Novel Techniques Targeting Fibroblasts after Ischemic Heart Injury
Abstract
1. Introduction
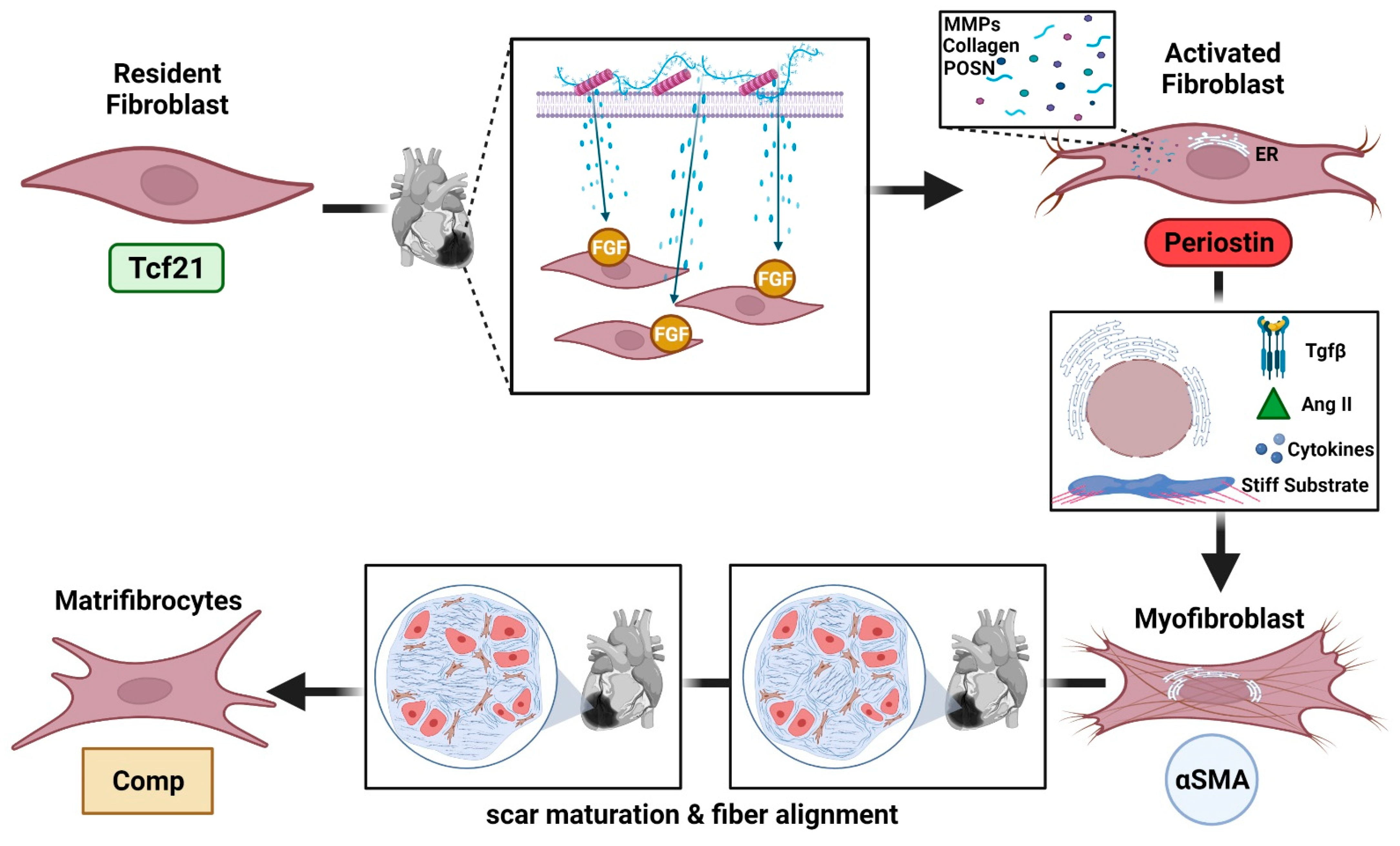
2. Cardiac Fibroblast Roles and Subpopulations
2.1. Cardiac Fibroblast Proinflammatory Actions after Ischemic Injury
2.2. Cardiac Fibroblasts during Resolution of Inflammation and Repair
2.3. Fibroblast Subpopulations
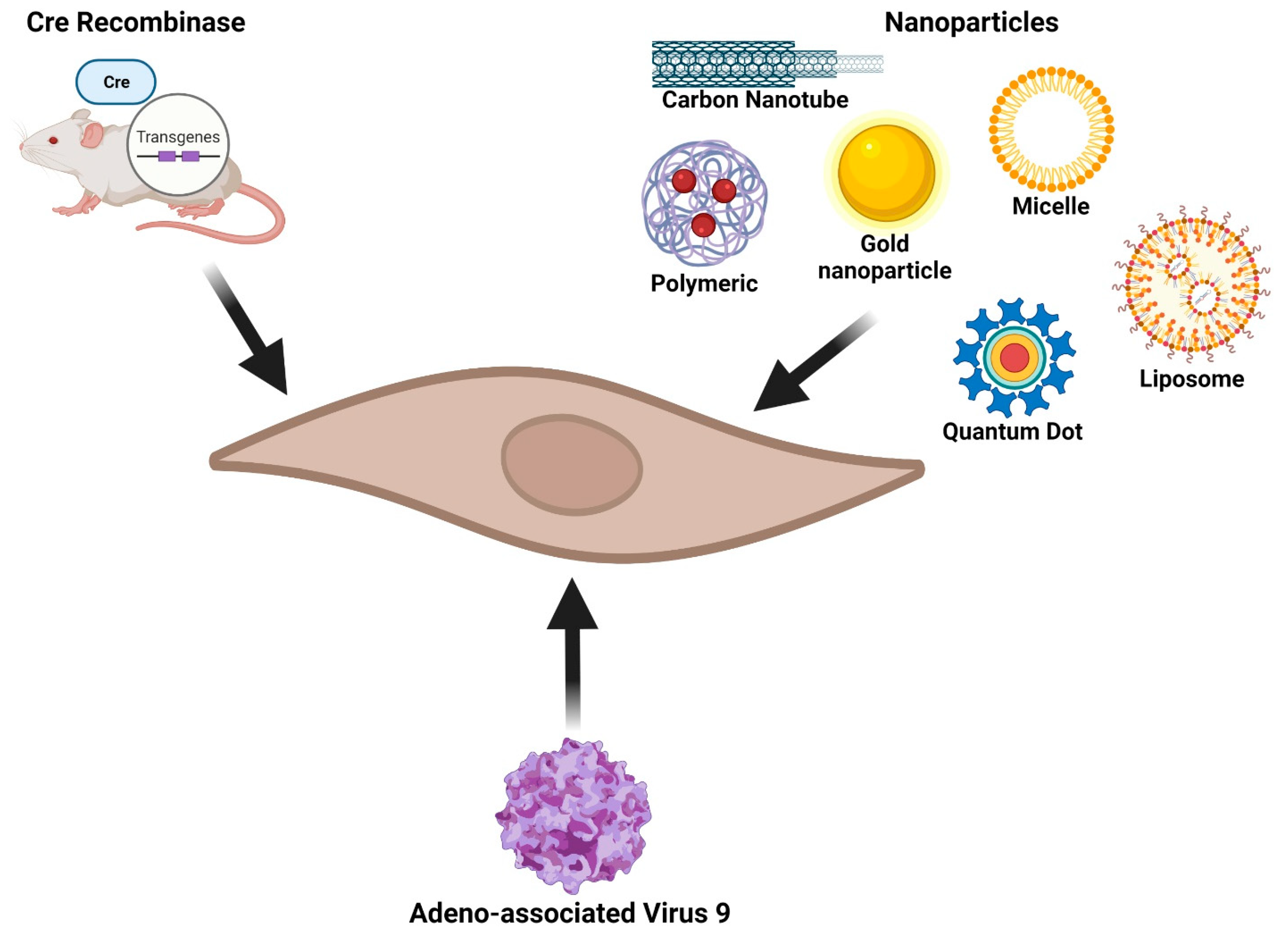
3. Fibroblast-Specific Research Tools
3.1. Transgenic Mice
3.2. Nanoparticles
3.3. Liposomes, PGLA, and Polymeric Micelles
3.4. Adeno-Associated Virus
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and Functional Characterisation of Cardiac Fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.A.; Bowers, S.L.K.; Baudino, T.A. Cardiac Fibroblast. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Chytil, A.; Plieth, D.; Gorska, A.E.; Dumont, N.; Shappell, S.; Washington, M.K.; Neilson, E.G.; Moses, H.L. TGF-ß Signaling in Fibroblasts Modulates the Oncogenic Potential of Adjacent Epithelia. Science 2004, 303, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. The Role of Cardiac Fibroblasts in Post-Myocardial Heart Tissue Repair. Exp. Mol. Pathol. 2016, 101, 231–240. [Google Scholar] [CrossRef]
- Lajiness, J.D.; Conway, S.J. Origin, Development, and Differentiation of Cardiac Fibroblasts. J. Mol. Cell. Cardiol. 2014, 70, 2–8. [Google Scholar] [CrossRef]
- McAnulty, R.J.; Laurent, G.J. Collagen Synthesis and Degradation In Vivo. Evidence for Rapid Rates of Collagen Turnover with Extensive Degradation of Newly Synthesized Collagen in Tissues of the Adult Rat. Coll. Relat. Res. 1987, 7, 93–104. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef]
- Rohr, S. Cardiac Fibroblasts in Cell Culture Systems: Myofibroblasts All Along? J. Cardiovasc. Pharmacol. 2011, 57, 389–399. [Google Scholar] [CrossRef]
- Lafontant, P.J.; Burns, A.R.; Donnachie, E.; Haudek, S.B.; Smith, C.W.; Entman, M.L. Oncostatin M Differentially Regulates CXC Chemokines in Mouse Cardiac Fibroblasts. Am. J. Physiol.-Cell Physiol. 2006, 291, C18–C26. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between Fibroblasts and Inflammatory Cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident Cardiac Mast Cells Degranulate and Release Preformed TNF-α, Initiating the Cytokine Cascade in Experimental Canine Myocardial Ischemia/Reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S.; Troidl, C.; Hamm, C.; Schulz, R. Ischemia and Reperfusion Related Myocardial Inflammation: A Network of Cells and Mediators Targeting the Cardiomyocyte. IUBMB Life 2015, 67, 110–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The Inflammatory Response in Myocardial Infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the Blood Microvascular System. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Iyer, R.P.; Zamilpa, R.; Yabluchanskiy, A.; DeLeon-Pennell, K.Y.; Hall, M.E.; Kaplan, A.; Zouein, F.A.; Bratton, D.; Flynn, E.R.; et al. A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis. J. Am. Coll. Cardiol. 2015, 66, 1364–1374. [Google Scholar] [CrossRef]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.-Y.; Jackson, C.J. Endogenous MMP-9 and Not MMP-2 Promotes Rheumatoid Synovial Fibroblast Survival, Inflammation and Cartilage Degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef]
