Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease
Abstract
:1. Introduction
2. Materials and Methods
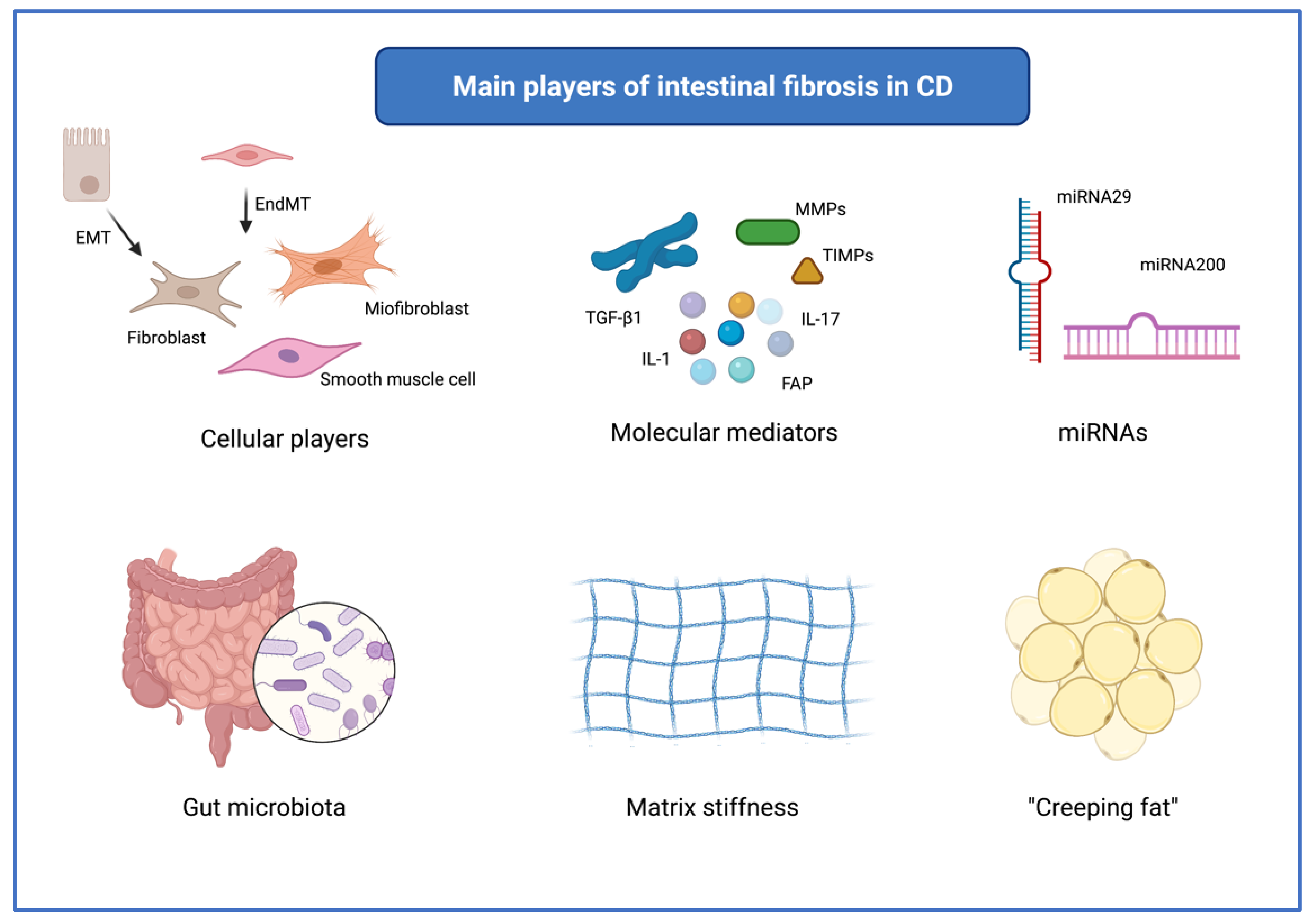
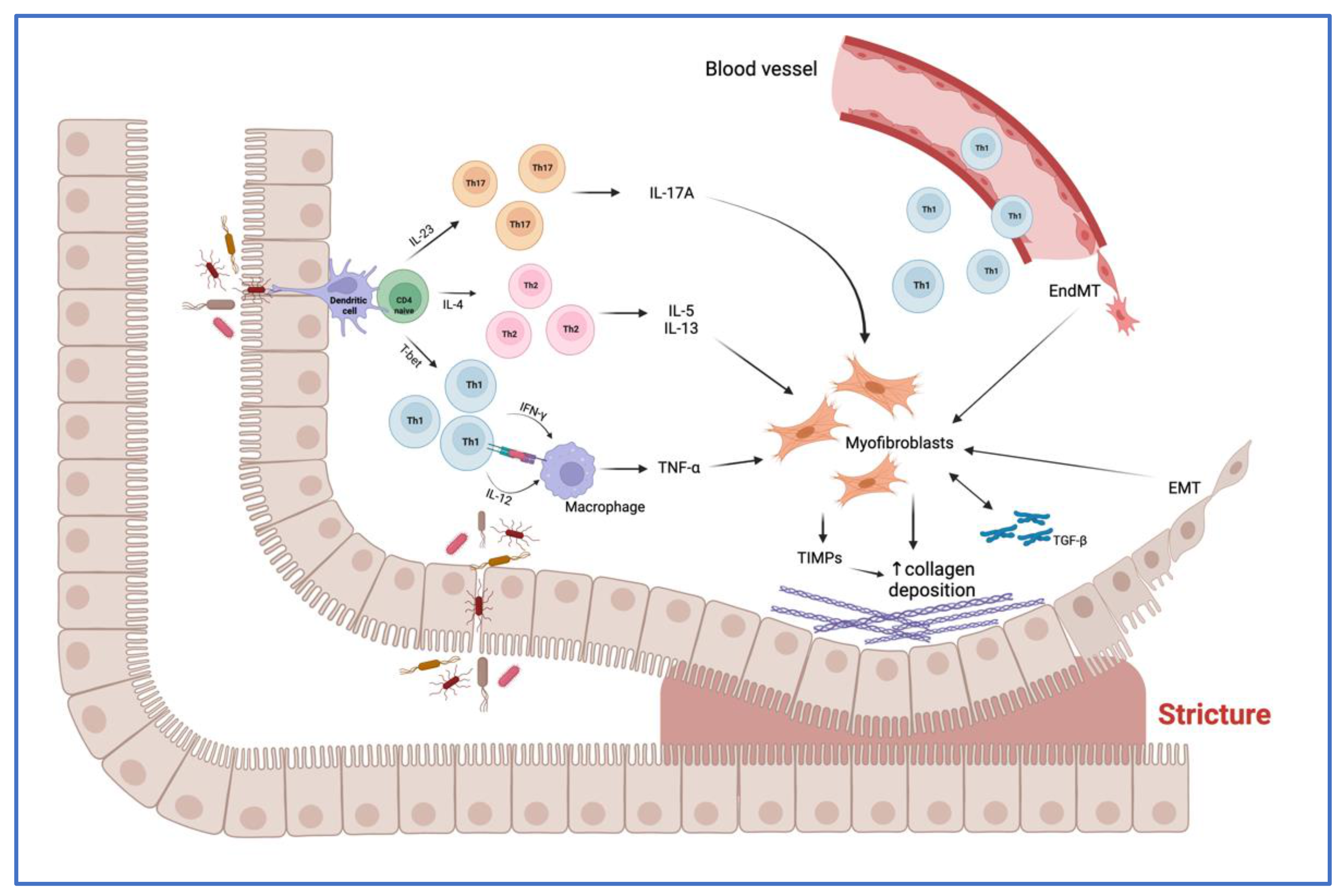
3. Overview of the Main Mechanisms of Intestinal Fibrosis in CD
3.1. Main Cells Involved in Fibrogenesis
3.2. Molecular Mediators of Fibrosis
3.3. MicroRNAs
3.4. The Role of Gut Microbiota
3.5. Matrix Stiffness
3.6. “Creeping Fat”
4. Diagnostic Tools
5. Therapeutic Approaches
5.1. Current Medical Options
5.2. Endoscopic and Surgical Management
5.3. Promising Anti-Fibrotic Therapy in CD
5.3.1. Targeting TGF-β Pathways
- Several studies on fibrosis of other tissues have shown that TGF-β1 production was strongly stimulated by the local activation of angiotensin II [72,73,74], the main effector of the renin-angiotensin system, whose activity is increased in the colonic mucosa of CD patients [75]. For this reason, it was assumed that angiotensin conversing enzyme (ACE) inhibitors and sartans (angiotensin II receptor antagonists), which typically act as anti-hypertensives, could also play a role in the process of intestinal fibrogenesis. The first ACE-inhibitor investigated was captopril, which showed to be effective in preventing colonic fibrosis in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Its anti-fibrotic action has been assumed to derive from blocking TGF-β1 overexpression and/or from a direct down-regulation of TGF-β1 transcripts [76]. Moreover, transanal administration of enalaprilat has been shown to be effective in preventing colonic fibrosis in a dextran sulfate sodium (DSS)-induced colitis model [77]. More recently, losartan, an antagonist of the angiotensin II receptor, was investigated and exhibited a pleiotropic effect, reducing TGF-β1 concentration and significantly improving the macro- and microscopic scores of fibrosis in the colonic wall of rats [78];
- Based on the known antagonistic relationship between the TGF-β/Smad pathway and the peroxisome proliferator-activated receptor (PPAR)γ, a member of ligand-activated transcription factors of nuclear hormone receptor superfamily [79,80], the effect of a novel 5-ASA analog (named GED-0507-34 Levo), able to activate PPARγ, has been investigated. GED-0507-34 Levo showed improvement of intestinal fibrosis in DSS-induced chronic colitis in mice, reducing the activation of myofibroblasts and the expression of the main pro-fibrotic molecules including TGF-β, Smad3, IL-13 and connective tissue growth factor (CTGF) [81]. Similarly, it has been shown that other PPARγ agonists, usually employed in the treatment of diabetes, such as troglitazone and rosiglitazone, may be useful in counteracting the fibrogenic process by suppressing TGF-β1-induced synthesis of collagen, fibronectin, and α-smooth muscle actin in human primary intestinal myofibroblasts [82];
- Another target signaling pathway induced by TGF-β1 but also by matrix stiffness is that of Rho/Rho chinase (ROCK) [83]. The first ROCK inhibitors studied were CCG-1423, CCG-100602, and CCG-203971, which, by inhibiting RhoA signaling in myofibroblasts, induced a significant anti-fibrotic activity [84,85]. These molecules, however, showed an unacceptable toxicity profile, especially with regard to cardiovascular side effects [86]. For this reason, the effect of a locally acting ROCK inhibitor (AMA0825) was investigated. This molecule prevented and reversed intestinal fibrosis in vitro and ex vivo by diminishing TGF-β1-induced activation of myocardine-related transcription factor and p38 mitogen-activated protein kinase (MAPK) and increasing autophagy in fibroblasts, with a good tolerability profile [87]. Combining AMA0825 with anti-inflammatory agents (such as anti-TNF-α) in vivo ameliorated inflammation but also prevented accumulation of fibrotic tissue, underscoring the importance of combination therapy;
