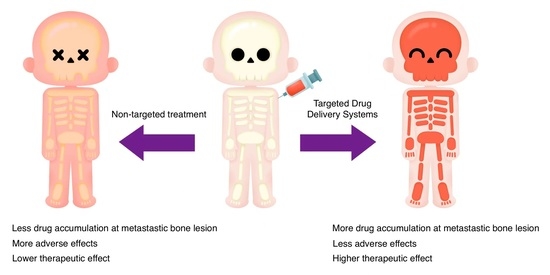
Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems
Abstract
:1. Introduction
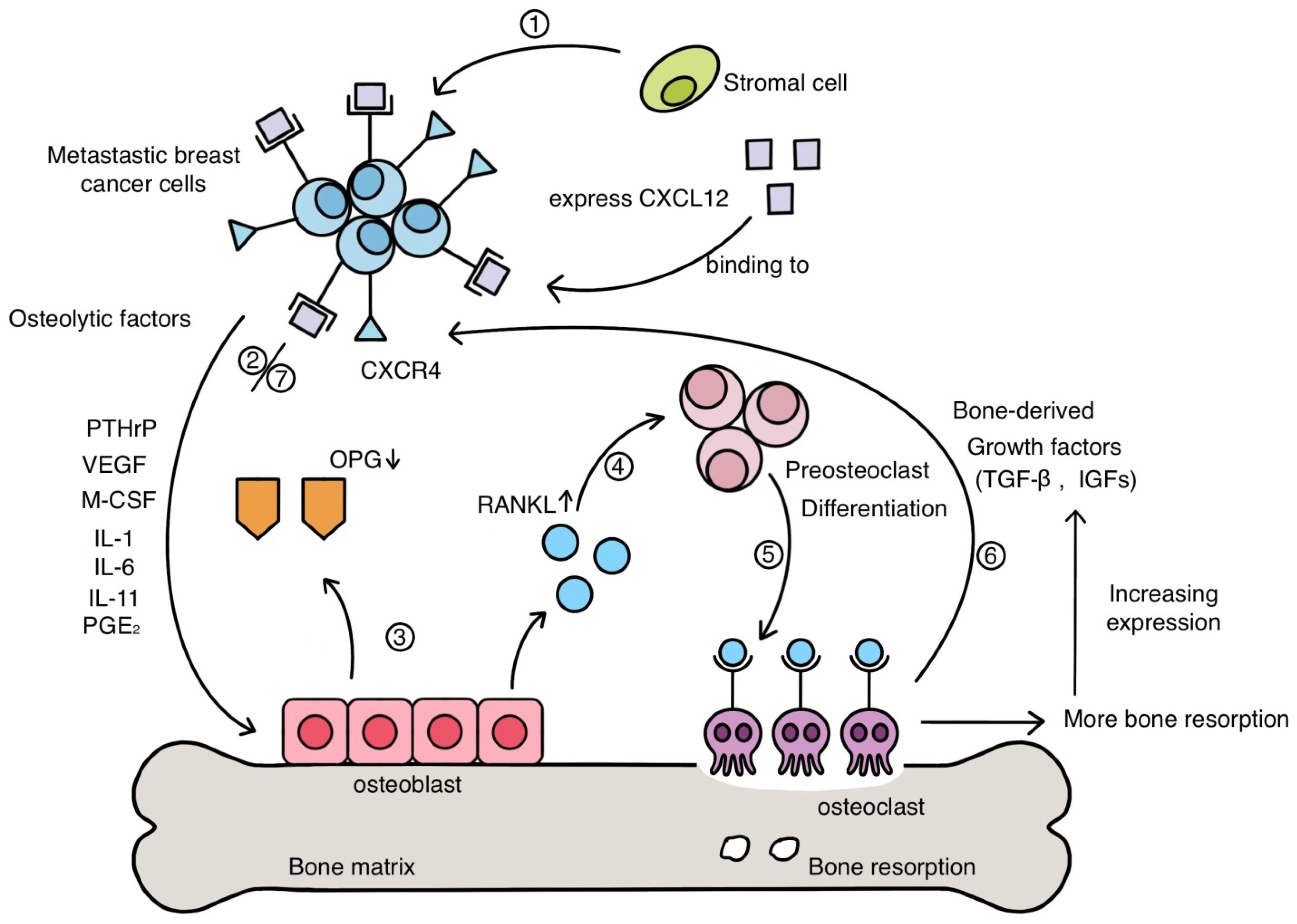
2. Mechanism of BC Bone Metastasis
2.1. Invasion
2.2. Migration
2.3. Adhesion
2.4. Survival
3. Conventional Treatments of BC Bone Metastasis
4. Targeting Agents for BC Bone Metastasis
4.1. Arginine-Glycine-Aspartic Acid (RGD) Peptide and Its Derivative
4.2. Bisphosphonate
4.2.1. Alendronate
4.2.2. Zoledronic Acid
5. Advanced Targeted DDSs
5.1. Targeted DDSs Loaded with Anticancer Agents
5.1.1. Cisplatin Prodrug
5.1.2. Bortezomib
5.1.3. Curcumin
5.1.4. Bortezomib and Curcumin
5.1.5. Doxorubicin
5.1.6. Docetaxel
5.1.7. Paclitaxel
5.1.8. Non-Cytotoxic Payloads
5.2. Targeted DDSs with Immunostimulatory Payloads
5.3. Targeted DDSs Loaded with Contrast Agents
5.4. Targeted DDSs Loaded with Photothermal Therapeutic Agents
5.5. Targeted DDSs Loaded with Photodynamic Therapeutic Agents
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Breast Cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 25 July 2021).