- Ohm, I.K.; Alfsnes, K.; Olsen, M.B.; Ranheim, T.; Sandanger, Ø.; Dahl, T.B.; Aukrust, P.; Finsen, A.V.; Yndestad, A.; Vinge, L.E. Toll-Like Receptor 9 Mediated Responses in Cardiac Fibroblasts. PLoS ONE 2014, 9, e104398. [Google Scholar] [CrossRef]
- Yang, R.; Song, Z.; Wu, S.; Wei, Z.; Xu, Y.; Shen, X. Toll-like Receptor 4 Contributes to a Myofibroblast Phenotype in Cardiac Fibroblasts and Is Associated with Autophagy after Myocardial Infarction in a Mouse Model. Atherosclerosis 2018, 279, 23–31. [Google Scholar] [CrossRef]
- Petrov, V.V.; Fagard, R.H.; Lijnen, P.J. Stimulation of Collagen Production by Transforming Growth Factor-Β1 During Differentiation of Cardiac Fibroblasts to Myofibroblasts. Hypertension 2002, 39, 258–263. [Google Scholar] [CrossRef]
- Li, Y.Y.; McTiernan, C.F.; Feldman, A.M. Proinflammatory Cytokines Regulate Tissue Inhibitors of Metalloproteinases and Disintegrin Metalloproteinase in Cardiac Cells. Cardiovasc. Res. 1999, 42, 162–172. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Chensue, S.W.; Smith, R.E.; Strieter, R.M.; Warmington, K.; Wilke, C.; Kunkel, S.L. Production of Monocyte Chemoattractant Protein-1 and Macrophage Inflammatory Protein-1 Alpha by Inflammatory Granuloma Fibroblasts. Am. J. Pathol. 1994, 144, 711–718. [Google Scholar] [PubMed]
- Rainger, G.E.; Stone, P.; Morland, C.M.; Nash, G.B. A Novel System for Investigating the Ability of Smooth Muscle Cells and Fibroblasts to Regulate Adhesion of Flowing Leukocytes to Endothelial Cells. J. Immunol. Methods 2001, 255, 73–82. [Google Scholar] [CrossRef]
- Tieu, B.C.; Lee, C.; Sun, H.; LeJeune, W.; Recinos, A.; Ju, X.; Spratt, H.; Guo, D.-C.; Milewicz, D.; Tilton, R.G.; et al. An Adventitial IL-6/MCP1 Amplification Loop Accelerates Macrophage-Mediated Vascular Inflammation Leading to Aortic Dissection in Mice. J. Clin. Investig. 2009, 119, 3637–3651. [Google Scholar] [CrossRef]
- Bräuninger, H.; Thottakara, T.; Schön, J.; Voss, S.; Dhople, V.; Warnke, S.; Scherschel, K.; Schrage, B.; Kirchhof, P.; Blankenberg, S.; et al. Cytokine-Mediated Alterations of Human Cardiac Fibroblast’s Secretome. Int. J. Mol. Sci. 2021, 22, 12262. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.; Zietsch, C.; Tank, J.; Sossalla, S.; Fluschnik, N.; Hinrichs, S.; Maier, L.; Poller, W.; Blankenberg, S.; Schultheiss, H.-P.; et al. Cardiac Fibroblasts Support Cardiac Inflammation in Heart Failure. Basic Res. Cardiol. 2014, 109, 428. [Google Scholar] [CrossRef]
- Daub, S.; Lutgens, E.; Münzel, T.; Daiber, A. CD40/CD40L and Related Signaling Pathways in Cardiovascular Health and Disease—The Pros and Cons for Cardioprotection. Int. J. Mol. Sci. 2020, 21, 8533. [Google Scholar] [CrossRef]
- Yellin, M.J.; Winikoff, S.; Fortune, S.M.; Baum, D.; Crow, M.K.; Lederman, S.; Chess, L. Ligation of CD40 on Fibroblasts Induces CD54 (ICAM-1) and CD106 (VCAM-1) up-Regulation and IL-6 Production and Proliferation. J. Leukoc. Biol. 1995, 58, 209–216. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef]
- Byun, J.Y.; Lee, G.Y.; Choi, H.Y.; Myung, K.B.; Choi, Y.W. The Expressions of TGF-Β1 and IL-10 in Cultured Fibroblasts after ALA-IPL Photodynamic Treatment. Ann. Dermatol. 2011, 23, 19–22. [Google Scholar] [CrossRef]