- Other compounds have been shown to downregulate the TGF-β signaling. These include cilengitide, which is an Arg-Gly-Asp (RGD)-containing αVβ3 integrin inhibitor, that is able to decrease TGF-β1 activation and development of fibrosis in chronic TNBS-induced colitis [88]. More recently, anti-fibrotic intestinal efficacy has been proposed for two molecules approved for the treatment of idiopathic pulmonary fibrosis, namely pirfenidone and nintedanib [89,90]. In particular, pirfenidone, an orally delivered pyridine derivative that suppresses TGF-β and TNF-α signals, inhibited, both in vivo and in vitro, intestinal fibroblast proliferation and motility and reduced collagen production through different TGF-β1 signaling pathways, including those of suppressor of mothers against decapentaplegic (Smad), phosphatidylinositol-3-kinase (PI3K)/AKT, MAPK, and mechanistic target of rapamycin (mTOR) [91,92,93,94]. Therefore, this molecule is of great interest and has important therapeutic potential, but needs further studies to better clarify its mechanism of action, efficacy, and safety [95]. No studies are yet available on the usefulness in intestinal fibrosis of nintedanib, a small oral molecule inhibitor of tyrosine kinase receptors, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) receptors. Finally, an anti-fibrotic action of maggot extract was described by downregulating the TGF-β1/Smad pathway via upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) expression [96].
5.3.2. Targeting TIMP/MMP Balance
5.3.3. Targeting VEGF
5.3.4. Targeting FAP
5.3.5. Targeting EMT
5.3.6. Targeting the Endogenous Cannabinoid System
5.3.7. Targeting IL-17
5.3.8. Targeting IL-36
5.3.9. Targeting TL1A
5.3.10. Targeting Both TNF-α and IL-17
5.3.11. Targeting AXL Pathway
5.3.12. Targeting NETs
5.3.13. Targeting miRNAs
5.3.14. Targeting Matrix Stiffness
5.3.15. Targeting Intestinal Microbiota
| TARGET | AGENT | MECHANISM | MODEL | REFERENCE |
|---|---|---|---|---|
| TGF-β pathways | Captopril | ↓ TGF-β1 expression and/or TGF-β1 transcript | TNBS-colitis | [76] |
| Transanal enalaprilat | ↓ TGF-β signaling pathway | DSS-colitis | [77] | |
| Losartan | ↓ TGF-β1 expression | TNBS-colitis | [78] | |
| GED-0507-34 Levo | PPAR-γ activation | DSS-colitis | [81] | |
| Troglitazone, Rosiglitazone | PPAR-γ activation | HIFs | [82] | |
| CCG-1423, CCG-100602, CCG-203971 | ROCK inhibition | CCD18-co HIFs | [85] | |
| AMA0825 | ROCK inhibition | DSS- and T-cell transfer-colitis, HIFs | [87] | |
| Cilengitide | αVβ3 integrin inhibition | TNBS-colitis | [88] | |
| Pirfenidone | Smad, PI3K/AKT, MAPK, and mTOR signaling pathways inhibition | HIFs, DSS-colitis, RIF | [91,92,93,94] | |
| Maggot extract | ↑ Nrf2 expression | DSS-colitis | [96] | |
| TIMP/MMP balance | Thalidomide | Altered TIMP/MMPs balance and ECM degradation | TNBS-colitis | [98] |
| VEGF | Bevacizumab | ↓ collagen deposition | n.a. | n.a. |
| FAP | Anti-FAP Ab | FAP inhibition | HIFs | [28] |
| EMT | rhBMP-7 | EMT inhibition | TNBS-colitis | [108] |
| miRNA200b-containing microvescicles | EMT inhibition | TNBS-colitis, IEC-6 | [109] | |
| Endogenous cannabinoid system | MAEA | ↓ collagen production and ↑ myofibroblasts migration | Human organ culture biopsies, LPMCs, and HIFs | [114] |
| IL-17 | Anti-IL17 Ab | ↓ profibrogenic cytokines and MMP/TIMPs balance alteration | TNBS-colitis | [116] |
| IL-36 | Anti-IL36R Ab | ↓ collagen production, MMPs, IL6 signaling, and EMT | DSS- and TNBS-colitis | [121] |
| TL1A | Anti-TL1A Ab | TGF-1/Smad3 signaling pathway inhibition | T-cell transfer-colitis | [31] |
| TNF-αand IL-17 | ABT-122 | n.a. | n.a. | n.a. |
| AXL pathway | BGB324 | ↓ matrix stiffness and TGF-β1-induced fibrogenesis | CCD-18co, TNBS-colitis | [127] |
| NETs | PAD4 inhibitors | ↓ NETs-derived fibrosis | n.a. | n.a. |
| miRNA | miRNA29 | ↓ TGF-β1-induced collagen expression | Human fibroblasts cultures | [36] |
| miRNA200 | ↓ ZEB1 and ZEB2, EMT inhibition | Intestinal epithelial cells | [38] | |