- How Common Is Breast Cancer? 2020. Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 25 July 2021).
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Parker, J. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, C.; Zhang, J.; Kong, L.; Zhu, H.; Yu, J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: A SEER based study. Oncotarget 2017, 8, 26368–26379. [Google Scholar] [CrossRef] [PubMed]
- Oner, G.; Altintas, S.; Canturk, Z.; Tjalma, W.; Verhoeven, Y.; Van Berckelaer, C.; Berneman, Z.; Peeters, M.; Pauwels, P.; van Dam, P.A. Triple-negative breast cancer-Role of immunology: A systemic review. Breast J. 2020, 26, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Alluri, P.; Newman, L.A. Basal-like and triple-negative breast cancers: Searching for positives among many negatives. Surg. Oncol. Clin. N. Am. 2014, 23, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef]
- Provenzano, E.; Ulaner, G.A.; Chin, S.F. Molecular Classification of Breast Cancer. PET Clin. 2018, 13, 325–338. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, Z.; Ben-David, Y.; Woodgett, J.R.; Zacksenhaus, E. Molecular stratification within triple-negative breast cancer subtypes. Sci. Rep. 2019, 9, 19107. [Google Scholar] [CrossRef] [Green Version]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Gerratana, L.; Fanotto, V.; Bonotto, M.; Bolzonello, S.; Minisini, A.M.; Fasola, G.; Puglisi, F. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 2015, 32, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Beca, F.; Santos, R.; Vieira, D.; Zeferino, L.; Dufloth, R.; Schmitt, F. Primary relapse site pattern in women with triple-negative breast cancer. Pathol. Res. Pract. 2014, 210, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.; Lundin, J.; Lundin, M.; Lehtimaki, T.; Ristimaki, A.; Holli, K.; Sailas, L.; Kataja, V.; Turpeenniemi-Hujanen, T.; Isola, J.; et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: A nationwide cohort study. Breast Cancer Res. BCR 2011, 13, R87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhu, W.; Biskup, E.; Yang, W.; Yang, Z.; Wang, H.; Qiu, X.; Zhang, C.; Hu, G.; Hu, G. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal-related events (SREs) in breast cancer patients: A systematic review of the real world data. J. Bone Oncol. 2018, 11, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Odle, T.G. Precision Medicine in Breast Cancer. Radiol. Technol. 2017, 88, 401M–421M. [Google Scholar] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis the seed and soil hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Silvestris, E.; Paradiso, A.; Cives, M.; Tucci, M. Role of Bone Targeting Agents in the Prevention of Bone Metastases from Breast Cancer. Int. J. Mol. Sci. 2020, 21, 3022. [Google Scholar] [CrossRef]
- Mareel, M.; Leroy, A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol. Rev. 2003, 83, 337–376. [Google Scholar] [CrossRef]
- Au, S.H.; Storey, B.D.; Moore, J.C.; Tang, Q.; Chen, Y.L.; Javaid, S.; Sarioglu, A.F.; Sullivan, R.; Madden, M.W.; O’Keefe, R.; et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 4947–4952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douma, S.; Van Laar, T.; Zevenhoven, J.; Meuwissen, R.; Van Garderen, E.; Peeper, D.S. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004, 430, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ting, D.T.; Stott, S.L.; Wittner, B.S.; Ozsolak, F.; Paul, S.; Ciciliano, J.C.; Smas, M.E.; Winokur, D.; Gilman, A.J.; et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 2012, 487, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Wang, J.; Schneider, A.; Sun, Y.X.; Koh-Paige, A.J.; Osman, N.I.; McCauley, L.K.; Taichman, R.S. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 2006, 38, 497–508. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Silva, I.; Branco, J.C. Rank/Rankl/opg: Literature review. Acta Reumatol. Port. 2011, 36, 209–218. [Google Scholar]