- Chen, W.; Saxena, A.; Li, N.; Sun, J.; Gupta, A.; Lee, D.-W.; Tian, Q.; Dobaczewski, M.; Frangogiannis, N.G. Endogenous IRAK-M Attenuates Postinfarction Remodeling Through Effects on Macrophages and Fibroblasts. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J. When Signaling Pathways Collide: Positive and Negative Regulation of Toll-like Receptor Signal Transduction. Immunity 2008, 29, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Shinde, A.V.; Haque, Z.; Wu, Y.-J.; Chen, W.; Su, Y.; Frangogiannis, N.G. The Role of Interleukin Receptor Associated Kinase (IRAK)-M in Regulation of Myofibroblast Phenotype in Vitro, and in an Experimental Model of Non-Reperfused Myocardial Infarction. J. Mol. Cell. Cardiol. 2015, 89, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gurantz, D.; Tran, V.; Cowling, R.T.; Greenberg, B.H. Tumor Necrosis Factor-α–Induced AT1 Receptor Upregulation Enhances Angiotensin II–Mediated Cardiac Fibroblast Responses That Favor Fibrosis. Circ. Res. 2002, 91, 1119–1126. [Google Scholar] [CrossRef]
- Furtado, M.B.; Costa, M.W.; Pranoto, E.A.; Salimova, E.; Pinto, A.R.; Lam, N.T.; Park, A.; Snider, P.; Chandran, A.; Harvey, R.P.; et al. Cardiogenic Genes Expressed in Cardiac Fibroblasts Contribute to Heart Development and Repair. Circ. Res. 2014, 114, 1422–1434. [Google Scholar] [CrossRef]
- Huebener, P.; Abou-Khamis, T.; Zymek, P.; Bujak, M.; Ying, X.; Chatila, K.; Haudek, S.; Thakker, G.; Frangogiannis, N.G. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J. Immunol. Baltim. Md 1950 2008, 180, 2625–2633. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From Adhesion Molecules to Signalling Regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Acharya, A.; Baek, S.T.; Huang, G.; Eskiocak, B.; Goetsch, S.; Sung, C.Y.; Banfi, S.; Sauer, M.F.; Olsen, G.S.; Duffield, J.S.; et al. The BHLH Transcription Factor Tcf21 Is Required for Lineage-Specific EMT of Cardiac Fibroblast Progenitors. Dev. Camb. Engl. 2012, 139, 2139–2149. [Google Scholar] [CrossRef]
- Ali, S.R.; Ranjbarvaziri, S.; Talkhabi, M.; Zhao, P.; Subat, A.; Hojjat, A.; Kamran, P.; Müller, A.M.S.; Volz, K.S.; Tang, Z.; et al. Developmental Heterogeneity of Cardiac Fibroblasts Does Not Predict Pathological Proliferation and Activation. Circ. Res. 2014, 115, 625–635. [Google Scholar] [CrossRef]
- Moore-Morris, T.; Guimarães-Camboa, N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M.; et al. Resident Fibroblast Lineages Mediate Pressure Overload–Induced Cardiac Fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, J.; Liu, Q.; Dodlapati, S.; Ming, H.; Wang, L.; Li, Y.; Li, R.; Jiang, Z.; Francis, J.; et al. The Landscape of Accessible Chromatin in Quiescent Cardiac Fibroblasts and Cardiac Fibroblasts Activated after Myocardial Infarction. Epigenetics 2021, 25, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, N.; Graf, K.; Do, Y.S.; Nunohiro, T.; Giachelli, C.M.; Meehan, W.P.; Tuan, T.L.; Hsueh, W.A. Osteopontin Is Produced by Rat Cardiac Fibroblasts and Mediates A(II)-Induced DNA Synthesis and Collagen Gel Contraction. J. Clin. Investig. 1996, 98, 2218–2227. [Google Scholar] [CrossRef]
- Komatsubara, I.; Murakami, T.; Kusachi, S.; Nakamura, K.; Hirohata, S.; Hayashi, J.; Takemoto, S.; Suezawa, C.; Ninomiya, Y.; Shiratori, Y. Spatially and Temporally Different Expression of Osteonectin and Osteopontin in the Infarct Zone of Experimentally Induced Myocardial Infarction in Rats. Cardiovasc. Pathol. 2003, 12, 186–194. [Google Scholar] [CrossRef]