| Matrix Stiffness | β-aminopropionitrile | ↑ MMP3 activity and ↓ ECM contraction | HIFs | [133] |
| Gut microbiota | Probiotics and prebiotics | Modulation fibrotic pathways | Mouse and cellular models | [134,135,136,137,138] |
6. Major Challenges for Anti-Fibrotic Agents Development
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Chron’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Rovedatti, L. Recent advances in understanding Crohn’s disease. Intern. Emerg. Med. 2013, 8, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.R.; Graff, L.A. Quality of life in inflammatory bowel disease: A systematic review and meta-analyses-Part I. Inflamm. Bowel Dis. 2018, 24, 742–751. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Rieder, F.; Bettenworth, D. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol. Ther. 2018, 48, 347–357. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Pakshir, P.; Boris, H. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 68-69, 81–93. [Google Scholar] [CrossRef]
- Lenti, M.V.; Di Sabatino, A. Intestinal fibrosis. Mol. Aspects Med. 2019, 65, 100–109. [Google Scholar] [CrossRef]
- Medina, C.; Santos-Martinez, M.J. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J. Pathol. 2011, 224, 461–472. [Google Scholar] [CrossRef]
- Chen, W.; Lu, C. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: A semiquantitative analysis by using a novel histological grading scheme. J. Crohn’s Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in IBD-a dynamic, multifactorial process. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 228–235. [Google Scholar] [CrossRef]
- Speca, S.; Giusti, I. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, J. Epithelial-mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018, 11, 294–303. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.P. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Jackson, C.L. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut 2009, 58, 777–789. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Drygiannakis, I.; Valatas, V. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: Implication in intestinal fibrosis. J. Crohn’s Colitis 2013, 7, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Scaldaferri, F. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.; Zhao, S. IL-36 in chronic inflammation and fibrosis—bridging the gap? J. Clin. Investig. 2021, 131, e144336. [Google Scholar] [CrossRef] [PubMed]
- Tindemans, I.; Joose, M.E. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Pohin, M. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latella, G.; Viscido, A. Controversial Contribution of Th17/IL-17 Toward the Immune Response in Intestinal Fibrosis. Dig. Dis. Sci. 2020, 65, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Biancheri, P.; Pender, S.L. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair. 2013, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, D.; Franzè, E. Interleukin-25 production is differently regulated by TNF-α and TGF-β1 in the human gut. Mucosal. Immunol. 2011, 4, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovedatti, L.; Di Sabatino, A. Fibroblast activation protein expression in Crohn’s disease strictures. Inflamm. Bowel Dis. 2011, 17, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Sorrentino, L. Inhibition of Fibroblast Activation Protein Restores a Balanced Extracellular Matrix and Reduces Fibrosis in Crohn’s Disease Strictures Ex Vivo. Inflamm. Bowel Dis. 2018, 24, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Di Sabatino, A.; Ciccocioppo, R. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn’s disease. Inflamm. Bowel Dis. 2004, 10, 573–577. [Google Scholar] [CrossRef]
- Shih, D.Q.; Zheng, L. Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014, 7, 1492–1503. [Google Scholar] [CrossRef]
