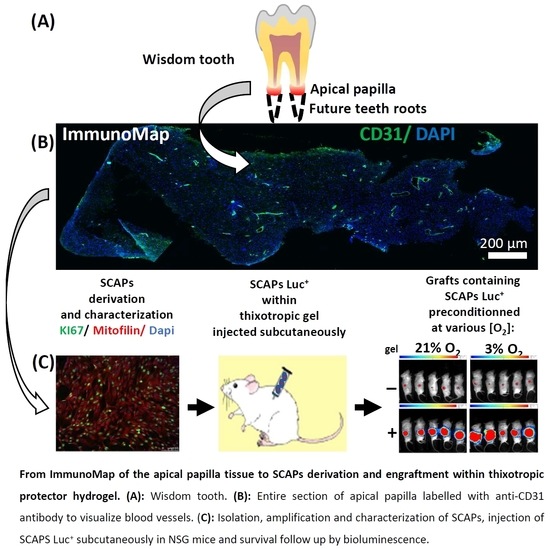
The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Native Tissue Preparation and Cryosectioning
2.2. Staining and Immunolabelling of Tissue Sections
2.3. Establishment of SCAP Banks, Cell Culture and Growth Curve Analysis
2.4. Flow Cytometry
2.5. Telomere Length and Telomerase Activity
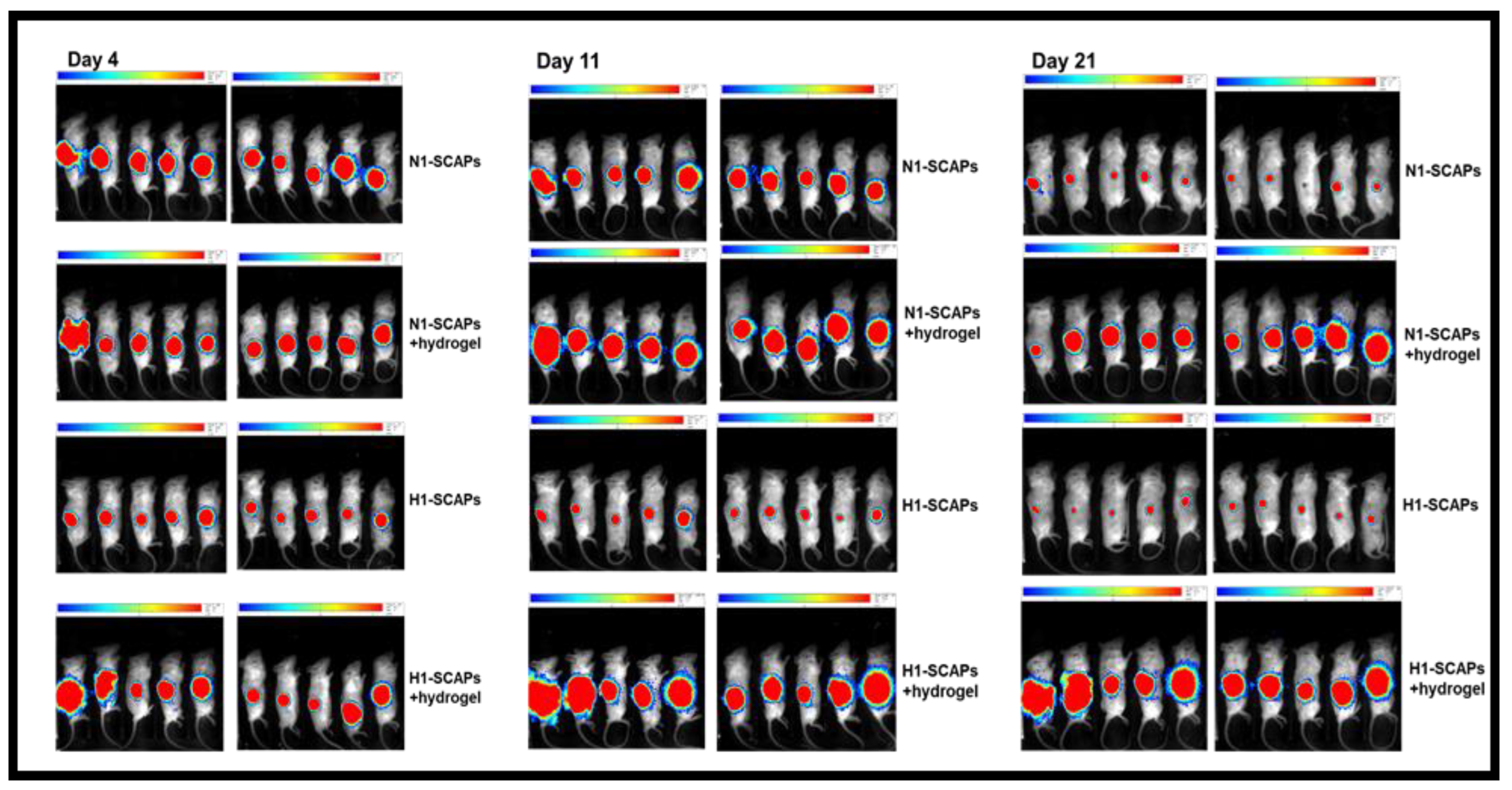
2.6. Lentiviral Infection and Engraftment in NSG Mice
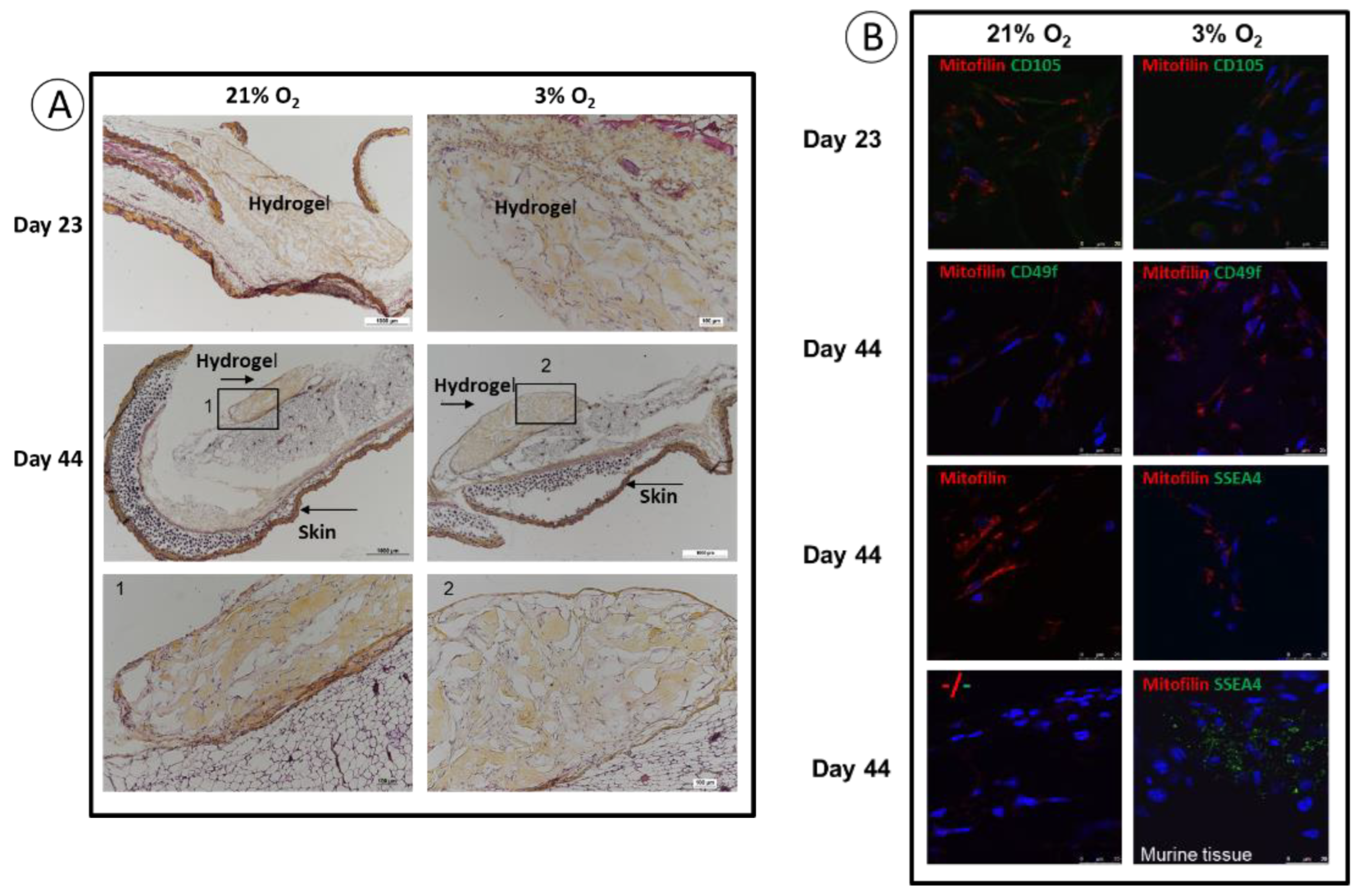
2.7. Analysis of the Grafts
2.8. Statistical Analysis
3. Results
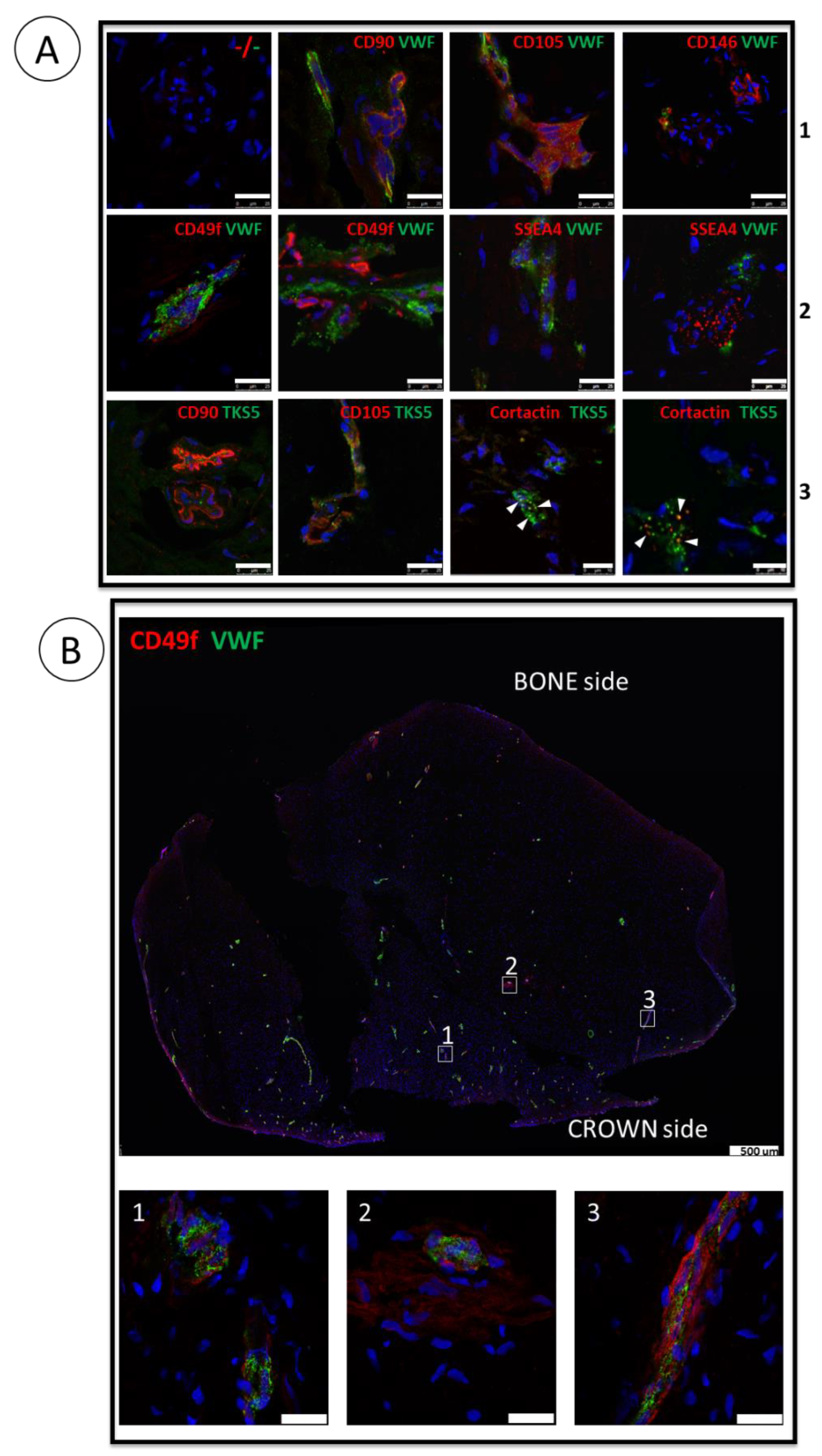
3.1. ImmunoMap of Native Apical Papilla Tissue Derived from Healthy Wisdom Teeth of Teenagers
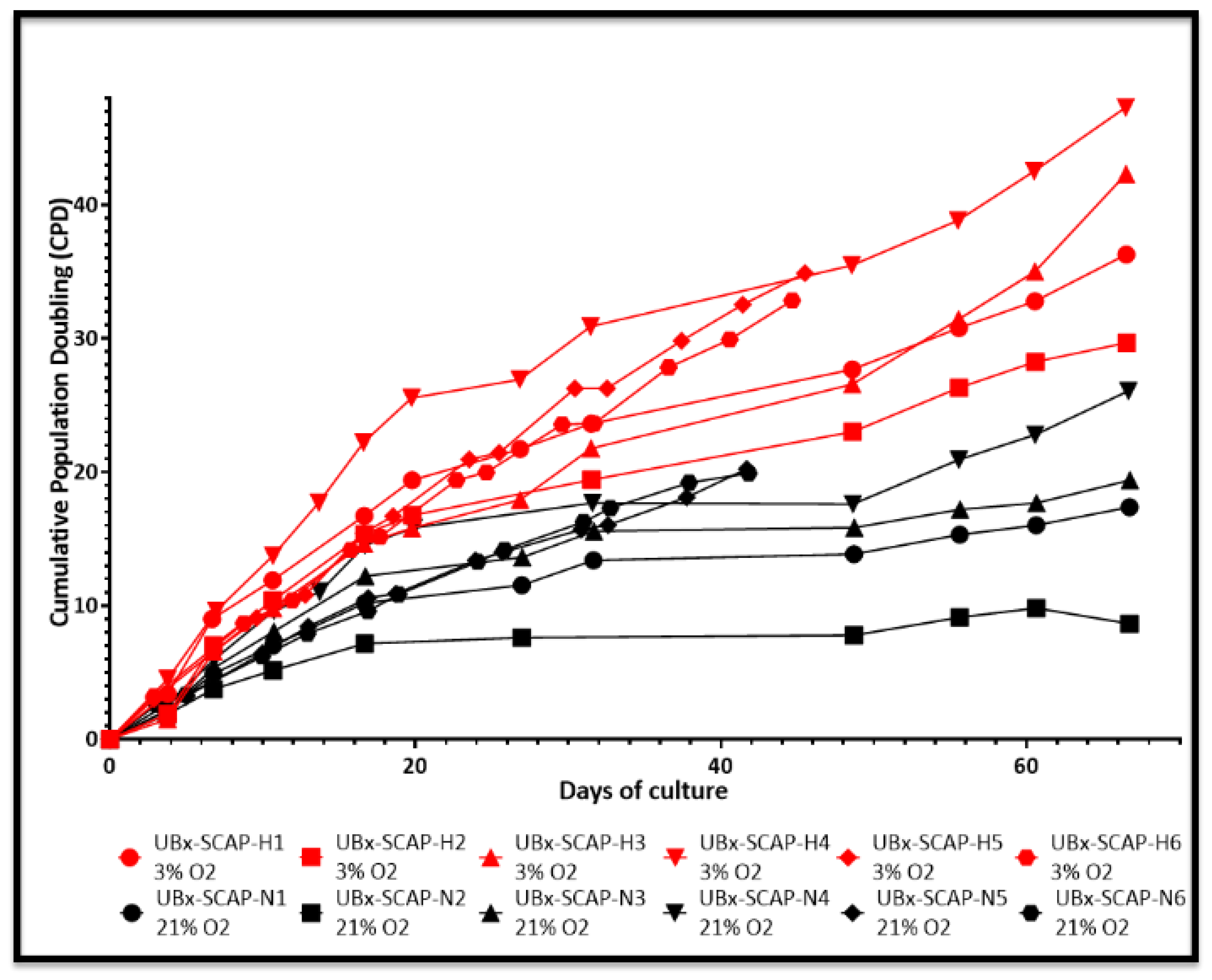
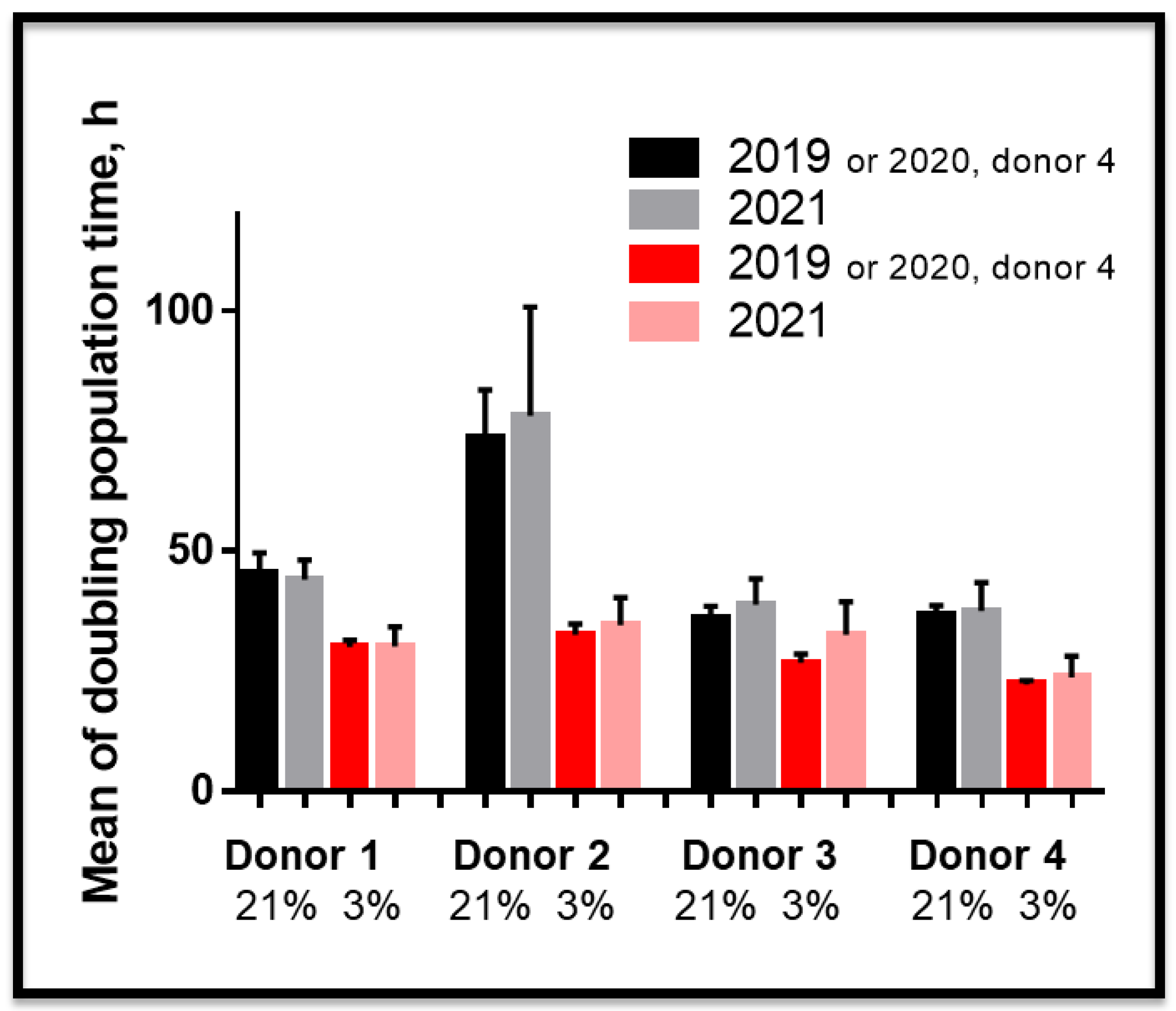
3.2. Proliferative Advantage of SCAPs Banks Derived at 3% O2 in Comparison with 21% O2 and Stability of SCAPs Banks over Time
3.3. Telomere Length and Telomerase Activity in SCAPs Are Not Critically Regulated by Oxygen Concentration
3.4. Implantation: Behavior of SCAPs Embedded within Thixotropic Biomaterials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Ippolito, G.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Isolation and Characterization of Marrow-Isolated Adult Multilineage Inducible (MIAMI) Cells. Exp. Hematol. 2006, 34, 1608–1610. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.; D’Ippolito, G.; Curtis, K.M.; Delcroix, G.J.-R.; Gomez, L.A.; El Hokayem, J.; Rieger, M.; Parrondo, R.; de Las Pozas, A.; Perez-Stable, C.; et al. Low Oxygen Modulates Multiple Signaling Pathways, Increasing Self-Renewal, while Decreasing Differentiation, Senescence, and Apoptosis in Stromal MIAMI Cells. Stem Cells Dev. 2016, 25, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, C.; Rochefort, G.Y.; Bascetin, R.; Ying, H.; Lesieur, J.; Sadoine, J.; Beckouche, N.; Berndt, S.; Novais, A.; Lesage, M.; et al. Priming Dental Pulp Stem Cells with Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs Through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion. Stem Cells Transl. Med. 2016, 5, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.; Lesieur, J.; Sadoine, J.; Slimani, L.; Baroukh, B.; Saubaméa, B.; Schmitt, A.; Vital, S.; Poliard, A.; Hélary, C.; et al. Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects. Stem Cells Transl. Med. 2019, 8, 844–857. [Google Scholar] [CrossRef] [Green Version]
