Evaluation of Atypical Chemokine Receptor Expression in T Cell Subsets
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Obtainment
2.2. Peripheral Blood Mononuclear Cell Preparation
2.3. Flow Cytometry Assays
2.4. T Cell Selection Using Magnetic Beads
2.5. Cell Sorting
2.6. T Cell Activation by Stimuli
2.7. Transwell Migration Assay
2.8. Confocal Imaging
2.9. ACKRs mRNA Evaluation on Database
2.10. Statistical Analysis
3. Results
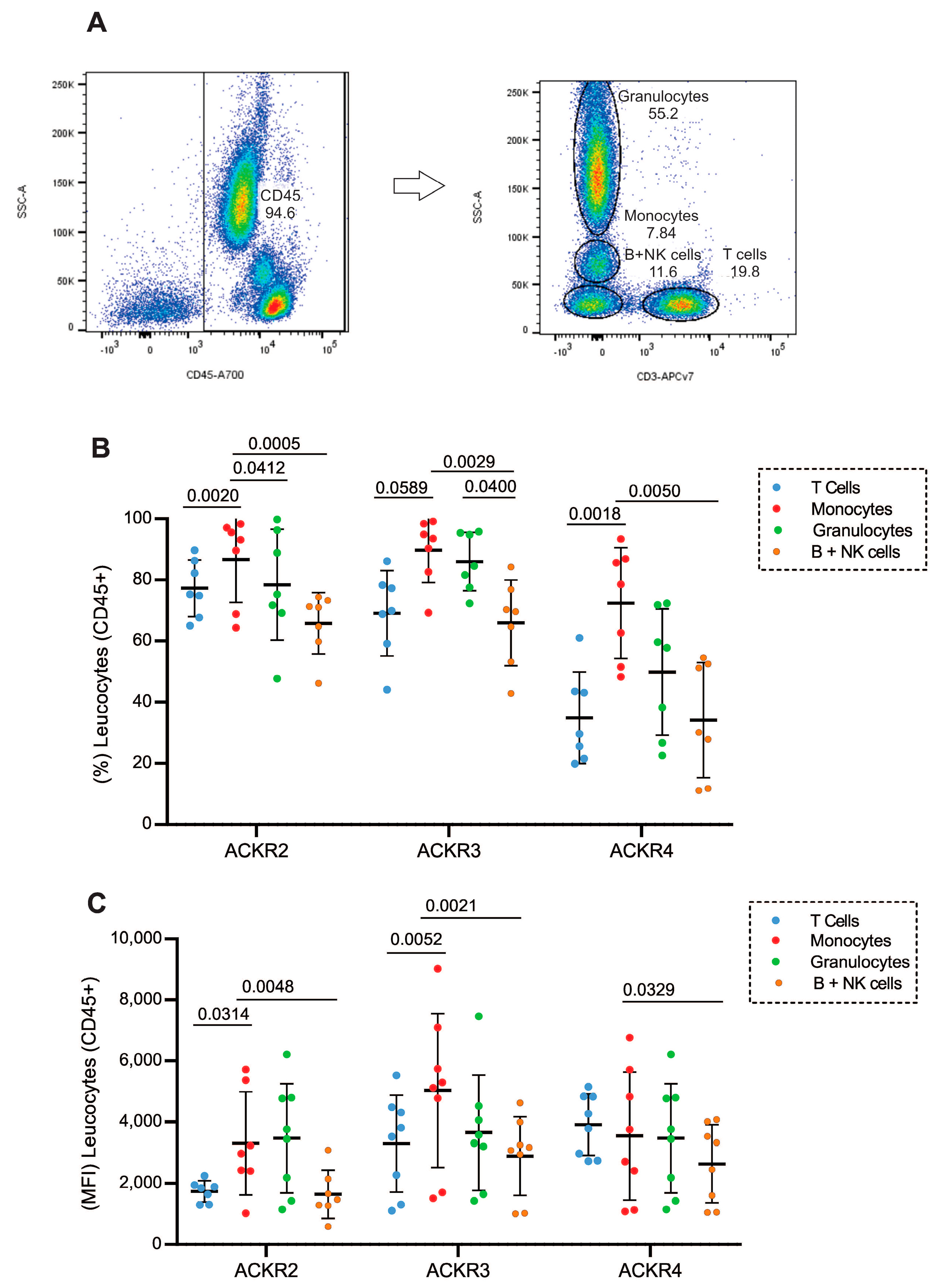
3.1. Expression of ACKR2, ACKR3 and ACKR4 in Different Leukocyte Populations
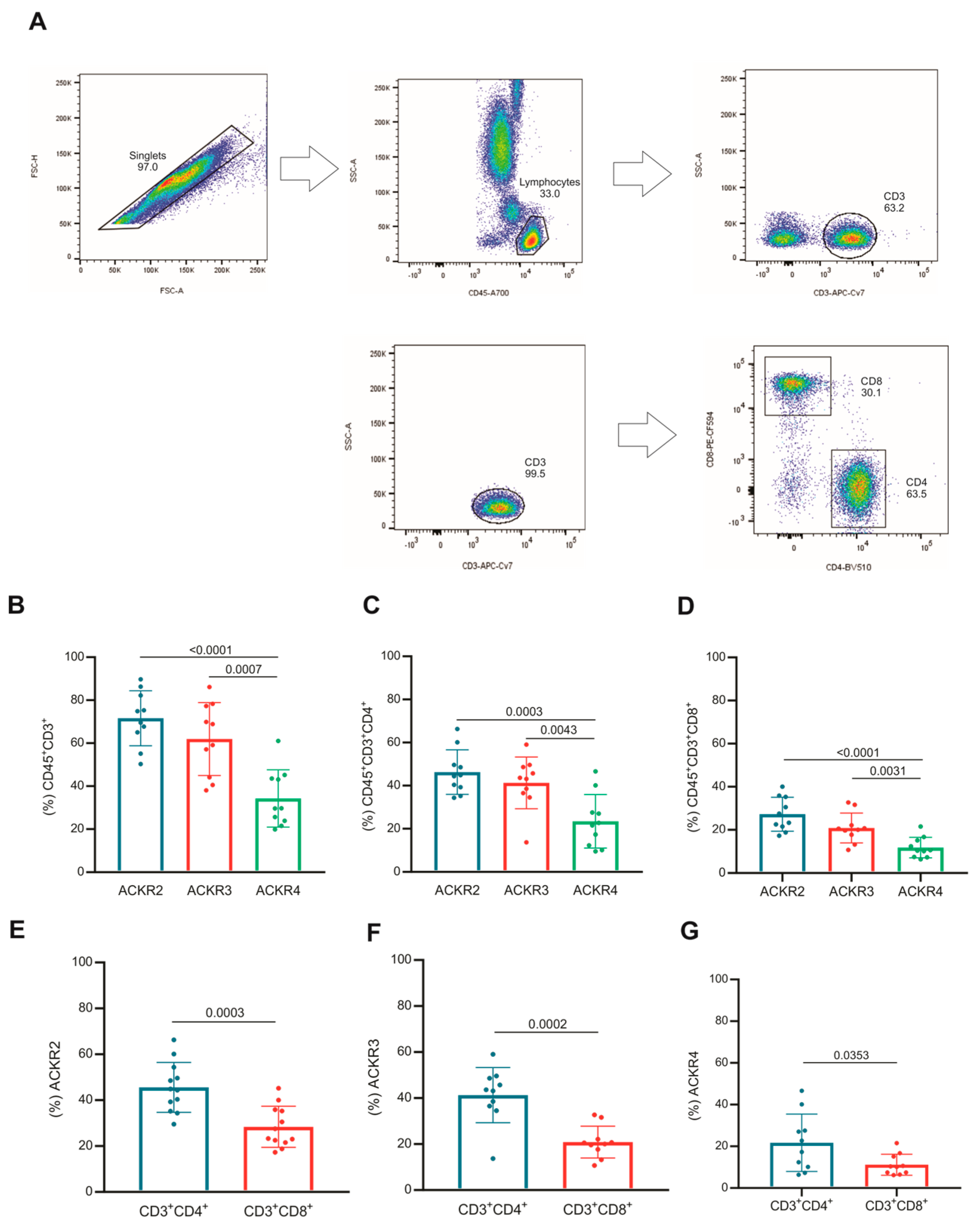
3.2. Subsets of T Lymphocytes That Express ACKR2, ACKR3 and ACKR4
3.3. Naive and Memory T Lymphocytes Express Atypical Chemokine Receptors
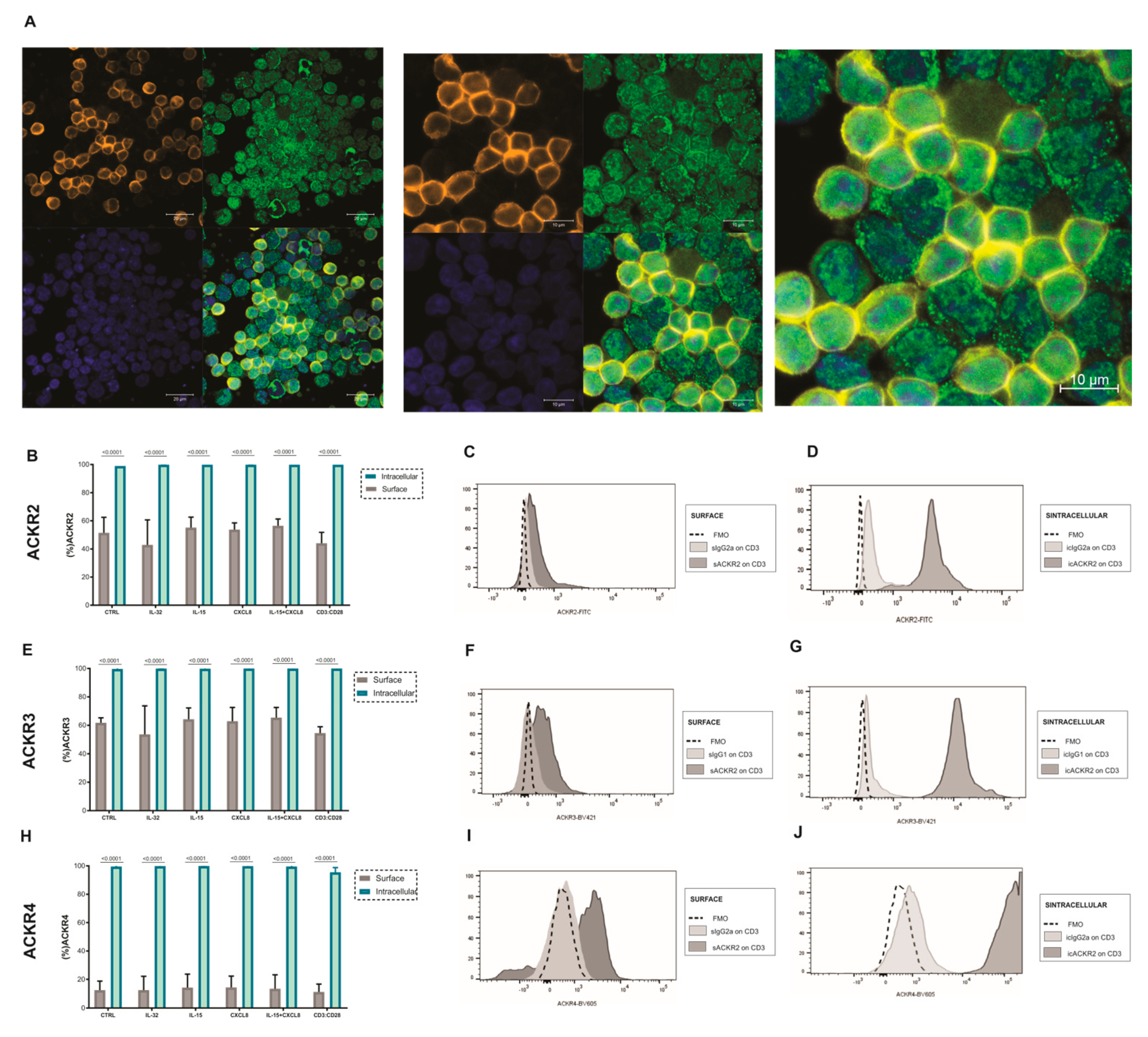
3.4. Expression of ACKR2, ACKR3 and ACKR4 in T Lymphocytes after Treatment with IL-32, CXCL8 and/or IL-15
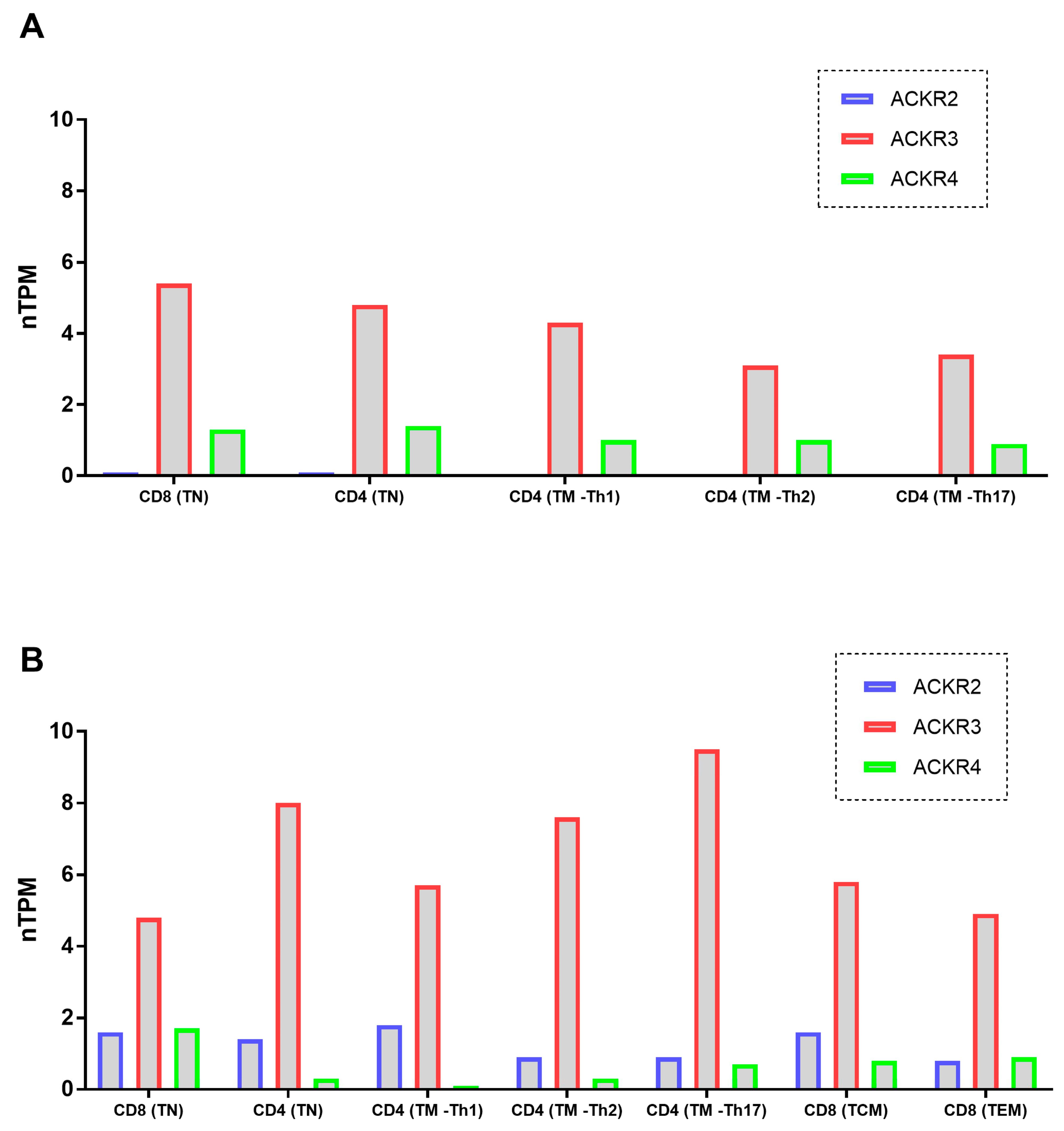
3.5. ACKRs mRNA Expression on T Cells Was Described on Databases
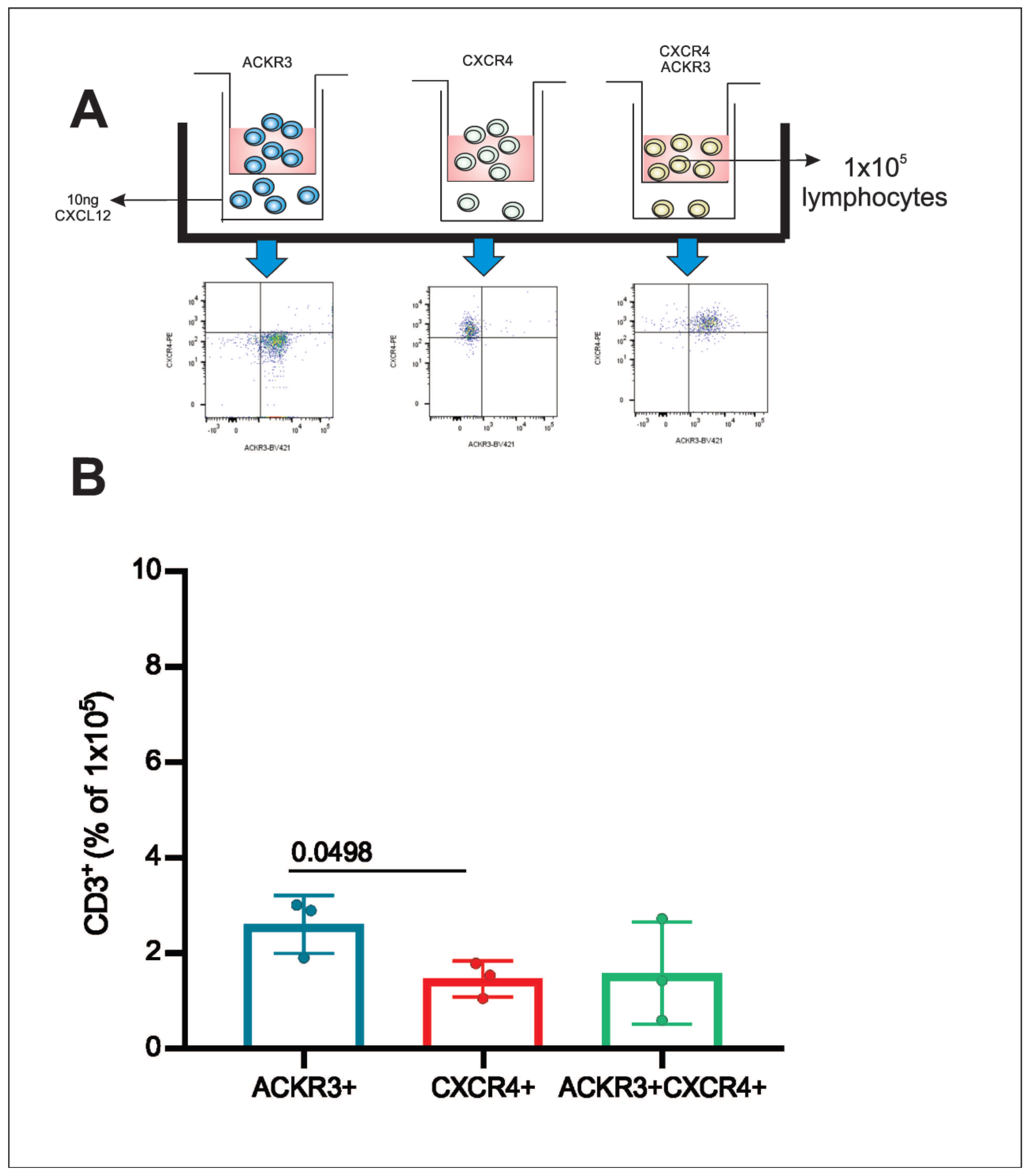
3.6. Post-Sorting Migration Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stone, M.J.; Hayward, J.A.; Huang, C.E.; Huma, Z.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Palomino, D.C.; Marti, L.C. Chemokines and immunity. Einstein 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Hub, E.; Rot, A. Atypical chemokine receptors. Exp. Cell Res. 2011, 317, 556–568. [Google Scholar] [CrossRef]