- Barrett, R.; Zhang, X. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am. J. Pathol. 2012, 180, 636–649. [Google Scholar] [CrossRef] [Green Version]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Chrysanthopoulou, A.; Mitroulis, I. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Dragoni, G.; De Hertogh, G. The Role of Citrullination in Inflammatory Bowel Disease: A Neglected Player in Triggering Inflammation and Fibrosis? Inflamm. Bowel Dis. 2021, 27, 134–144. [Google Scholar] [CrossRef]
- Voglova, K.; Bezakova, J. Micro RNAs: An arguable appraisal in medicine. Endocr. Regul. 2016, 50, 106–124. [Google Scholar] [CrossRef] [Green Version]
- Nijhuis, A.; Biancheri, P. In Crohn’s disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin. Sci. 2014, 127, 341–350. [Google Scholar] [CrossRef]
- Mongroo, P.S.; Rustgi, A.K. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol. Ther. 2010, 10, 219–222. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, W. miR-200b is involved in intestinal fibrosis of Crohn’s disease. Int. J. Mol. Med. 2012, 29, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rieder, F. The gut microbiome in intestinal fibrosis: Environmental protector or provocateur? Sci. Transl. Med. 2013, 5, 1–90. [Google Scholar] [CrossRef] [Green Version]
- Rieder, F.; Bhilocha, S. Activation of toll-like receptor (TLR) 5 induces a pro-fibrogenic phenotype on human intestinal myofibroblasts (HIF)—A novel pathway mediated by Caspase 1. Gastroenterology 2011, 140, S114. [Google Scholar] [CrossRef]
- Imai, J.; Kitamoto, S. Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019, 12, 632–643. [Google Scholar] [CrossRef]
- Abreu, M.T.; Taylor, K.D. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn’s disease. Gastroenterology 2002, 123, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Grassl, G.A.; Valdez, Y. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology 2008, 134, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.; Salas, A. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology 1998, 114, 519–526. [Google Scholar] [CrossRef]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Johnson, L.A.; Rodansky, E.S. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis. 2013, 19, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Luke, A. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: Impact of a "Top-Down" approach to intestinal fibrosis in mice. Inflamm. Bowel Dis. 2012, 18, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Chamaillard, M. Mesenteric fat in Crohn’s disease: A pathogenetic hallmark or an innocent bystander? Gut 2007, 56, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Kredel, L.I.; Batra, A. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut 2013, 62, 852–862. [Google Scholar] [CrossRef]
- Mao, R.; Kurada, S. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Bettenworth, D.; Bokemeyer, A. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: A systematic review. Gut 2019, 68, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Onali, S.; Calabrese, E. Endoscopic vs ultrasonographic findings related to Crohn’s disease recurrence: A prospective longitudinal study at 3 years. J. Crohn’s Colitis 2010, 4, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Baraty, B. Systematic review: Medical therapy for fibrostenosing Crohn’s disease. Aliment Pharmacol. Ther. 2020, 51, 1233–1246. [Google Scholar] [CrossRef]
- Yaffe, B.H.; Korelitz, B.I. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J. Clin. Gastroenterol. 1983, 5, 211–215. [Google Scholar] [CrossRef]
- Vasilopoulos, S.; Kugathasan, S. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. Am. J. Gastroenterol. 2000, 95, 2503. [Google Scholar] [CrossRef]
- Toy, L.S.; Scherl, E.J. Complete bowel obstruction following initial response to infliximab therapy for Crohn’s disease: A series of a newly described complication. Gastroenterology 2000, 118, A569. [Google Scholar] [CrossRef]