- Wobma, H.M.; Tamargo, M.A.; Goeta, S.; Brown, L.M.; Duran-Struuck, R.; Vunjak-Novakovic, G. The Influence of Hypoxia and IFN-γ on the Proteome and Metabolome of Therapeutic Mesenchymal Stem Cells. Biomaterials 2018, 167, 226–234. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem Cell-Based Tooth and Periodontal Regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.-J.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The Hidden Treasure in Apical Papilla: The Potential Role in Pulp/Dentin Regeneration and Bioroot Engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.L.; Forner, L.; Almudéver, A.; Guerrero-Gironés, J.; Llena, C. Viability and Stimulation of Human Stem Cells from the Apical Papilla (HSCAPs) Induced by Silicate-Based Materials for Their Potential Use in Regenerative Endodontics: A Systematic Review. Materials 2020, 13, E974. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-Y.; Chen, X.; Yue, L.; Huang, G.T.-J.; Zou, X.-Y. CXC Chemokine Receptor 4 Is Expressed Paravascularly in Apical Papilla and Coordinates with Stromal Cell-Derived Factor-1α during Transmigration of Stem Cells from Apical Papilla. J. Endod. 2015, 41, 1430–1436. [Google Scholar] [CrossRef]
- Driesen, R.B.; Hilkens, P.; Smisdom, N.; Vangansewinkel, T.; Dillen, Y.; Ratajczak, J.; Wolfs, E.; Gervois, P.; Ameloot, M.; Bronckaers, A.; et al. Dental Tissue and Stem Cells Revisited: New Insights from the Expression of Fibroblast Activation Protein-Alpha. Front. Cell Dev. Biol. 2019, 7, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, S.; Lin, X.; Wang, L.; Dong, R.; Du, J.; Yang, D.; Fan, Z. Analysis of Gene Expression Profiles between Apical Papilla Tissues, Stem Cells from Apical Papilla and Cell Sheet to Identify the Key Modulators in MSCs Niche. Cell Prolif. 2017, 50, 12337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rémy, M.; Ferraro, F.; Le Salver, P.; Rey, S.; Genot, E.; Djavaheri-Mergny, M.; Thébaud, N.; Boiziau, C.; Boeuf, H. Isolation and Culture of Human Stem Cells from Apical Papilla under Low Oxygen Concentration Highlight Original Properties. Cells 2019, 8, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saller, M.M.; Prall, W.C.; Docheva, D.; Schönitzer, V.; Popov, T.; Anz, D.; Clausen-Schaumann, H.; Mutschler, W.; Volkmer, E.; Schieker, M.; et al. Increased Stemness and Migration of Human Mesenchymal Stem Cells in Hypoxia Is Associated with Altered Integrin Expression. Biochem. Biophys. Res. Commun. 2012, 423, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Paccola Mesquita, F.C.; Hochman-Mendez, C.; Morrissey, J.; Sampaio, L.C.; Taylor, D.A. Laminin as a Potent Substrate for Large-Scale Expansion of Human Induced Pluripotent Stem Cells in a Closed Cell Expansion System. Stem Cells Int. 2019, 2019, 9704945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.K.; Chen, C.-B.; Xu, C.; Xu, Y.; Yao, X.; Huang, L.; Liang, J.-J.; Cheung, H.S.; Pang, C.P.; Huang, Y. Attenuated Regenerative Properties in Human Periodontal Ligament-Derived Stem Cells of Older Donor Ages with Shorter Telomere Length and Lower SSEA4 Expression. Cell Tissue Res. 2020, 381, 71–81. [Google Scholar] [CrossRef]