- Patel, M.; McInnes, I.B.; Graham, G. Atypical chemokine receptors in inflammatory disease. Curr. Mol. Med. 2009, 9, 86–93. [Google Scholar] [CrossRef]
- Bonecchi, R.; Graham, G.J. Atypical Chemokine Receptors and Their Roles in the Resolution of the Inflammatory Response. Front. Immunol. 2016, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Borroni, E.M.; Anselmo, A.; Doni, A.; Savino, B.; Mirolo, M. Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood 2008, 112, 493–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Fra, A.M.; Locati, M.; Otero, K.; Sironi, M.; Signorelli, P.; Massardi, M.L. Cutting edge: Scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J. Immunol. 2003, 170, 2279–2282. [Google Scholar] [CrossRef]
- Weber, M.; Blair, E.; Simpson, C.V.; O’Hara, M.; Blackburn, P.E.; Rot, A. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol. Biol. Cell 2004, 15, 2492–2508. [Google Scholar] [CrossRef] [PubMed]
- Bideak, A.; Blaut, A.; Hoppe, J.M.; Müller, M.B.; Federico, G.; Eltrich, N. The atypical chemokine receptor 2 limits renal inflammation and fibrosis in murine progressive immune complex glomerulonephritis. Kidney Int. 2018, 93, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.; Gilchrist, D.S.; King, V.; Ferra, A.; Forrow, S.; Hunter, K.D. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J. Clin. Investig. 2007, 117, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.; Wilson, G.J.; Singh, M.; van den Bogaard, E.H.; Le Brocq, M.L.; Holmes, S. Spread of Psoriasiform Inflammation to Remote Tissues Is Restricted by the Atypical Chemokine Receptor ACKR2. J. Investig. Dermatol. 