- Asano, T.; Okamoto, K.; Nakai, Y.; Tsutsumi, M.; Muro, R.; Suematsu, A.; Hashimoto, K.; Okamura, T.; Ehata, S.; Nitta, T.; et al. Soluble RANKL is physiologically dispensable but accelerates tumour metastasis to bone. Nat. Metab. 2019, 1, 868–875. [Google Scholar] [CrossRef]
- Demirkan, B. The Roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in Breast Cancer Bone Metastasis: Potential Targets for Prevention and Treatment. J. Clin. Med. 2013, 2, 264–282. [Google Scholar] [CrossRef] [Green Version]
- Shao, G.; Gu, W.; Guo, M.; Zang, S.; Fu, J.; Liu, S.; Wang, F.; Wang, Z. Clinical study of 99mTc-3P-RGD2 peptide imaging in osteolytic. Oncotarget 2017, 8, 75587. [Google Scholar] [CrossRef] [Green Version]
- Chilla, A.; Bianconi, D.; Geetha, N.; Dorda, A.; Poettler, M.; Unseld, M.; Sykoutri, D.; Redlich, K.; Zielinski, C.C.; Prager, G.W. Effects of cilengitide in osteoclast maturation and behavior. Exp. Cell Res. 2015, 337, 68–75. [Google Scholar] [CrossRef]
- Zou, W.; Kitaura, H.; Reeve, J.; Long, F.; Tybulewicz, V.L.; Shattil, S.J.; Ginsberg, M.H.; Ross, F.P.; Teitelbaum, S.L. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007, 176, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Felding-Habermann, B.; O’Toole, T.E.; Smith, J.W.; Fransvea, E.; Ruggeri, Z.M.; Ginsberg, M.H.; Hughes, P.E.; Pampori, N.; Shattil, S.J.; Saven, A.; et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillett, C.E.; Miles, D.W.; Ryder, K.; Skilton, D.; Liebman, R.D.; Springall, R.J.; Barnes, D.M.; Hanby, A.M. Retention of the expression of E-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J. Pathol. 2001, 193, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Gamallo, C.A.; Palacios, J.; Suarez, A.; Pizarro, A.; Navarro, P.; Quintanilla, M.A.; Cano, A. Correlation of E-cadherin Expression With Differentiation Grade and Histological Type in Breast Carcinoma. Am. J. Pathol. 1993, 142, 987–993. [Google Scholar]
- Lim, S.; Lee, M. Significance of E-cadherin/β-catenin complex and cyclin D1 in breast cancer. Oncol. Rep. 2002, 9, 915–928. [Google Scholar] [CrossRef]
- Yoshida, R.; Kimura, N.; Harada, Y.; Ohuchi, N. The loss of E-cadherin, alpha- and beta-catenin expression is associated with metastasis and poor prognosis in invasive breast cancer. Int. J. Oncol. 2001, 18, 513–520. [Google Scholar]
- Saha, B.; Chaiwun, B.; Imam, S.S.; Tsao-Wei, D.D.; Groshen, S.; Naritoku, W.Y.; Imam, S.A. Overexpression of E-cadherin Protein in Metastatic Breast Cancer Cells in Bone. Anticancer Res. 2007, 27, 3903–3908. [Google Scholar]
- Wang, H.; Yu, C.; Gao, X.; Welte, T.; Muscarella, A.M.; Tian, L.; Zhao, H.; Zhao, Z.; Du, S.; Tao, J.; et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 2015, 27, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Satriyo, P.B.; Bamodu, O.A.; Chen, J.H.; Aryandono, T.; Haryana, S.M.; Yeh, C.T.; Chao, T.Y. Cadherin 11 Inhibition Downregulates beta-catenin, Deactivates the Canonical WNT Signalling Pathway and Suppresses the Cancer Stem Cell-Like Phenotype of Triple Negative Breast Cancer. J. Clin. Med. 2019, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Kozlow, W.; Guise, T.A. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J. Mammary Gland. Biol. Neoplasia 2005, 10, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Waning, D.L.; Guise, T.A.; Mohammad, K.S. A “Connexin” Responsible for the Fatal Attraction of Cancer to Bone. Cell Metab. 2019, 29, 6–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK System-Based Mechanism for Breast Cancer Bone Metastasis and Related Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Syed, V. TGF-beta Signaling in Cancer. J. Cell Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soki, F.N.; Park, S.I.; McCauley, L.K. The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol. 2012, 8, 803–817. [Google Scholar] [CrossRef] [Green Version]