- Georges-Labouesse, E.; Messaddeq, N.; Yehia, G.; Cadalbert, L.; Dierich, A.; Le Meur, M. Absence of Integrin Alpha 6 Leads to Epidermolysis Bullosa and Neonatal Death in Mice. Nat. Genet. 1996, 13, 370–373. [Google Scholar] [CrossRef]
- De Arcangelis, A.; Hamade, H.; Alpy, F.; Normand, S.; Bruyère, E.; Lefebvre, O.; Méchine-Neuville, A.; Siebert, S.; Pfister, V.; Lepage, P.; et al. Hemidesmosome Integrity Protects the Colon against Colitis and Colorectal Cancer. Gut 2017, 66, 1748–1760. [Google Scholar] [CrossRef]
- Yu, K.-R.; Yang, S.-R.; Jung, J.-W.; Kim, H.; Ko, K.; Han, D.W.; Park, S.-B.; Choi, S.W.; Kang, S.-K.; Schöler, H.; et al. CD49f Enhances Multipotency and Maintains Stemness through the Direct Regulation of OCT4 and SOX2. Stem Cells Dayt. Ohio 2012, 30, 876–887. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, P.; Fu, X.; Li, Q.; Ma, S.; Wu, D.; Kang, N.; Liu, X.; Yan, L.; Xiao, R. CD49f Acts as an Inflammation Sensor to Regulate Differentiation, Adhesion, and Migration of Human Mesenchymal Stem Cells. Stem Cells Dayt. Ohio 2015, 33, 2798–2810. [Google Scholar] [CrossRef]
- Au, H.-K.; Peng, S.-W.; Guo, C.-L.; Lin, C.-C.; Wang, Y.-L.; Kuo, Y.-C.; Law, T.-Y.; Ho, H.-N.; Ling, T.-Y.; Huang, Y.-H. Niche Laminin and IGF-1 Additively Coordinate the Maintenance of Oct-4 Through CD49f/IGF-1R-Hif-2α Feedforward Loop in Mouse Germline Stem Cells. Front. Cell Dev. Biol. 2021, 9, 646644. [Google Scholar] [CrossRef]
- Driesen, R.B.; Gervois, P.; Vangansewinkel, T.; Lambrichts, I. Unraveling the Role of the Apical Papilla During Dental Root Maturation. Front. Cell Dev. Biol. 2021, 9, 665600. [Google Scholar] [CrossRef] [PubMed]
- Nada, O.A.; El Backly, R.M. Stem Cells from the Apical Papilla (SCAP) as a Tool for Endogenous Tissue Regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, M.; Ono, N.; Ono, W. Unveiling Diversity of Stem Cells in Dental Pulp and Apical Papilla Using Mouse Genetic Models: A Literature Review. Cell Tissue Res. 2021, 383, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Spuul, P.; Génot, E. Podosomes in Endothelial Cell—Microenvironment Interactions. Curr. Opin. Hematol. 2020, 27, 197–205. [Google Scholar] [CrossRef]
- Genot, E. ARF1 at the Crossroads of Podosome Construction and Function. J. Cell Biol. 2017, 216, 13–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasco, M.A. Telomeres and Human Disease: Ageing, Cancer and Beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and Telomerase in Stem Cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [Green Version]