- Allocca, M.; Bonifacio, C. Efficacy of tumour necrosis factor antagonists in stricturing Crohn’s disease: A tertiary center real-life experience. Dig. Liver Dis. 2017, 49, 872–877. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Carbonnel, F. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Engel, T.; Ungar, B. Vedolizumab in IBD-Lessons from Real-world Experience; A Systematic Review and Pooled Analysis. J. Crohn’s Colitis 2018, 12, 245–257. [Google Scholar] [CrossRef]
- Ma, C.; Fedorak, R.N. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: Real world experience from a multicentre cohort. Aliment Pharmacol. Ther. 2017, 45, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M. 5-Aminosalicylates to maintain remission in Crohn’s disease: Interpreting conflicting systematic review evidence. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Latella, G. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohns Colitis 2016, 10, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Andoh, A. Efficacy of Endoscopic Balloon Dilation for Small Bowel Strictures in Patients With Crohn’s Disease: A Nationwide, Multi-centre, Open-label, Prospective Cohort Study. J. Crohns Colitis 2018, 12, 394–401. [Google Scholar] [CrossRef]
- Bettenworth, D.; Gustavsson, A. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 133–142. [Google Scholar] [CrossRef]
- Siddiqui, U.D.; Banerjee, S. Tools for endoscopic stricture dilation. Gastrointest. Endosc. 2013, 78, 391–404. [Google Scholar] [CrossRef]
- Bemelman, W.A.; Warusavitarne, J. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J. Crohns Colitis 2018, 12, 1–16. [Google Scholar]
- Katsuno, H.; Maeda, K. Novel antimesenteric functional end-to-end handsewn (Kono-S) anastomoses for Crohn’s disease: A report of surgical procedure and short-term outcomes. Dig. Surg. 2015, 32, 39–44. [Google Scholar] [CrossRef]
- Coffey, C.J.; Kiernan, M.G. Inclusion of the Mesentery in Ileocolic Resection for Crohn’s Disease is Associated with Reduced Surgical Recurrence. J. Crohns Colitis 2018, 12, 1139–1150. [Google Scholar] [CrossRef]
- Sica, G.S.; Iaculli, E. Laparoscopic versus open ileo-colonic resection in Crohn’s disease: Short-and long-term results from a prospective longitudinal study. J. Gastrointest. Surg. 2008, 12, 1094–1102. [Google Scholar] [CrossRef]
- Campbell, S.E.; Katwa, L.C. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J. Mol. Cell Cardiol. 1997, 29, 1947–1958. [Google Scholar] [CrossRef]
- Warner, F.J.; Lubel, J.S. Liver fibrosis: A balance of ACEs? Clin. Sci. 2007, 113, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Uhal, B.D.; Kim, J.K. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: Autocrine mechanisms in myofibroblasts and macrophages. Curr. Pharm. Des. 2007, 13, 1247–1256. [Google Scholar] [CrossRef]
- Jaszewski, R.; Tolia, V. Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology 1990, 98, 1543–1548. [Google Scholar] [CrossRef]
- Wengrower, D.; Zanninelli, G. Prevention of fibrosis in experimental colitis by captopril: The role of tgf-beta1. Inflamm. Bowel Dis. 2004, 10, 536–545. [Google Scholar] [CrossRef]
- Koga, H.; Yang, H. Transanal delivery of angiotensin converting enzyme inhibitor prevents colonic fibrosis in a mouse colitis model: Development of a unique mode of treatment. Surgery 2008, 144, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Wengrower, D.; Zanninelli, G. Losartan reduces trinitrobenzene sulphonic acid-induced colorectal fibrosis in rats. Can. J. Gastroenterol. 2012, 26, 33–39. [Google Scholar] [CrossRef]
- Wei, J.; Ghosh, A.K. PPARγ downregulation by TGFß in fibroblast and impaired expression and function in systemic sclerosis: A novel mechanism for progressive fibrogenesis. PLoS ONE 2010, 5, e13778. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.K.; Bhattacharyya, S. Peroxisome proliferator-activated receptor-gamma abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 2009, 23, 2968–2977. [Google Scholar] [CrossRef] [Green Version]