- Le Pape, F.; Vargas, G.; Clezardin, P. The role of osteoclasts in breast cancer bone metastasis. J. Bone Oncol. 2016, 5, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Kent, C.L.; McDuff, S.G.R.; Salama, J.K. Oligometastatic breast cancer: Where are we now and where are we headed?—A narrative review. Ann. Palliat. Med. 2020, 9, 62–72. [Google Scholar] [CrossRef]
- Leto, G. Current status and future directions in the treatment of bone metastases from breast cancer. Clin. Exp. Pharmacol. Physiol. 2019, 46, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, F.; Orlandi, A.; Manfrida, S.; Masiello, V.; Di Leone, A.; Massaccesi, M.; Moschella, F.; Franceschini, G.; Bria, E.; Gambacorta, M.A.; et al. Diagnosis and Treatment of Bone Metastases in Breast Cancer: Radiotherapy, Local Approach and Systemic Therapy in a Guide for Clinicians. Cancers 2020, 12, 2390. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.; Trippa, F.; Barone, G.; Maranzano, E. Prevention and Treatment of Bone Metastases in Breast Cancer. J. Clin. Med. 2013, 2, 151–175. [Google Scholar] [CrossRef]
- Katsumi, H.; Yamashita, S.; Morishita, M.; Yamamoto, A. Bone-Targeted Drug Delivery Systems and Strategies for Treatment of Bone Metastasis. Chem. Pharm. Bull. 2020, 68, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cai, X.; Yang, J.; Wang, C.; Tong, L.; Xiao, J.; Li, L. A Targeted and pH-Responsive Bortezomib Nanomedicine in the Treatment of Metastatic Bone Tumors. ACS Appl. Mater. Interfaces 2018, 10, 41003–41011. [Google Scholar] [CrossRef]
- Liu, T.; Romanova, S.; Wang, S.; Hyun, M.A.; Zhang, C.; Cohen, S.M.; Singh, R.K.; Bronich, T.K. Alendronate-Modified Polymeric Micelles for the Treatment of Breast Cancer Bone Metastasis. Mol. Pharm. 2019, 16, 2872–2883. [Google Scholar] [CrossRef]
- Sun, W.; Ge, K.; Jin, Y.; Han, Y.; Zhang, H.; Zhou, G.; Yang, X.; Liu, D.; Liu, H.; Liang, X.J.; et al. Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano 2019, 13, 7556–7567. [Google Scholar] [CrossRef]
- Foubert, P.; Varner, J.A. Integrins in tumor angiogenesis and lymphangiogenesis. Methods Mol. Biol. 2012, 757, 471–486. [Google Scholar]
- Wang, L.; Song, L.; Li, J.; Wang, Y.; Yang, C.; Kou, X.; Xiao, B.; Zhang, W.; Li, L.; Liu, S.; et al. Bone sialoprotein-alphavbeta3 integrin axis promotes breast cancer metastasis to the bone. Cancer Sci. 2019, 110, 3157–3172. [Google Scholar] [CrossRef]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Sloan, E.K.; Pouliot, N.; Stanley, K.L.; Chia, J.; Moseley, J.M.; Hards, D.K.; Anderson, R.L. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. BCR 2006, 8, R20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danhier, F.; Le Breton, A.; Preat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; Amend, S.R.; Weilbaecher, K.N. Integrins and bone metastasis: Integrating tumor cell and stromal cell interactions. Bone 2011, 48, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Z.; Xiang, L.; Li, L.; Zhang, H.; Lin, F.; Cao, H. Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer. Drug Des. Dev. Ther. 2019, 13, 1357–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, M.; Gordon, A.; Shnyder, S.D.; Patterson, L.H.; Sheldrake, H.M. RGD-Binding Integrins in Prostate Cancer: Expression Patterns and Therapeutic Prospects against Bone Metastasis. Cancers 2012, 4, 1106–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Pignatello, R.; Cenni, E.; Micieli, D.; Fotia, C.; Salerno, M.; Granchi, D.; Avnet, S.; Sarpietro, M.G.; Castelli, F.; Baldini, N. A novel biomaterial for osteotropic drug nanocarriers: Synthesis and biocompatibility evaluation of a PLGA–ALE conjugate. Nanomedicine 2009, 4, 161–175. [Google Scholar] [CrossRef]