- Serakinci, N.; Graakjaer, J.; Kolvraa, S. Telomere Stability and Telomerase in Mesenchymal Stem Cells. Biochimie 2008, 90, 33–40. [Google Scholar] [CrossRef]
- Markiewicz-Potoczny, M.; Lobanova, A.; Loeb, A.M.; Kirak, O.; Olbrich, T.; Ruiz, S.; Lazzerini Denchi, E. TRF2-Mediated Telomere Protection Is Dispensable in Pluripotent Stem Cells. Nature 2021, 589, 110–115. [Google Scholar] [CrossRef]
- Lambricht, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Goldansaz, H.; Bouzin, C.; Préat, V.; Dupont-Gillain, C.; des Rieux, A. The Type and Composition of Alginate and Hyaluronic-Based Hydrogels Influence the Viability of Stem Cells of the Apical Papilla. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, e349–e361. [Google Scholar] [CrossRef]
- Chatterjee, K.; Lin-Gibson, S.; Wallace, W.E.; Parekh, S.H.; Lee, Y.J.; Cicerone, M.T.; Young, M.F.; Simon, C.G. The Effect of 3D Hydrogel Scaffold Modulus on Osteoblast Differentiation and Mineralization Revealed by Combinatorial Screening. Biomaterials 2010, 31, 5051–5062. [Google Scholar] [CrossRef] [Green Version]
- Ziane, S.; Schlaubitz, S.; Miraux, S.; Patwa, A.; Lalande, C.; Bilem, I.; Lepreux, S.; Rousseau, B.; Le Meins, J.-F.; Latxague, L.; et al. A Thermosensitive Low Molecular Weight Hydrogel as Scaffold for Tissue Engineering. Eur. Cell. Mater. 2012, 23, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Aussel, A.; Boiziau, C.; L’Azou, B.; Siadous, R.; Delmond, S.; Montembault, A.; David, L.; Bordenave, L.; Thébaud, N.-B. Cell and Tissue Responses at the Interface with a Chitosan Hydrogel Intended for Vascular Applications: In Vitro and in Vivo Exploration. Biomed. Mater. Bristol Engl. 2019, 14, 025009. [Google Scholar] [CrossRef]
- Germain, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Jacobs, D.; Vandermeulen, G.; Bouzin, C.; Préat, V.; Dupont-Gillain, C.; et al. Fibrin Hydrogels to Deliver Dental Stem Cells of the Apical Papilla for Regenerative Medicine. Regen. Med. 2015, 10, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, A.; Vanacker, J.; Germain, L.; Leprince, J.G.; Diogenes, A.; Shakesheff, K.M.; White, L.J.; des Rieux, A. Extracellular Matrix-Derived Hydrogels for Dental Stem Cell Delivery. J. Biomed. Mater. Res. A 2017, 105, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Qiu, J.; Kuang, R.; Zhang, B.; Wang, W.; Yu, Q. Synergistic Effects of Stromal Cell-Derived Factor-1α and Bone Morphogenetic Protein-2 Treatment on Odontogenic Differentiation of Human Stem Cells from Apical Papilla Cultured in the VitroGel 3D System. Cell Tissue Res. 2019, 378, 207–220. [Google Scholar] [CrossRef]