2017, 137, 85–94. [Google Scholar] [CrossRef]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J. International union of pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014, 66, 1–79. [Google Scholar] [CrossRef]
- Bazzan, E.; Saetta, M.; Turato, G.; Borroni, E.M.; Cancellieri, C.; Baraldo, S. Expression of the atypical chemokine receptor D6 in human alveolar macrophages in COPD. Chest 2013, 143, 98–106. [Google Scholar] [CrossRef]
- Balabanian, K.; Lagane, B.; Infantino, S.; Chow, K.Y.; Harriague, J.; Moepps, B. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005, 280, 35760–35766. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Sánchez-Mateos, P.; Cabañas, C. CXCR7 impact on CXCL12 biology and disease. Trends Mol. Med. 2013, 19, 12–22. [Google Scholar] [CrossRef]
- Karin, N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J. Leukoc. Biol. 2010, 88, 463–473. [Google Scholar] [CrossRef]
- Melo, R.C.C.; Ferro, K.P.V.; Duarte, A.D.S.S.; Olalla Saad, S.T. CXCR7 participates in CXCL12-mediated migration and homing of leukemic and normal hematopoietic cells. Stem Cell Res. Ther. 2018, 9, 34. [Google Scholar] [CrossRef]
- García-Cuesta, E.M.; Santiago, C.A.; Vallejo-Díaz, J.; Juarranz, Y.; Rodríguez-Frade, J.M.; Mellado, M. The Role of the CXCL12/CXCR4/ACKR3 Axis in Autoimmune Diseases. Front. Endocrinol. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Matti, C.; Salnikov, A.; Artinger, M.; D’Agostino, G.; Kindinger, I.; Uguccioni, M. ACKR4 Recruits GRK3 Prior to β-Arrestins but Can Scavenge Chemokines in the Absence of β-Arrestins. Front. Immunol. 2020, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Werth, K.; Braun, A.; Kelay, P.; Hub, E.; Eller, K. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014, 15, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Kara, E.E.; Bastow, C.R.; McKenzie, D.R.; Gregor, C.E.; Fenix, K.A.; Babb, R.; Norton, T.S.; Zotos, D.; Rodda, L.B.; Hermes, J.R.; et al. Atypical chemokine receptor 4 shapes activated B cell fate. J. Exp. Med. 2018, 215, 801–813. [Google Scholar] [CrossRef]
- Bryce, S.A.; Wilson, R.A.; Tiplady, E.M.; Asquith, D.L.; Bromley, S.K.; Luster, A.D. ACKR4 on Stromal Cells Scavenges CCL19 To Enable CCR7-Dependent Trafficking of APCs from Inflamed Skin to Lymph Nodes. J. Immunol. 2016, 196, 3341–3353. [Google Scholar] [CrossRef]
- Comerford, I.; Milasta, S.; Morrow, V.; Milligan, G.; Nibbs, R. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur. J. Immunol. 2006, 36, 1904–1916. [Google Scholar] [CrossRef]
- Schmiedel, B.J.; Singh, D.; Madrigal, A.; Valdovino-Gonzalez, A.G.; White, B.M.; Zapardiel-Gonzalo, J.; Ha, B.; Altay, G.; Greenbaum, J.A.; McVicker, G.; et al. Impact of Genetic Polymorphisms on Human Immune Cell Gene Expression. Cell 2018, 175, 1701–1715. [Google Scholar] [CrossRef]