- Bai, S.B.; Liu, D.Z.; Cheng, Y.; Cui, H.; Liu, M.; Cui, M.X.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Osteoclasts and tumor cells dual targeting nanoparticle to treat bone metastases of lung cancer. Nanomedicine 2019, 21, 102054. [Google Scholar] [CrossRef]
- Celin, M.R.; Simon, J.C.; Krzak, J.J.; Fial, A.V.; Kruger, K.M.; Smith, P.A.; Harris, G.F. Do Bisphosphonates Alleviate Pain in Children? A Systematic Review. Curr. Osteoporos Rep. 2020, 18, 486–504. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007, 119 (Suppl. 2), S150–S162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.; Weber, D.R.; Kaplan, P.; Hummel, K.; Monk, H.M.; Levine, M.A. Short-Term Safety of Zoledronic Acid in Young Patients with Bone Disorders: An Extensive Institutional Experience. J. Clin. Endocrinol. Metab. 2015, 100, 4163–4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampson, G.; Fogelman, I. Clinical role of bisphosphonate therapy. Int. J. Women’s Health 2012, 4, 455–469. [Google Scholar]
- Lin, T.-J. Predicting binding affinities of nitrogen-containing bisphosphonates on hydroxyapatite surface by molecular dynamics. Chem. Phys. Lett. 2019, 716, 83–92. [Google Scholar] [CrossRef]
- Benyettou, F.; Lalatonne, Y.; Sainte-Catherine, O.; Monteil, M.; Motte, L. Superparamagnetic nanovector with anti-cancer properties: Gamma Fe2O3@Zoledronate. Int. J. Pharm. 2009, 379, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.L.; Zhao, Y.P.; Li, H.Q.; Na, R.; Li, F.; Mei, Q.B.; Zhao, M.G.; Zhou, S.Y. Doxorubicin-poly (ethylene glycol)-alendronate self-assembled micelles for targeted therapy of bone metastatic cancer. Sci. Rep. 2015, 5, 14614. [Google Scholar] [CrossRef] [PubMed]
- Rouach, V.; Goldshtein, I.; Wolf, I.; Catane, R.; Chodick, G.; Iton, A.; Stern, N.; Cohen, D. Exposure to alendronate is associated with a lower risk of bone metastases in osteoporotic women with early breast cancer. J. Bone Oncol. 2018, 12, 91–95. [Google Scholar] [CrossRef]
- Rudnick-Glick, S.; Corem-Salkmon, E.; Grinberg, I.; Margel, S. Targeted drug delivery of near IR fluorescent doxorubicin-conjugated poly(ethylene glycol) bisphosphonate nanoparticles for diagnosis and therapy of primary and metastatic bone cancer in a mouse model. J. Nanobiotechnol. 2016, 14, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Cornelissen, D.; Silverman, S.; Pinto, D.; Si, L.; Kremer, I.; Bours, S.; de Bot, R.; Boonen, A.; Evers, S.; et al. An Updated Systematic Review of Cost-Effectiveness Analyses of Drugs for Osteoporosis. Pharmacoeconomics 2021, 39, 181–209. [Google Scholar] [CrossRef]
- Wardley, A.; Davidson, N.; Barrett-Lee, P.; Hong, A.; Mansi, J.; Dodwell, D.; Murphy, R.; Mason, T.; Cameron, D. Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: A randomised, crossover study of community vs hospital bisphosphonate administration. Br. J. Cancer 2005, 92, 1869–1876. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H.J.P.R. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 9, 2539–2555. [Google Scholar]
- Forrest, M.L.; Kwon, G.S. Clinical developments in drug delivery nanotechnology. Adv. Drug Deliv. Rev. 2008, 60, 861–862. [Google Scholar] [CrossRef]