- Wang, F.; Nan, L.-P.; Zhou, S.-F.; Liu, Y.; Wang, Z.-Y.; Wang, J.-C.; Feng, X.-M.; Zhang, L. Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells Int. 2019, 2019, 8496025. [Google Scholar] [CrossRef]
- Sugioka, Y.; Nakamura, J.; Ohtsuki, C.; Sugawara-Narutaki, A. Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides. Int. J. Mol. Sci. 2021, 22, 4104. [Google Scholar] [CrossRef]
- Latxague, L.; Ramin, M.A.; Appavoo, A.; Berto, P.; Maisani, M.; Ehret, C.; Chassande, O.; Barthélémy, P. Control of Stem-Cell Behavior by Fine Tuning the Supramolecular Assemblies of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed Engl. 2015, 54, 4517–4521. [Google Scholar] [CrossRef] [PubMed]
- Devillard, R.; Rémy, M.; Kalisky, J.; Bourget, J.-M.; Kérourédan, O.; Siadous, R.; Bareille, R.; Amédée-Vilamitjana, J.; Chassande, O.; Fricain, J.-C. In Vitro Assessment of a Collagen/Alginate Composite Scaffold for Regenerative Endodontics. Int. Endod. J. 2017, 50, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Latxague, L.; Gaubert, A.; Maleville, D.; Baillet, J.; Ramin, M.A.; Barthélémy, P. Carbamate-Based Bolaamphiphile as Low-Molecular-Weight Hydrogelators. Gels 2016, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansode, N.D.; Sindhu, K.R.; Morel, C.; Rémy, M.; Verget, J.; Boiziau, C.; Barthélémy, P. A Disulfide Based Low Molecular Weight Gel for the Selective Sustained Release of Biomolecules. Biomater. Sci. 2020, 8, 3186–3192. [Google Scholar] [CrossRef]
- Zanna, N.; Tomasini, C. Peptide-Based Physical Gels Endowed with Thixotropic Behaviour. Gels 2017, 3, 39. [Google Scholar] [CrossRef]
- Smyrek, I.; Stelzer, E.H.K. Quantitative Three-Dimensional Evaluation of Immunofluorescence Staining for Large Whole Mount Spheroids with Light Sheet Microscopy. Biomed. Opt. Express 2017, 8, 484–499. [Google Scholar] [CrossRef] [Green Version]
- Acharya, A.; Brungs, S.; Lichterfeld, Y.; Hescheler, J.; Hemmersbach, R.; Boeuf, H.; Sachinidis, A. Parabolic, Flight-Induced, Acute Hypergravity and Microgravity Effects on the Beating Rate of Human Cardiomyocytes. Cells 2019, 8, 352. [Google Scholar] [CrossRef] [Green Version]
- Hammoud, A.A.; Kirstein, N.; Mournetas, V.; Darracq, A.; Broc, S.; Blanchard, C.; Zeineddine, D.; Mortada, M.; Boeuf, H. Murine Embryonic Stem Cell Plasticity Is Regulated through Klf5 and Maintained by Metalloproteinase MMP1 and Hypoxia. PLoS ONE 2016, 11, e0146281. [Google Scholar] [CrossRef] [Green Version]
- Ropio, J.; Chebly, A.; Ferrer, J.; Prochazkova-Carlotti, M.; Idrissi, Y.; Azzi-Martin, L.; Cappellen, D.; Pham-Ledard, A.; Soares, P.; Merlio, J.-P.; et al. Reliable Blood Cancer Cells’ Telomere Length Evaluation by QPCR. Cancer Med. 2020, 9, 3153–3162. [Google Scholar] [CrossRef] [Green Version]