- Speca, S.; Rousseaux, C. Novel PPARγ Modulator GED-0507-34 Levo Ameliorates Inflammation-driven Intestinal Fibrosis. Inflamm. Bowel Dis. 2016, 22, 279–292. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.B.; Nam, M.O. Anti-fibrogenic effect of PPAR-γ agonists in human intestinal myofibroblasts. BMC Gastroenterol. 2017, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Bourgier, C.; Haydont, V. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut 2005, 54, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandbo, N.; Kregel, S. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am. J. Respir. Cell Mol. Biol. 2009, 41, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.A.; Rodansky, E.S. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm. Bowel Dis. 2014, 20, 154–165. [Google Scholar] [CrossRef]
- Bian, H.; Zhou, Y. Rho-kinase signaling pathway promotes the expression of PARP to accelerate cardiomyocyte apoptosis in ischemia/reperfusion. Mol. Med. Rep. 2017, 16, 2002–2008. [Google Scholar] [CrossRef] [Green Version]
- Holvoet, T.; Devriese, S. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology 2017, 153, 1054–1067. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Flynn, R.S. Increased activation of latent TGF-β1 by αVβ3 in human Crohn’s disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm. Bowel Dis. 2013, 19, 2829–2839. [Google Scholar] [CrossRef] [Green Version]
- Sathiyamoorthy, G.; Sehgal, S. Pirfenidone and Nintedanib for Treatment of Idiopathic Pulmonary Fibrosis. South Med. J. 2017, 110, 393–398. [Google Scholar] [CrossRef]
- Ma, C.; Jairath, V. Targeting anti-fibrotic pathways in Crohn’s disease—The final frontier? Best Pract. Res. Clin. Gastroenterol. 2019, 38-39, 101603. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M. Pirfenidone Inhibits Cell Proliferation and Collagen I Production of Primary Human Intestinal Fibroblasts. Cells. 2020, 9, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhang, Y. Pirfenidone suppresses TGF-β1-induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 18, 3907–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Ren, J. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-β signaling in a murine colitis model. Biochem. Pharmacol. 2016, 117, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.W.; Zhang, Y.Y. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-β1/Smad/CTGF signaling pathway. Eur. J. Pharmacol. 2018, 822, 199–206. [Google Scholar] [CrossRef]
- Latella, G.; Viscido, A. Could Pirfenidone Also be Effective in Treating Intestinal Fibrosis? Cells 2020, 9, 1762. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D. Therapeutic Targeting of Nrf2 Signaling by Maggot Extracts Ameliorates Inflammation-Associated Intestinal Fibrosis in Chronic DSS-Induced Colitis. Front. Immunol. 2021, 12, 670159. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, M.; Shu, X. Thalidomide is a therapeutic agent that is effective in inducing and maintaining endoscopic remission in adult CD patients. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 210–216. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H. Thalidomide Prevented and Ameliorated Pathogenesis of Crohn’s Disease in Mice via Regulation of Inflammatory Response and Fibrosis. Front. Pharmacol. 2019, 10, 1486. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.J.; Zhang, Z.H. Effect of bevacizumab on the expression of fibrosis-related inflammatory mediators in ARPE-19 cells. Int. J. Ophthalmol. 2017, 10, 366–371. [Google Scholar]
- Alkim, C.; Alkim, H. Angiogenesis in Inflammatory Bowel Disease. Int. J. Inflam. 2015, 2015, 970890. [Google Scholar] [CrossRef] [Green Version]
- Juillerat-Jeanneret, L.; Gerber-Lemaire, S. The prolyl-aminodipeptidases and their inhibitors as therapeutic targets for fibrogenic disorders. Mini Rev. Med. Chem. 2009, 9, 215–226. [Google Scholar] [CrossRef]