- Monaco, G.; Lee, B.; Xu, W.; Mustafah, S.; Hwang, Y.Y.; Carré, C.; Burdin, N.; Visan, L.; Ceccarelli, M.; Poidinger, M.; et al. RNA-Seq Signatures Normalized by mRNA Abundance Allow Absolute Deconvolution of Human Immune Cell Types. Cell Rep. 2019, 26, 1627–1640. [Google Scholar] [CrossRef]
- Comerford, I.; Nibbs, R.J.; Litchfield, W.; Bunting, M.; Harata-Lee, Y.; Haylock-Jacobs, S. The atypical chemokine receptor CCX-CKR scavenges homeostatic chemokines in circulation and tissues and suppresses Th17 responses. Blood 2010, 116, 4130–4140. [Google Scholar] [CrossRef]
- Rot, A.; McKimmie, C.; Burt, C.L.; Pallas, K.J.; Jamieson, T.; Pruenster, M.; Horuk, R.; Nibbs, R.J.B.; Graham, G.J. Cell-autonomous regulation of neutrophil migration by the D6 chemokine decoy receptor. J. Immunol. 2013, 190, 6450–6456. [Google Scholar] [CrossRef]
- Kunkel, E.J.; Kim, C.H.; Lazarus, N.H.; Vierra, M.A.; Soler, D.; Bowman, E.P. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Investig. 2003, 111, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yoo, I.; Lee, S.; Jung, W.; Kim, H.J.; Hyun, S.H. Atypical chemokine receptors 1, 2, 3 and 4: Expression and regulation in the endometrium during the estrous cycle and pregnancy and with somatic cell nucleus transfer-cloned embryos in pigs. Theriogenology 2019, 129, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Koenen, J.; Bachelerie, F.; Balabanian, K.; Schlecht-Louf, G.; Gallego, C. Atypical Chemokine Receptor 3 (ACKR3): A Comprehensive Overview of its Expression and Potential Roles in the Immune System. Mol. Pharmacol. 