- Chevret, E.; Andrique, L.; Prochazkova-Carlotti, M.; Ferrer, J.; Cappellen, D.; Laharanne, E.; Idrissi, Y.; Boettiger, A.; Sahraoui, W.; Ruiz, F.; et al. Telomerase Functions beyond Telomere Maintenance in Primary Cutaneous T-Cell Lymphoma. Blood 2014, 123, 1850–1859. [Google Scholar] [CrossRef]
- Alonso, F.; Spuul, P.; Daubon, T.; Kramer, I.; Génot, E. Variations on the Theme of Podosomes: A Matter of Context. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D. Clinical Translation of Stem Cells for Regenerative Medicine. Circ. Res. 2019, 124, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.B.; Yong, K.W.; Poon, C.T.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Xu, F.; Wan Safwani, W.K.Z. In Situ Normoxia Enhances Survival and Proliferation Rate of Human Adipose Tissue-Derived Stromal Cells without Increasing the Risk of Tumourigenesis. PLoS ONE 2015, 10, e0115034. [Google Scholar] [CrossRef]
- Ivanovic, Z. Respect the Anaerobic Nature of Stem Cells to Exploit Their Potential in Regenerative Medicine. Regen. Med. 2013, 8, 677–680. [Google Scholar] [CrossRef]
- Linder, S.; Wiesner, C. Feel the Force: Podosomes in Mechanosensing. Exp. Cell Res. 2016, 343, 67–72. [Google Scholar] [CrossRef]
- Linder, S.; Cervero, P. The Podosome Cap: Past, Present, Perspective. Eur. J. Cell Biol. 2020, 99, 151087. [Google Scholar] [CrossRef]
- Murphy, D.A.; Diaz, B.; Bromann, P.A.; Tsai, J.H.; Kawakami, Y.; Maurer, J.; Stewart, R.A.; Izpisúa-Belmonte, J.C.; Courtneidge, S.A. A Src-Tks5 Pathway Is Required for Neural Crest Cell Migration during Embryonic Development. PLoS ONE 2011, 6, e22499. [Google Scholar] [CrossRef] [Green Version]
- Kudlik, G.; Takács, T.; Radnai, L.; Kurilla, A.; Szeder, B.; Koprivanacz, K.; Merő, B.L.; Buday, L.; Vas, V. Advances in Understanding TKS4 and TKS5: Molecular Scaffolds Regulating Cellular Processes from Podosome and Invadopodium Formation to Differentiation and Tissue Homeostasis. Int. J. Mol. Sci. 2020, 21, E8117. [Google Scholar] [CrossRef]
- Crimaldi, L.; Courtneidge, S.A.; Gimona, M. Tks5 Recruits AFAP-110, P190RhoGAP, and Cortactin for Podosome Formation. Exp. Cell Res. 2009, 315, 2581–2592. [Google Scholar] [CrossRef]
- Dourado, D.G.; Lima, C.C.B.; Silva, R.N.C.; Tajra, F.S.; Moura, M.S.; Lopes, T.S.P.; De Deus Moura, L.D.F.A.; de Lima, M.D.D.M. Molar-Incisor Hypomineralization in Quilombola Children and Adolescents: A Study of Prevalence and Associated Factors. J. Public Health Dent. 2021, 81, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pandey, R.K. Molar Incisor Hypomineralization: An Epidemiological Study with Prevalence and Etiological Factors in Indian Pediatric Population. Int. J. Clin. Pediatr. Dent. 2016, 9, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.; Waldron, J.M. Molar Incisor Hypomineralisation: Clinical Management of the Young Patient. J. Ir. Dent. Assoc. 2009, 55, 83–86. [Google Scholar] [PubMed]