- Cleutjens, J.P.M.; Kandala, J.C.; Guarda, E.; Guntaka, R.V.; Weber, K.T. Regulation of Collagen Degradation in the Rat Myocardium after Infarction. J. Mol. Cell. Cardiol. 1995, 27, 1281–1292. [Google Scholar] [CrossRef]
- Akamatsu, T.; Arai, Y.; Kosugi, I.; Kawasaki, H.; Meguro, S.; Sakao, M.; Shibata, K.; Suda, T.; Chida, K.; Iwashita, T. Direct Isolation of Myofibroblasts and Fibroblasts from Bleomycin-Injured Lungs Reveals Their Functional Similarities and Differences. Fibrogenesis Tissue Repair 2013, 6, 15. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Hinz, B. Formation and Function of the Myofibroblast during Tissue Repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized Fibroblast Differentiated States Underlie Scar Formation in the Infarcted Mouse Heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef]
- Rai, V.; Sharma, P.; Agrawal, S.; Agrawal, D.K. Relevance of Mouse Models of Cardiac Fibrosis and Hypertrophy in Cardiac Research. Mol. Cell. Biochem. 2017, 424, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Q.; Li, C.; Li, Y.; Wang, L. Cardiac Fibrosis and Cardiac Fibroblast Lineage-Tracing: Recent Advances. Front. Physiol. 2020, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.G.; Klinghoffer, R.A.; Corrin, P.D.; Soriano, P. Evolutionary Divergence of Platelet-Derived Growth Factor Alpha Receptor Signaling Mechanisms. Mol. Cell. Biol. 2003, 23, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Teng, Y.; O’Connell, J.T.; Charytan, D.; Müller, G.A.; Müller, C.A.; Sugimoto, H.; Kalluri, R. Identification of Human Epididymis Protein-4 as a Fibroblast-Derived Mediator of Fibrosis. Nat. Med. 2013, 19, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B. Inducible Gene Targeting in Mice Using the Cre/Lox System. Methods 1998, 14, 381–392. [Google Scholar] [CrossRef]
- Soriano, P. Generalized LacZ Expression with the ROSA26 Cre Reporter Strain. Nat. Genet. 1999, 21, 70–71. [Google Scholar] [CrossRef]
- Wendling, O.; Bornert, J.-M.; Chambon, P.; Metzger, D. Efficient Temporally-Controlled Targeted Mutagenesis in Smooth Muscle Cells of the Adult Mouse. Genesis 2009, 47, 14–18. [Google Scholar] [CrossRef]
- Ubil, E.; Duan, J.; Pillai, I.C.L.; Rosa-Garrido, M.; Wu, Y.; Bargiacchi, F.; Lu, Y.; Stanbouly, S.; Huang, J.; Rojas, M.; et al. Mesenchymal-Endothelial Transition Contributes to Cardiac Neovascularization. Nature 2014, 514, 585–590. [Google Scholar] [CrossRef]
- Acharya, A.; Baek, S.T.; Banfi, S.; Eskiocak, B.; Tallquist, M.D. Efficient Inducible Cre-Mediated Recombination in Tcf21 Cell Lineages in the Heart and Kidney. Genesis 2011, 49, 870–877. [Google Scholar] [CrossRef]
