Identification of Spinal Inhibitory Interneurons Required for Attenuating Effect of Duloxetine on Neuropathic Allodynia-like Signs in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Recombinant Adeno-Associated Virus (rAAV) Vector Production
2.3. In Vivo AAV-NpyP+ Neurons-Specific Knockdown with AAV Vector Encoding shRNA
2.4. Intra-SDH Injection of rAAV Vector
2.5. Immunohistochemistry
2.6. Whole-Cell Patch-Clamp Recordings
2.7. Neuropathic Pain Model
2.8. Light Illumination of Hind paw
2.9. Von Frey Test
2.10. Intraperitoneally Administration of Duloxetine
2.11. Statistical Analysis
3. Results
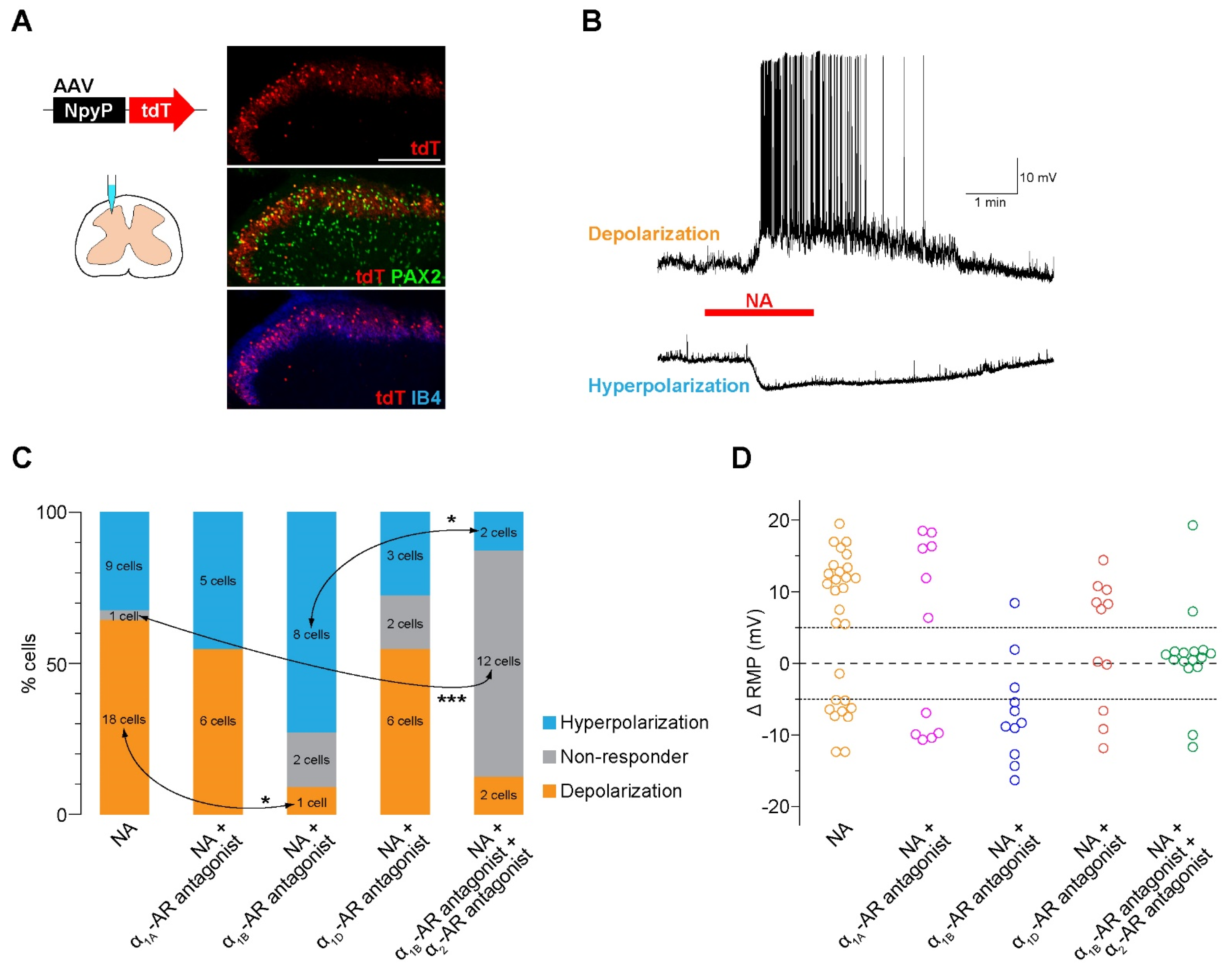
3.1. NA Excites the Majority of AAV-NpyP+ Neurons Via α1B-AR
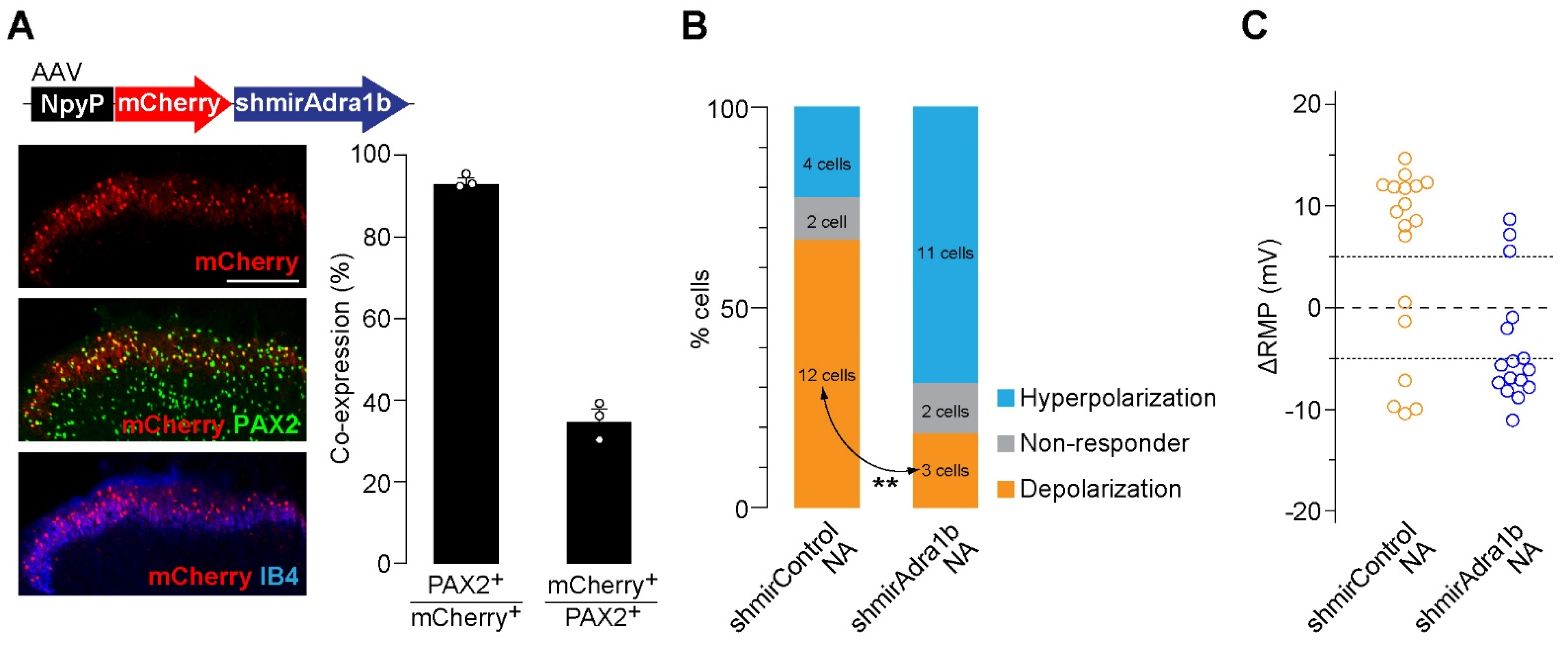
3.2. Knockdown of α1B-AR in AAV-NpyP+ Neurons Suppresses NA-Evoked Depolarization
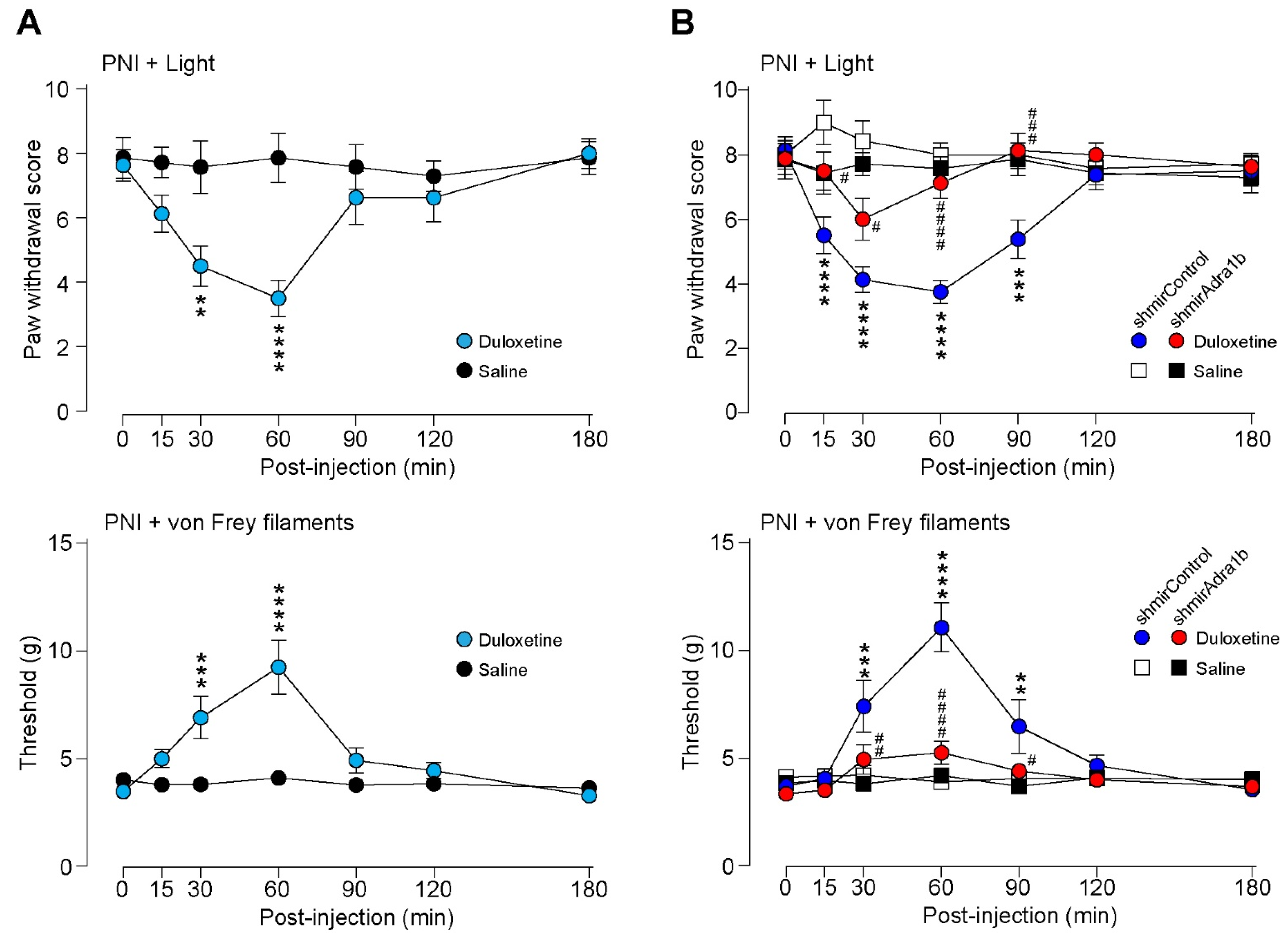