- Ivanovic, Z. Hypoxia or in Situ Normoxia: The Stem Cell Paradigm. J. Cell. Physiol. 2009, 219, 271–275. [Google Scholar] [CrossRef]
- Ivanovic, Z.; Vlaski-Lafarge, M. Low O2 Concentrations and the Maintenance of Stem Cells Ex Vivo. In Anaerobiosis and Stemness. An Evolutionary Paradigm; Elsevier: Amsterdam, The Netherlands, 2016; pp. 40–71. [Google Scholar]
- Beegle, J.; Lakatos, K.; Kalomoiris, S.; Stewart, H.; Isseroff, R.R.; Nolta, J.A.; Fierro, F.A. Hypoxic Preconditioning of Mesenchymal Stromal Cells Induces Metabolic Changes, Enhances Survival, and Promotes Cell Retention in Vivo. Stem Cells Dayt. Ohio 2015, 33, 1818–1828. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, P.; Zhu, S.; Liu, Z.; Wang, W.; Geng, T.; Dissanayaka, W.L.; Jin, L.; Zhang, C. Overexpression of EphrinB2 in Stem Cells from Apical Papilla Accelerates Angiogenesis. Oral Dis. 2019, 25, 848–859. [Google Scholar] [CrossRef]
- Bolli, R.A.; Dasari, C.; Arshia, A.; Devadoss, D.; Guo, Y.; Ashraf, U.; Li, Q. Physiological Oxygen Tension Enhances Competence and Functional Properties of Murine Cardiac Mesenchymal Cells. Stem Cell Rev. Rep. 2021, 17, 900–910. [Google Scholar] [CrossRef]
- Dan, J.; Zhou, Z.; Wang, F.; Wang, H.; Guo, R.; Keefe, D.L.; Liu, L. Zscan4 Contributes to Telomere Maintenance in Telomerase-Deficient Late Generation Mouse ESCs and Human ALT Cancer Cells. Cells 2022, 11, 456. [Google Scholar] [CrossRef]
- Le, R.; Huang, Y.; Zhang, Y.; Wang, H.; Lin, J.; Dong, Y.; Li, Z.; Guo, M.; Kou, X.; Zhao, Y.; et al. Dcaf11 Activates Zscan4-Mediated Alternative Telomere Lengthening in Early Embryos and Embryonic Stem Cells. Cell Stem Cell 2021, 28, 732–747.e9. [Google Scholar] [CrossRef]
- Zalzman, M.; Falco, G.; Sharova, L.V.; Nishiyama, A.; Thomas, M.; Lee, S.-L.; Stagg, C.A.; Hoang, H.G.; Yang, H.-T.; Indig, F.E.; et al. Zscan4 Regulates Telomere Elongation and Genomic Stability in ES Cells. Nature 2010, 464, 858–863. [Google Scholar] [CrossRef]
- Rivera, T.; Haggblom, C.; Cosconati, S.; Karlseder, J. A Balance between Elongation and Trimming Regulates Telomere Stability in Stem Cells. Nat. Struct. Mol. Biol. 2017, 24, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and Aging: Lessons from Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Huang, X.; Luo, Z.; He, J.; Haider, F.; Song, C.; Peng, L.; Chen, T.; Wu, B. Hypoxia-Activated PI3K/Akt Inhibits Oxidative Stress via the Regulation of Reactive Oxygen Species in Human Dental Pulp Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6595189. [Google Scholar] [CrossRef]