- Kisanuki, Y.Y.; Hammer, R.E.; Miyazaki, J.; Williams, S.C.; Richardson, J.A.; Yanagisawa, M. Tie2-Cre Transgenic Mice: A New Model for Endothelial Cell-Lineage Analysis In Vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.-C.J.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic Lineage Tracing Defines Myofibroblast Origin and Function in the Injured Heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Zhang, C.; Li, P.; Wu, Y.; Wang, C.; Lau, W.B.; Ma, X.; Du, J. Cardiac Fibroblast-Specific Activating Transcription Factor 3 Protects Against Heart Failure by Suppressing MAP2K3-P38 Signaling. Circulation 2017, 135, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.L.; Duenas, E.T.; Park, A.; Daugherty, A.; Kahn, J.; Kowalski, J.; Cuthbertson, A. Development of Poly-(d,l-Lactide–Coglycolide) Microsphere Formulations Containing Recombinant Human Vascular Endothelial Growth Factor to Promote Local Angiogenesis. J. Controll Release 2001, 72, 13–24. [Google Scholar] [CrossRef]
- Dvir, T.; Bauer, M.; Schroeder, A.; Tsui, J.H.; Anderson, D.G.; Langer, R.; Liao, R.; Kohane, D.S. Nanoparticles Targeting the Infarcted Heart. Nano Lett. 2011, 11, 4411–4414. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Kim, Y.; Duvall, C.L.; Bellamkonda, R.V.; Gupta, D.; Lin, A.S.; Weiss, D.; Robert Taylor, W.; Guldberg, R.E. Sustained VEGF Delivery via PLGA Nanoparticles Promotes Vascular Growth. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H1959–H1965. [Google Scholar] [CrossRef]
- Harel-Adar, T.; Mordechai, T.B.; Amsalem, Y.; Feinberg, M.S.; Leor, J.; Cohen, S. Modulation of Cardiac Macrophages by Phosphatidylserine-Presenting Liposomes Improves Infarct Repair. Proc. Natl. Acad. Sci. USA 2011, 108, 1827–1832. [Google Scholar] [CrossRef]
- Rocha, F.G.; Sundback, C.A.; Krebs, N.J.; Leach, J.K.; Mooney, D.J.; Ashley, S.W.; Vacanti, J.P.; Whang, E.E. The Effect of Sustained Delivery of Vascular Endothelial Growth Factor on Angiogenesis in Tissue-Engineered Intestine. Biomaterials 2008, 29, 2884–2890. [Google Scholar] [CrossRef]
- Scott, R.C.; Rosano, J.M.; Ivanov, Z.; Wang, B.; Chong, P.L.-G.; Issekutz, A.C.; Crabbe, D.L.; Kiani, M.F. Targeting VEGF-Encapsulated Immunoliposomes to MI Heart Improves Vascularity and Cardiac Function. FASEB J. 2009, 23, 3361–3367. [Google Scholar] [CrossRef]
- Ho, Y.T.; Poinard, B.; Kah, J.C.Y. Nanoparticle Drug Delivery Systems and Their Use in Cardiac Tissue Therapy. Nanomed. 2016, 11, 693–714. [Google Scholar] [CrossRef]
- Shevach, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold Nanoparticle-Decellularized Matrix Hybrids for Cardiac Tissue Engineering. Nano Lett. 2014, 14, 5792–5796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, H.; Chen, J.; Hu, Q.; Yang, S.; Liu, X. Silica-Coated Magnetic Nanoparticles Labeled Endothelial Progenitor Cells Alleviate Ischemic Myocardial Injury and Improve Long-Term Cardiac Function with Magnetic Field Guidance in Rats with Myocardial Infarction. J. Cell. Physiol. 2019, 234, 18544–18559. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Vieira, L.F.; Lins, M.P.; Viana, I.M.M.N.; dos Santos, J.E.; Smaniotto, S.; dos Santos Reis, M.D. Metallic Nanoparticles Reduce the Migration of Human Fibroblasts In Vitro. Nanoscale Res. Lett. 2017, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Koshman, Y.E.; Waters, S.B.; Walker, L.A.; Los, T. Delivery and Visualization of Proteins Conjugated to Quantum Dots in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2008, 45, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Allijn, I.E.; Czarny, B.M.S.; Wang, X.; Chong, S.Y.; Weiler, M.; da Silva, A.E.; Metselaar, J.M.; Lam, C.S.P.; Pastorin, G.; de Kleijn, D.P.V.; et al. Liposome Encapsulated Berberine Treatment Attenuates Cardiac Dysfunction after Myocardial Infarction. J. Controll Release 2017, 247, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Vij, N.; Min, T.; Marasigan, R.; Belcher, C.N.; Mazur, S.; Ding, H.; Yong, K.