer Rate of secondary oophorectomy and associated morbidity Proteomic profile of tissue from radical fimbriectomy | Endocrine function/menopause Quality of life Satisfaction/regret Surgical morbidity Psychological health Incidence of STIC and invasive carcinoma Utility score Cost effectiveness | Severity of menopausal symptoms Quality of life Mental health Completion of oophorectomy | Patient-reported acceptance of the intervention Compliance (serum monitoring) |
| Estimated primary completion date | February 2040 | October 2032 | December 2019 | July 2028 | May 2041 | December 2023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, D.; Diaz-Barbe, A.; Pinto, A.; Schlumbrecht, M.; George, S. Hereditary Ovarian Carcinoma: Cancer Pathogenesis Looking beyond BRCA1 and BRCA2. Cells 2022, 11, 539. https://doi.org/10.3390/cells11030539
Samuel D, Diaz-Barbe A, Pinto A, Schlumbrecht M, George S. Hereditary Ovarian Carcinoma: Cancer Pathogenesis Looking beyond BRCA1 and BRCA2. Cells. 2022; 11(3):539. https://doi.org/10.3390/cells11030539
Chicago/Turabian StyleSamuel, David, Alexandra Diaz-Barbe, Andre Pinto, Matthew Schlumbrecht, and Sophia George. 2022. "Hereditary Ovarian Carcinoma: Cancer Pathogenesis Looking beyond BRCA1 and BRCA2" Cells 11, no. 3: 539. https://doi.org/10.3390/cells11030539
APA StyleSamuel, D., Diaz-Barbe, A., Pinto, A., Schlumbrecht, M., & George, S. (2022). Hereditary Ovarian Carcinoma: Cancer Pathogenesis Looking beyond BRCA1 and BRCA2. Cells, 11(3), 539. https://doi.org/10.3390/cells11030539