- Lababidi, N.; Sigal, V.; Koenneke, A.; Schwarzkopf, K.; Manz, A.; Schneider, M. Microfluidics as tool to prepare size-tunable PLGA nanoparticles with high curcumin encapsulation for efficient mucus penetration. Beilstein J. Nanotechnol. 2019, 10, 2280–2293. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Chen, S.H.; Liu, T.I.; Chuang, C.L.; Chen, H.H.; Chiang, W.H.; Chiu, H.C. Alendronate/folic acid-decorated polymeric nanoparticles for hierarchically targetable chemotherapy against bone metastatic breast cancer. J. Mater. Chem. B 2020, 8, 3789–3800. [Google Scholar] [CrossRef]
- Lim, C.W.; Kim, D. Bone targeting nano-aggregates prepared from self-assembled polyaspartamide graft copolymers for pH sensitive DOX delivery. Biomater. Sci. 2021, 9, 1660–1667. [Google Scholar] [CrossRef]
- Qiao, H.; Cui, Z.; Yang, S.; Ji, D.; Wang, Y.; Yang, Y.; Han, X.; Fan, Q.; Qin, A.; Wang, T.; et al. Targeting Osteocytes to Attenuate Early Breast Cancer Bone Metastasis by Theranostic Upconversion Nanoparticles with Responsive Plumbagin Release. ACS Nano 2017, 11, 7259–7273. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, Z.; Guan, Z.; Shen, Y.; Jiang, Y.; Xu, X.; Huang, Z.; Zhao, C. A light-triggered self-reinforced nanoagent for targeted chemo-photodynamic therapy of breast cancer bone metastases via ER stress and mitochondria mediated apoptotic pathways. J. Control. Release 2020, 319, 119–134. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Huang, Z.; Jiang, Y.; Sun, X.; Shen, Y.; Chu, W.; Zhao, C. Bisphosphonate-functionalized coordination polymer nanoparticles for the treatment of bone metastatic breast cancer. J. Control. Release 2017, 264, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, Z.; Guan, Z.; Zeng, Z.; Shen, Y.; Xu, X.; Zhao, C. Bone-seeking nanoplatform co-delivering cisplatin and zoledronate for synergistic therapy of breast cancer bone metastasis and bone resorption. Acta Pharm. Sin. B 2020, 10, 2384–2403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huo, Q.; Xu, M.; Yang, F.; Li, Y.; Shi, H.; Niu, Y.; Liu, Y. Bortezomib-catechol conjugated prodrug micelles: Combining bone targeting and aryl boronate-based pH-responsive drug release for cancer bone-metastasis therapy. Nanoscale 2018, 10, 18387–18397. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zou, S.; Guo, C.; Wang, K.; Zhao, F.; Fan, H.; Yin, J.; Chen, D. Multifunctional redox-responsive and CD44 receptor targeting polymer-drug nanomedicine based curcumin and alendronate: Synthesis, characterization and in vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Guo, C.; Dong, X.; Yu, Y.; Wang, B.; Liu, W.; Chen, D. In Vivo Evaluation of Reduction-Responsive Alendronate-Hyaluronan-Curcumin Polymer-Drug Conjugates for Targeted Therapy of Bone Metastatic Breast Cancer. Mol. Pharm. 2018, 15, 2764–2769. [Google Scholar] [CrossRef]
- Thamake, S.I.; Raut, S.L.; Gryczynski, Z.; Ranjan, A.P.; Vishwanatha, J.K. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials 2012, 33, 7164–7173. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Ye, W.L.; Liu, D.Z.; Cui, H.; Cheng, Y.; Liu, M.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Redox and pH dual sensitive bone targeting nanoparticles to treat breast cancer bone metastases and inhibit bone resorption. Nanoscale 2017, 9, 6264–6277. [Google Scholar] [CrossRef]