-T.; Roy, I. Development of PEGylated PLGA Nanoparticle for Controlled and Sustained Drug Delivery in Cystic Fibrosis. J. Nanobiotechnol. 2010, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Croy, S.R.; Kwon, G.S. Polymeric Micelles for Drug Delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an Important Inorganic Nanoparticle Applied in Drug Carrier Systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef]
- Meyers, M.W.; Rink, J.S.; Jiang, Q.; Kelly, M.E.; Vercammen, J.M.; Thaxton, C.S.; Kibbe, M.R. Systemically Administered Collagen-targeted Gold Nanoparticles Bind to Arterial Injury Following Vascular Interventions. Physiol. Rep. 2017, 5, e13128. [Google Scholar] [CrossRef]
- Radaic, A.; Joo, N.E.; Jeong, S.-H.; Yoo, S.-I.; Kotov, N.; Kapila, Y.L. Phosphatidylserine-Gold Nanoparticles (PS-AuNP) Induce Prostate and Breast Cancer Cell Apoptosis. Pharmaceutics 2021, 13, 1094. [Google Scholar] [CrossRef]
- Saha, S.; Xiong, X.; Chakraborty, P.K.; Shameer, K.; Arvizo, R.R.; Kudgus, R.A.; Dwivedi, S.K.D.; Hossen, M.N.; Gillies, E.M.; Robertson, J.D.; et al. Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth. ACS Nano 2016, 10, 10636–10651. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. Cy5.5-Gly-Pro-Leu-Gly-Val-Arg-Gly-Cys-Gold Nanoparticles. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Liu, L.; Cai, R.; Wang, Y.; Tao, G.; Ai, L.; Wang, P.; Yang, M.; Zuo, H.; Zhao, P.; He, H. Polydopamine-Assisted Silver Nanoparticle Self-Assembly on Sericin/Agar Film for Potential Wound Dressing Application. Int. J. Mol. Sci. 2018, 19, 2875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, L.; Tong, T.; Zhang, W.; Wang, Z.; Wang, J.; Wang, S. Antifouling and Antibacterial Behavior of Polyethersulfone Membrane Incorporating Polyaniline@silver Nanocomposites. Environ. Sci. Water Res. Technol. 2017, 3, 710–719. [Google Scholar] [CrossRef]
- Veeraraghavan, V.P.; Periadurai, N.D.; Karunakaran, T.; Hussain, S.; Surapaneni, K.M.; Jiao, X. Green Synthesis of Silver Nanoparticles from Aqueous Extract of Scutellaria Barbata and Coating on the Cotton Fabric for Antimicrobial Applications and Wound Healing Activity in Fibroblast Cells (L929). Saudi J. Biol. Sci. 2021, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Becak DP, H.N.; Shannahan, J.H. Cardiac Ischemia Reperfusion Injury Following Instillation of 20 Nm Citrate-Capped Nanosilver. J. Nanomed. Nanotechnol. 2015, 6 (Suppl. 6), 6. [Google Scholar] [CrossRef]
- Martin, M.E.; Reaves, D.K.; Jeffcoat, B.; Enders, J.R.; Costantini, L.M.; Yeyeodu, S.T.; Botta, D.; Kavanagh, T.J.; Fleming, J.M. Silver Nanoparticles Alter Epithelial Basement Membrane Integrity, Cell Adhesion Molecule Expression, and TGF-Β1 Secretion. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102070. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Jiang, X.; Wang, J.; Chen, C.; Liu, R.-S. Nano–Bio Effects: Interaction of Nanomaterials with Cells. Nanoscale 2013, 5, 3547–3569. [Google Scholar] [CrossRef]