2019, 96, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; von Ungern-Sternberg, S.N.; Seizer, P.; Schlegel, F.; Büttcher, M.; Sindhu, N.A. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis. 2015, 6, e1989. [Google Scholar] [CrossRef]
- Hartmann, T.N.; Grabovsky, V.; Pasvolsky, R.; Shulman, Z.; Buss, E.C.; Spiegel, A. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34 cells. J. Leukoc. Biol. 2008, 84, 1130–1140. [Google Scholar] [CrossRef]
- Heinzel, K.; Benz, C.; Bleul, C.C. A silent chemokine receptor regulates steady-state leukocyte homing in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8421–8426. [Google Scholar] [CrossRef]
- Sprent, J.; Surh, C.D. Normal T cell homeostasis: The conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011, 12, 478–484. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Berard, M.; Tough, D.F. Qualitative differences between naïve and memory T cells. Immunology 2002, 106, 127–138. [Google Scholar] [CrossRef]
- Salimi, P.; Esmaeili, A.; Hashemi, M.; Behjati, M. Endogenous expression of the atypical chemokine receptor CCX-CKR (CCRL1) gene in human embryonic kidney (HEK 293) cells. Mol. Cell Biochem. 2016, 412, 229–233. [Google Scholar] [CrossRef]
- Caccamo, N.; Joosten, S.A.; Ottenhoff, T.H.M.; Dieli, F. Atypical Human Effector/Memory CD4(+) T Cells With a Naive-Like Phenotype. Front. Immunol. 2018, 9, 2832. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Baars, P.A.; Rep, M.H.; Hooibrink, B.; Kerkhof-Garde, S.R.; Klein, M.R. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997, 186, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.E.; York, J.; Jensen, E.R. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992, 145, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Brummelman, J.; Pilipow, K.; Lugli, E. The Single-Cell Phenotypic Identity of Human CD8(+) and CD4(+) T Cells. Int. Rev. Cell Mol. Biol. 2018, 341, 63–124. [Google Scholar]
- Sjöberg, E.; Meyrath, M.; Chevigné, A.; Östman, A.; Augsten, M.; Szpakowska, M. The diverse and complex roles of atypical chemokine receptors in cancer: From molecular biology to clinical relevance and therapy. Adv. Cancer Res. 2020, 145, 99–138. [Google Scholar]
- Heuninck, J.; Perpiñá Viciano, C.; Işbilir, A.; Caspar, B.; Capoferri, D.; Briddon, S.J. Context-Dependent Signaling of CXC Chemokine Receptor 4 and Atypical Chemokine Receptor 3. Mol. Pharmacol. 2019, 96, 778–793. [Google Scholar] [CrossRef]
- Naumann, U.; Cameroni, E.; Pruenster, M.; Mahabaleshwar, H.; Raz, E.; Zerwes, H.G. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE 2010, 5, e9175. [Google Scholar] [CrossRef]
- Vetrano, S.; Borroni, E.M.; Sarukhan, A.; Savino, B.; Bonecchi, R.; Correal, C.; Arena, V.; Fantini, M.; Roncalli, M.; Malesci, A.; et al. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut 2010, 59, 197–206. [Google Scholar] [CrossRef]