- Egger, C.; Cannet, C. Effects of the fibroblast activation protein inhibitor, PT100, in a murine model of pulmonary fibrosis. Eur. J. Pharmacol. 2017, 809, 64–72. [Google Scholar] [CrossRef]
- Hill, C.; Li, J. Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis. Cell Death Dis. 2019, 10, 591. [Google Scholar] [CrossRef]
- Yao, L.; Conforti, F. Paracrine signalling during ZEB1-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in lung fibrosis. Cell Death Differ. 2019, 26, 943–957. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, M.; Yang, C. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, E.M.; Tarnavski, O. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Zeisberg, M.; Hanai, J. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003, 9, 964–968. [Google Scholar] [CrossRef]
- Flier, S.N.; Tanjore, H. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J. Biol. Chem. 2010, 285, 20202–20212. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhou, C.Z. miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. J. Gastroenterol. Hepatol. 2017, 32, 1966–1974. [Google Scholar] [CrossRef]
- Muccioli, G.G. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today 2010, 15, 474–483. [Google Scholar] [CrossRef]
- Petrosino, S.; Ligresti, A. Endocannabinoid chemical biology: A tool for the development of novel therapies. Curr. Opin. Chem. Biol. 2009, 13, 309–320. [Google Scholar] [CrossRef]
- Izzo, A.A.; Camilleri, M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut 2008, 57, 1140–1155. [Google Scholar] [CrossRef]
- Julien, B.; Grenard, P. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Battista, N. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011, 4, 574–583. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, L. Role of Interleukin-17 in Pathogenesis of Intestinal Fibrosis in Mice. Dig. Dis. Sci. 2020, 65, 1971–1979. [Google Scholar] [CrossRef]
- Paul, J.; Singh, A.K. IL-17-driven intestinal fibrosis is inhibited by Itch-mediated ubiquitination of HIC-5. Mucosal Immunol. 2018, 11, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Hueber, W.; Sands, B.E. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar] [CrossRef] [Green Version]
- Targan, S.R.; Feagan, B. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn’s Disease. Am. J. Gastroenterol. 2016, 111, 1599–1607. [Google Scholar] [CrossRef]
- Zhang, B.L.; Liang, T.S. Interleukin-17 as a Therapy Target in Intestinal Fibrosis. Dig. Dis. Sci. 2020, 65, 3054–3055. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.; Qiu, H. Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis. Int. J. Mol. Sci. 2020, 21, 6458. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Kersten, C. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice with Chronic Intestinal Inflammation. Gastroenterology 2019, 156, 1082–1097. [Google Scholar] [CrossRef] [Green Version]
- Danese, S.; Klopocka, M. Anti-TL1A Antibody PF-06480605 Safety and Efficacy for Ulcerative Colitis: A Phase 2a Single-Arm Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; Wagner, F. Safety, Tolerability, and Pharmacodynamics of ABT-122, a Tumor Necrosis Factor- and Interleukin-17-Targeted Dual Variable Domain Immunoglobulin, in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 2283–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, P.J.; Genovese, M.C. Phase II Study of ABT-122, a Tumor Necrosis Factor- and Interleukin-17A-Targeted Dual Variable Domain Immunoglobulin, in Patients with Psoriatic Arthritis with an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2018, 70, 1778–1789. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.F.; Isaacs, J.D. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef]