- Kittana, N.; Assali, M.; Abu-Rass, H.; Lutz, S.; Hindawi, R.; Ghannam, L.; Zakarneh, M.; Mousa, A. Enhancement of Wound Healing by Single-Wall/Multi-Wall Carbon Nanotubes Complexed with Chitosan. Int. J. Nanomed. 2018, 13, 7195–7206. [Google Scholar] [CrossRef]
- Stout, D.A.; Raimondo, E.; Marostica, G.; Webster, T.J. Growth Characteristics of Different Heart Cells on Novel Nanopatch Substrate during Electrical Stimulation. Biomed. Mater. Eng. 2014, 24, 2101–2107. [Google Scholar] [CrossRef]
- Pinaud, F.; Michalet, X.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Iyer, G.; Weiss, S. Advances in Fluorescence Imaging with Quantum Dot Bio-Probes. Biomaterials 2006, 27, 1679–1687. [Google Scholar] [CrossRef]
- Haghshenas, M.; Hoveizi, E.; Mohammadi, T.; Kazemi Nezhad, S.R. Use of Embryonic Fibroblasts Associated with Graphene Quantum Dots for Burn Wound Healing in Wistar Rats. In Vitro Cell. Dev. Biol.-Anim. 2019, 55, 312–322. [Google Scholar] [CrossRef]
- Yang, L.; Xue, S.; Du, M.; Lian, F. Highly Efficient MicroRNA Delivery Using Functionalized Carbon Dots for Enhanced Conversion of Fibroblasts to Cardiomyocytes. Int. J. Nanomed. 2021, 16, 3741–3754. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Kwon, S.P.; Hwang, B.-H.; Park, E.-H.; Kim, H.Y.; Lee, J.-R.; Kang, M.; Song, S.Y.; Jung, M.; Sohn, H.S.; Kim, E.; et al. Nanoparticle-Mediated Blocking of Excessive Inflammation for Prevention of Heart Failure Following Myocardial Infarction. Small Weinh. Bergstr. Ger. 2021, 17, e2101207. [Google Scholar] [CrossRef]
- Li, M.; Tang, X.; Liu, X.; Cui, X.; Lian, M.; Zhao, M.; Peng, H.; Han, X. Targeted MiR-21 Loaded Liposomes for Acute Myocardial Infarction. J. Mater. Chem. B 2020, 8, 10384–10391. [Google Scholar] [CrossRef]
- Dasa, S.S.K.; Suzuki, R.; Gutknecht, M.; Brinton, L.T.; Tian, Y.; Michaelsson, E.; Lindfors, L.; Klibanov, A.L.; French, B.A.; Kelly, K.A. Development of Target-Specific Liposomes for Delivering Small Molecule Drugs after Reperfused Myocardial Infarction. J. Control. Release 2015, 220, 556–567. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, W.; Zhang, Z.; Zhu, J.; Fu, Y.; Lin, Q.; Kuang, S.; Zhang, M.; Shan, Z. MicroRNA-199a-3p enhances expressions of fibrosis-associated genes through targeting Smad1 in mouse cardiac fibroblasts. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 1203–1208. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Zhang, M.; Tian, X.-Q.; Zheng, L.; Lu, C.-T. Using Basic Fibroblast Growth Factor Nanoliposome Combined with Ultrasound-Introduced Technology to Early Intervene the Diabetic Cardiomyopathy. Int. J. Nanomed. 2016, 11, 675–686. [Google Scholar] [CrossRef][Green Version]
- Yaşacan, M.; Erikçi, A.; Eylem, C.C.; Çiftçi, S.Y.; Nemutlu, E.; Ulubayram, K.; Eroğlu, İ. Polymeric Nanoparticle Versus Liposome Formulations: Comparative Physicochemical and Metabolomic Studies as l-Carnitine Delivery Systems. AAPS PharmSciTech 2020, 21, 308. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block Copolymer Micelles as Long-Circulating Drug Vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C. Micellar Solubilization of Drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–165. [Google Scholar] [PubMed]