- Salerno, M.; Cenni, E.; Fotia, C.; Avnet, S.; Granchi, D.; Castelli, F.; Micieli, D.; Pignatello, R.; Capulli, M.; Rucci, N.; et al. Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr. Cancer Drug Targets 2010, 10, 649–659. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, H.T.; Phung, C.D.; Pathak, S.; Regmi, S.; Ha, D.-H.; Kim, J.O.; Yong, C.S.; Kim, S.K.; Choi, J.-E.; et al. Targeted delivery of doxorubicin for the treatment of bone metastasis from breast cancer using alendronate-functionalized graphene oxide nanosheets. J. Ind. Eng. Chem. 2019, 76, 310–317. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef]
- Otaka, A.; Yamaguchi, T.; Saisho, R.; Hiraga, T.; Iwasaki, Y. Bone-targeting phospholipid polymers to solubilize the lipophilic anticancer drug. J. Biomed. Mater. Res. A 2020, 108, 2090–2099. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Kumar, A.; Khandelwal, V.K.M.; Mishra, A.K.; Monkkonen, J.; Murthy, R.S.R. Targeting Efficiency and Biodistribution of Zoledronate Conjugated Docetaxel Loaded Pegylated PBCA Nanoparticles for Bone Metastasis. Adv. Funct. Mater. 2012, 22, 4101–4114. [Google Scholar] [CrossRef]
- Ross, M.H.; Esser, A.K.; Fox, G.C.; Schmieder, A.H.; Yang, X.; Hu, G.; Pan, D.; Su, X.; Xu, Y.; Novack, D.V.; et al. Bone-Induced Expression of Integrin beta3 Enables Targeted Nanotherapy of Breast Cancer Metastases. Cancer Res. 2017, 77, 6299–6312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonzi, G.; Salmaso, S.; Scomparin, A.; Eldar-Boock, A.; Satchi-Fainaro, R.; Caliceti, P. Novel pullulan bioconjugate for selective breast cancer bone metastases treatment. Bioconjug. Chem. 2015, 26, 489–501. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Y.; Xie, C.; Chen, C.; Lin, D.; Wang, S.; Lin, D.; Cui, X.; Guo, Z.; Zhou, J. Dual-active targeting liposomes drug delivery system for bone metastatic breast cancer: Synthesis and biological evaluation. Chem. Phys. Lipids 2019, 223, 104785. [Google Scholar] [CrossRef] [PubMed]
- Vanderburgh, J.; Hill, J.L.; Gupta, M.K.; Kwakwa, K.A.; Wang, S.K.; Moyer, K.; Bedingfield, S.K.; Merkel, A.R.; d’Arcy, R.; Guelcher, S.A.; et al. Tuning Ligand Density To Optimize Pharmacokinetics of Targeted Nanoparticles for Dual Protection against Tumor-Induced Bone Destruction. ACS Nano 2020, 14, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Fu, Y.; Li, C.; Wu, Z.; Cao, W.; Hu, X.; Sun, X.; He, W.; Cao, X.; Ling, D.; et al. Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer-Associated Osteolysis. Nano Lett. 2020, 20, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef]
- Cai, S.; Yan, J.; Xiong, H.; Xing, H.; Liu, Y.; Liu, S.; Liu, Z. Aptamer-Functionalized Molybdenum Disulfide Nanosheets for Tumor Cell Targeting and Lysosomal Acidic Environment/NIR Laser Responsive Drug Delivery to Realize Synergetic Chemo-Photothermal Therapeutic Effects. Int. J. Pharm. 2020, 590, 119948. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Chen, S.; Guo, Q.; Jing, Z.; Huang, B.; Pan, Y.; Wang, L.; Hu, Y. Zoledronate and SPIO dual-targeting nanoparticles loaded with ICG for photothermal therapy of breast cancer tibial metastasis. Sci. Rep. 2020, 10, 13675. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]


| Therapeutic Options | Main Indications |
|---|---|
| Systemic endocrine therapy | Disease control |
| Systemic chemotherapy | |
| Systemic targeted therapy | |
| Adjuvant bone-targeted therapy (bisphosphonates, denosumab) | SREs, bone loss and metastasis prevention |
| Radiotherapy | Bone pain relief Bone recalcification Metastatic spinal cord compression control (administered with concomitant steroids) |
| Surgical intervention | Bone pain relief Independence/mobility improvement SREs prevention |
| Analgesics | Chronic pain relief |
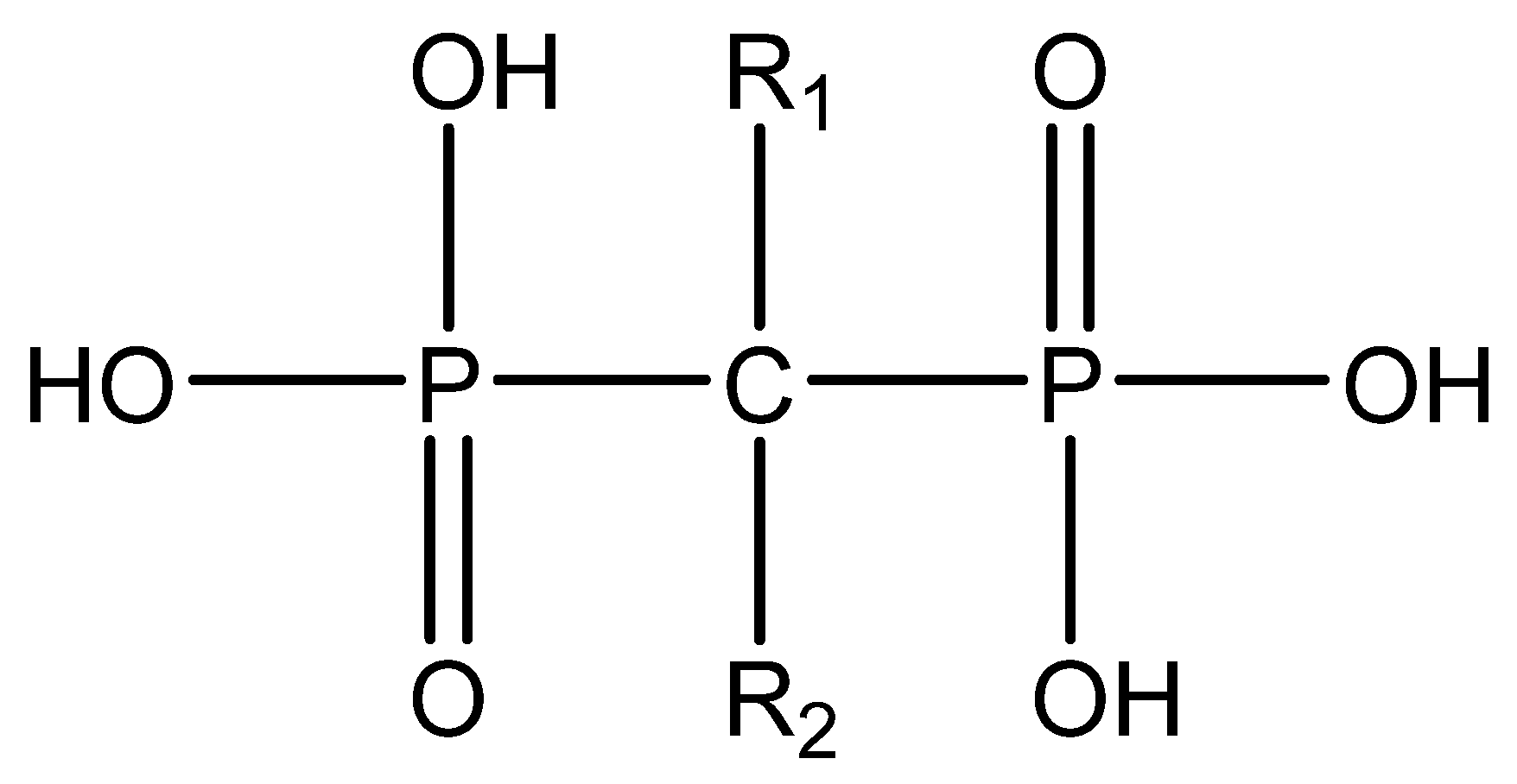
| Bisphosphonates | Generation | Name | R1 | R2 |
|---|---|---|---|---|
| Non-Nitrogenous | First | Etidronate | -OH | -CH3 |