- Zou, T.; Jiang, S.; Zhang, Y.; Liu, J.; Yi, B.; Qi, Y.; Dissanayaka, W.L.; Zhang, C. In Situ Oxygen Generation Enhances the SCAP Survival in Hydrogel Constructs. J. Dent. Res. 2021, 100, 1127–1135. [Google Scholar] [CrossRef]
- Niu, H.; Li, C.; Guan, Y.; Dang, Y.; Li, X.; Fan, Z.; Shen, J.; Ma, L.; Guan, J. High Oxygen Preservation Hydrogels to Augment Cell Survival under Hypoxic Condition. Acta Biomater. 2020, 105, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.-M.; Lesieur, J.; Vacher, C.; Chaussain, C.; Rochefort, G.Y. Strategies Developed to Induce, Direct, and Potentiate Bone Healing. Front. Physiol. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collignon, A.-M.; Castillo-Dali, G.; Gomez, E.; Guilbert, T.; Lesieur, J.; Nicoletti, A.; Acuna-Mendoza, S.; Letourneur, D.; Chaussain, C.; Rochefort, G.Y.; et al. Mouse Wnt1-CRE-RosaTomato Dental Pulp Stem Cells Directly Contribute to the Calvarial Bone Regeneration Process. Stem Cells 2019, 37, 701–711. [Google Scholar] [CrossRef]
- Collignon, A.-M.; Lesieur, J.; Anizan, N.; Azzouna, R.B.; Poliard, A.; Gorin, C.; Letourneur, D.; Chaussain, C.; Rouzet, F.; Rochefort, G.Y. Early Angiogenesis Detected by PET Imaging with 64Cu-NODAGA-RGD Is Predictive of Bone Critical Defect Repair. Acta Biomater. 2018, 82, 111–121. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavinga, M.; Palmier, M.; Rémy, M.; Jeannière, C.; Lenoir, S.; Rey, S.; Saint-Marc, M.; Alonso, F.; Génot, E.; Thébaud, N.; et al. The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration. Cells 2022, 11, 4098. https://doi.org/10.3390/cells11244098
Mavinga M, Palmier M, Rémy M, Jeannière C, Lenoir S, Rey S, Saint-Marc M, Alonso F, Génot E, Thébaud N, et al. The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration. Cells. 2022; 11(24):4098. https://doi.org/10.3390/cells11244098
Chicago/Turabian StyleMavinga, Marine, Mathilde Palmier, Murielle Rémy, Caroline Jeannière, Solène Lenoir, Sylvie Rey, Martine Saint-Marc, Florian Alonso, Elisabeth Génot, Noélie Thébaud, and et al. 2022. "The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration" Cells 11, no. 24: 4098. https://doi.org/10.3390/cells11244098
APA StyleMavinga, M., Palmier, M., Rémy, M., Jeannière, C., Lenoir, S., Rey, S., Saint-Marc, M., Alonso, F., Génot, E., Thébaud, N., Chevret, E., Mournetas, V., Rousseau, B., Boiziau, C., & Boeuf, H. (2022). The Journey of SCAPs (Stem Cells from Apical Papilla), from Their Native Tissue to Grafting: Impact of Oxygen Concentration. Cells, 11(24), 4098. https://doi.org/10.3390/cells11244098