- Bárcena, C.; Stefanovic, M. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J. Hepatol. 2015, 63, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Steiner, C.A.; Rodansky, E.S. AXL Is a Potential Target for the Treatment of Intestinal Fibrosis. Inflamm. Bowel Dis. 2021, 27, 303–316. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Elhasid, R. Neutrophil extracellular traps in pediatric inflammatory bowel disease. Pathol. Int. 2018, 68, 517–523. [Google Scholar] [CrossRef]
- He, Z.; Si, Y. Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb. Haemost. 2016, 115, 738–751. [Google Scholar]
- Angelidou, I.; Chrysanthopoulou, A. REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J. Immunol. 2018, 200, 3950–3961. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Suzuki, M.; Ikari, J. PAD4 Deficiency Improves Bleomycin-induced Neutrophil Extracellular Traps and Fibrosis in Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2020, 63, 806–818. [Google Scholar] [CrossRef]
- de Bruyn, J.R.; van den Brink, G.R. Fibrostenotic Phenotype of Myofibroblasts in Crohn’s Disease is Dependent on Tissue Stiffness and Reversed by LOX Inhibition. J. Crohn’s Colitis 2018, 12, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Kashima, S.; Fujiya, M. Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis. Transl. Res. 2015, 166, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Choi, J. A probiotic complex, rosavin, zinc, and prebiotics ameliorate intestinal inflammation in an acute colitis mouse model. J. Transl. Med. 2018, 16, 37. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X. Protective effects of a novel probiotic strain, Lactococcus lactis ML2018, in colitis: In vivo and in vitro evidence. Food Funct. 2019, 10, 1132–1145. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, C. Treatment with a probiotic combination reduces abdominal adhesion in rats by decreasing intestinal inflammation and restoring microbial composition. Oncol. Rep. 2020, 43, 986–998. [Google Scholar] [CrossRef]
- Lombardi, F.; Augello, F.R. Soluble Fraction from Lysate of a High Concentration Multi-Strain Probiotic Formulation Inhibits TGF-β1-Induced Intestinal Fibrosis on CCD-18Co Cells. Nutrients 2021, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Pinzani, M. Anti-fibrotic therapy: Lost in translation? J. Hepatol. 2012, 56, S66–S74. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y. Robust bioengineered 3D functional human intestinal epithelium. Sci. Rep. 2015, 5, 13708. [Google Scholar] [CrossRef]
- Giuffrida, P.; Curti, M. Decellularized Human Gut as a Natural 3D Platform for Research in Intestinal Fibrosis. Inflamm. Bowel Dis. 2019, 25, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, P.; Pinzani, M. Biomarkers of intestinal fibrosis—One step towards clinical trials for stricturing inflammatory bowel disease. United Eur. Gastroenterol J. 2016, 4, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, C.A.; Berinstein, J.A. Biomarkers for the Prediction and Diagnosis of Fibrostenosing Crohn’s Disease: A Systematic Review. Clin. Gastroenterol. Hepatol. 2021, 21, S1542–S3565. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Bonovas, S. Identification of Endpoints for Development of Antifibrosis Drugs for Treatment of Crohn’s Disease. Gastroenterology 2018, 155, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Rieder, F. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santacroce, G.; Lenti, M.V.; Di Sabatino, A. Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease. Cells 2022, 11, 429. https://doi.org/10.3390/cells11030429
Santacroce G, Lenti MV, Di Sabatino A. Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease. Cells. 2022; 11(3):429. https://doi.org/10.3390/cells11030429
Chicago/Turabian StyleSantacroce, Giovanni, Marco Vincenzo Lenti, and Antonio Di Sabatino. 2022. "Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease" Cells 11, no. 3: 429. https://doi.org/10.3390/cells11030429
APA StyleSantacroce, G., Lenti, M. V., & Di Sabatino, A. (2022). Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease. Cells, 11(3), 429. https://doi.org/10.3390/cells11030429