- O’Neil, C.P.; van der Vlies, A.J.; Velluto, D.; Wandrey, C.; Demurtas, D.; Dubochet, J.; Hubbell, J.A. Extracellular Matrix Binding Mixed Micelles for Drug Delivery Applications. J. Controll Release 2009, 137, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Piras, B.A.; O’Connor, D.M.; French, B.A. Systemic Delivery of ShRNA by AAV9 Provides Highly Efficient Knockdown of Ubiquitously Expressed GFP in Mouse Heart, but not Liver. PLoS ONE 2013, 8, e75894. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, L.; Wen, X.; Gao, L.; Li, G.; Chang, G.; Qin, S.; Zhang, D. TNAP Is a Novel Regulator of Cardiac Fibrosis after Myocardial Infarction by Mediating TGF-β/Smads and ERK1/2 Signaling Pathways. EBioMedicine 2021, 67, 103370. [Google Scholar] [CrossRef]
- Liu, C.; Hu, T.; Cai, Z.; Xie, Q.; Yuan, Y.; Li, N.; Xie, S.; Yao, Q.; Zhao, J.; Wu, Q.Q.; et al. Nucleotide-Binding Oligomerization Domain-Like Receptor 3 Deficiency Attenuated Isoproterenol-Induced Cardiac Fibrosis via Reactive Oxygen Species/High Mobility Group Box 1 Protein Axis. Front. Cell Dev. Biol. 2020, 8, 713. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Li, X.; Zhao, L.; Xi, H.; Hu, W.; Li, S. Lefty1 Ameliorates Post-Infarction Fibrosis by Suppressing p-Smad2 and p-ERK1/2 Signaling Pathways. J Cardiovasc. Transl. Res. 2021, 14, 636–646. [Google Scholar] [CrossRef]
- Prasad, K.-M.R.; Xu, Y.; Yang, Z.; Acton, S.T.; French, B.A. Robust Cardiomyocyte-Specific Gene Expression Following Systemic Injection of AAV: In Vivo Gene Delivery Follows a Poisson Distribution. Gene Ther. 2011, 18, 43–52. [Google Scholar] [CrossRef]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV Serotypes 1–9 Mediated Gene Expression and Tropism in Mice After Systemic Injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef]
- Piras, B.A.; Tian, Y.; Xu, Y.; Thomas, N.A.; O’Connor, D.M.; French, B.A. Systemic Injection of AAV9 Carrying a Periostin Promoter Targets Gene Expression to a Myofibroblast-like Lineage in Mouse Hearts after Reperfused Myocardial Infarction. Gene Ther. 2016, 23, 469–478. [Google Scholar] [CrossRef]
- Kawakami, T.; Kanazawa, H.; Satoh, T.; Ieda, M.; Ieda, Y.; Kimura, K.; Mochizuki, H.; Shimada, T.; Yokoyama, C.; Ogawa, S.; et al. AAV-PGIS Gene Transfer Improves Hypoxia-Induced Pulmonary Hypertension in Mice. Biochem. Biophys. Res. Commun. 2007, 363, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Hu, J.; Pratt, R.E.; Hodgkinson, C.P.; Asokan, A.; Dzau, V.J. Optimizing Delivery for Efficient Cardiac Reprogramming. Biochem. Biophys. Res. Commun. 2020, 533, 9–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cakir, S.N.; Whitehead, K.M.; Hendricks, H.K.L.; de Castro Brás, L.E. Novel Techniques Targeting Fibroblasts after Ischemic Heart Injury. Cells 2022, 11, 402. https://doi.org/10.3390/cells11030402
Cakir SN, Whitehead KM, Hendricks HKL, de Castro Brás LE. Novel Techniques Targeting Fibroblasts after Ischemic Heart Injury. Cells. 2022; 11(3):402. https://doi.org/10.3390/cells11030402
Chicago/Turabian StyleCakir, Sirin N., Kaitlin M. Whitehead, Hanifah K. L. Hendricks, and Lisandra E. de Castro Brás. 2022. "Novel Techniques Targeting Fibroblasts after Ischemic Heart Injury" Cells 11, no. 3: 402. https://doi.org/10.3390/cells11030402
APA StyleCakir, S. N., Whitehead, K. M., Hendricks, H. K. L., & de Castro Brás, L. E. (2022). Novel Techniques Targeting Fibroblasts after Ischemic Heart Injury. Cells, 11(3), 402. https://doi.org/10.3390/cells11030402