| Second | Clodronate | -Cl | -Cl | |
| Tiludronate | -H | 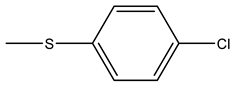 | ||
| Nitrogenous | Pamidronate | -OH | 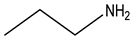 | |
| Third | Alendronate | -OH | 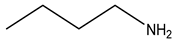 | |
| Neridronate | -OH | 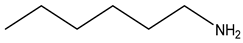 | ||
| Olpadronate | -OH |  | ||
| Ibandronate | -OH | 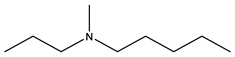 | ||
| Risedronate | -OH | 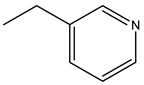 | ||
| Zoledronic acid | -OH |  |
| Nanoparticle | Particle Size (nm) | Particle Type | Zeta Potential (mV) | Targeting Agent | Loaded Compound |
|---|---|---|---|---|---|
| Zn@PEG-ALN NPs | About 55 * | Polymeric nanoparticle | About −25 * | Alendronate | Cisplatin prodrug |
| DZ@ALN | 61 ± 0.78 | Polymeric nanoparticle | −23.5 ± 0.41 | Alendronate | Cisplatin prodrug and Zoledronate |
| ALN-NPs | 95 ± 15 | Micelle | −11.7 ± 4.3 | Alendronate | Bortezomib |
| ALN-oHA-S-S-CUR | 179 ± 23 | Micelle | −25.7 ± 0.7 | Alendronate | Curcumin |
| ALN-oHA-S-S-CUR | 180 | Micelle | / | Alendronate | Curcumin |
| Alendronate coated PLGA nanoparticles | 235.5 ± 71.3 | Polymeric nanoparticle | / | Alendronate | Bortezomib and Curcumin |
| DOX@ALN-(HA-PASP)CL | 110 ± 9 | Polymeric nanoparticle | −16.3 ± 3.7 | Alendronate | Doxorubicin |
| NGO-ALs | 60–150 | Nanosheet | / | Alendronate | Doxorubicin |
| A1-L-DOX-Lip A10-L-DOX-Lip | 107.2 ± 4.8 106.5 ± 3.5 | Liposome | −11.5 ± 1.96 −12.3 ± 2.01 | Alendronate | Doxorubicin |
| ALN-PEG/C18/HYD-DOX-g-PASPAM | About 200 | Micelle | / | Alendronate | Doxorubicin |
| ALN-m/DTX | 84 ± 5 | Micelle | −30 ± 2 | Alendronate | Docetaxel |
| PMBA-DTX | 27.0 ± 0.1 | Micelle | −11.8 ± 1.6 | Alendronate | Docetaxel |
| PTX-AFTPNs (A to F ratio: 0.67) | 125.9 ± 0.95 | Polymeric nanoparticle | −29.6 ± 1.21 | Alendronate | Paclitaxel |
| Pull-(GGPNle-φ-PTX)-(PEG-ALN) | 163.3 ± 18.3 (pH = 5.5) | Micelle | / | Alendronate | Paclitaxel |
| GANT58-BTNPs | About 100 | Micelle | / | Alendronate | Small molecule inhibitors of Gli2 |
| BTZ@ZnPc-ALN | About 60 | Polymeric nanoparticle | −18 mV | Alendronate | Bortezomib and Zinc phthalocyanine |
| Au@MSNs-ZOL | About 70 | Mesoporous silica nanoparticle | +24.3 | Zoledronic acid | Gold nanorods |
| BT-isMOF | 228 ± 12 | Metal−organic framework nanoparticle | / | Zoledronic acid | Immunostimulatory oligonucleotide |
| PBCA-PEG-ZOL NPs | 82 ± 6.35 | Polymeric nanoparticle | From −8.26 ± 1.26 to −23.51 ± 3.37 | Zoledronic acid | Docetaxel |
| UCZP | About 60 | Mesoporous silica nanoparticle | −18.9 | Zoledronic acid | Gadolinium |
| ICG/Fe3O4@PLGA-ZOL | 313.9 | Polymeric nanoparticle | −15.0 | Zoledronic acid | Iron oxide (Fe3O4) and indocyanine green |
| DPA−G5-PEG−cRGD/BTZ | 78.02 * | Polymeric nanoparticle | −3.425 * | RGD peptide | Bortezomib |
| PTX-Glu6-RGD-Lip | 121.9 ± 4.7 | Liposome | −14.37 ± 4.85 | RGD peptide (Glu6-RGD) derivative | Paclitaxel |
| αvβ3-MPs | 12.5 ± 0.8 | Micelle | −3.82 ± 1.23 | Quinolone nonpeptide | Docetaxel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, H.; Varamini, P. Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems. Cells 2022, 11, 388. https://doi.org/10.3390/cells11030388
Shao H, Varamini P. Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems. Cells. 2022; 11(3):388. https://doi.org/10.3390/cells11030388
Chicago/Turabian StyleShao, Huimin, and Pegah Varamini. 2022. "Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems" Cells 11, no. 3: 388. https://doi.org/10.3390/cells11030388
APA StyleShao, H., & Varamini, P. (2022). Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems. Cells, 11(3), 388. https://doi.org/10.3390/cells11030388