- Zabel, B.A.; Wang, Y.; Lewen, S.; Berahovich, R.D.; Penfold, M.E.; Zhang, P.; Powers, J.; Summers, B.C.; Miao, Z.; Zhao, B.; et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4- mediated tumor cell transendothelial migration by CXCR7 ligands. J. Immunol. 2009, 183, 3204–3211. [Google Scholar] [CrossRef]
- Yamada, K.; Maishi, N.; Akiyama, K.; Towfik Alam, M.; Ohga, N.; Kawamoto, T.; Shindoh, M.; Takahashi, N.; Kamiyama, T.; Hida, H.; et al. CXCL12-CXCR7 axis is important for tumor endothelial cell angiogenic property. Int. J. Cancer 2015, 137, 2825–2836. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.O.; Rocha, F.A.; Aloia, T.P.A.; Marti, L.C. Evaluation of Atypical Chemokine Receptor Expression in T Cell Subsets. Cells 2022, 11, 4099. https://doi.org/10.3390/cells11244099
Pacheco MO, Rocha FA, Aloia TPA, Marti LC. Evaluation of Atypical Chemokine Receptor Expression in T Cell Subsets. Cells. 2022; 11(24):4099. https://doi.org/10.3390/cells11244099
Chicago/Turabian StylePacheco, Messias Oliveira, Fernanda Agostini Rocha, Thiago Pinheiro Arrais Aloia, and Luciana Cavalheiro Marti. 2022. "Evaluation of Atypical Chemokine Receptor Expression in T Cell Subsets" Cells 11, no. 24: 4099. https://doi.org/10.3390/cells11244099
APA StylePacheco, M. O., Rocha, F. A., Aloia, T. P. A., & Marti, L. C. (2022). Evaluation of Atypical Chemokine Receptor Expression in T Cell Subsets. Cells, 11(24), 4099. https://doi.org/10.3390/cells11244099