3.3. Duloxetine Alleviates Neuropathic Allodynia-like Behavior Via α1B-AR in AAV-NpyP+ Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Reviews. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.; Ginty, D.D. The mechanosensory neurons of touch and their mechanisms of activation. Nat. Rev. Neurosci. 2021, 22, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Duan, B.; Huang, T.; Zhang, Y.; Chen, Y.; Britz, O.; Garcia-Campmany, L.; Ren, X.; Vong, L.; Lowell, B.B.; et al. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat. Neurosci. 2017, 20, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Tashima, R.; Koga, K.; Sekine, M.; Kanehisa, K.; Kohro, Y.; Tominaga, K.; Matsushita, K.; Tozaki-Saitoh, H.; Fukazawa, Y.; Inoue, K.; et al. Optogenetic Activation of Non-Nociceptive Aβ Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats. eNeuro 2018, 5, 0450-0417. [Google Scholar] [CrossRef]
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef]
- Tsuda, M. New approach for investigating neuropathic allodynia by optogenetics. Pain 2019, 160 (Suppl. 1), S53–S58. [Google Scholar] [CrossRef]
- Hughes, D.I.; Todd, A.J. Central Nervous System Targets: Inhibitory Interneurons in the Spinal Cord. Neurother.: J. Am. Soc. Exp. NeuroTherapeutics 2020, 17, 874–885. [Google Scholar] [CrossRef]
- Peirs, C.; Dallel, R.; Todd, A.J. Recent advances in our understanding of the organization of dorsal horn neuron populations and their contribution to cutaneous mechanical allodynia. J. Neural Trasm. 2020, 127, 505–525. [Google Scholar] [CrossRef]
- Tashima, R.; Koga, K.; Yoshikawa, Y.; Sekine, M.; Watanabe, M.; Tozaki-Saitoh, H.; Furue, H.; Yasaka, T.; Tsuda, M. A subset of spinal dorsal horn interneurons crucial for gating touch-evoked pain-like behavior. Proc. Natl. Acad. Sci. USA 2021, 118, e2021220118. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Yoshimura, M.; Furue, H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006, 101, 107–117. [Google Scholar] [CrossRef]
- Hayashida, K.I.; Obata, H. Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System. Int. J. Mol. Sci. 2019, 20, 822. [Google Scholar] [CrossRef]
- Hiroki, T.; Suto, T.; Saito, S.; Obata, H. Repeated Administration of Amitriptyline in Neuropathic Pain: Modulation of the Noradrenergic Descending Inhibitory System. Anesth. Analg. 2017, 125, 1281–1288. [Google Scholar] [CrossRef]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef]
- Kremer, M.; Yalcin, I.; Goumon, Y.; Wurtz, X.; Nexon, L.; Daniel, D.; Megat, S.; Ceredig, R.A.; Ernst, C.; Turecki, G. A Dual Noradrenergic Mechanism for the Relief of Neuropathic Allodynia by the Antidepressant Drugs Duloxetine and Amitriptyline. J. Neurosci. 2018, 38, 9934–9954. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet. Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Murai, N.; Aoki, T.; Tamura, S.; Sekizawa, T.; Kakimoto, S.; Tsukamoto, M.; Oe, T.; Enomoto, R.; Hamakawa, N.; Matsuoka, N. AS1069562, the (+)-isomer of indeloxazine, exerts analgesic effects in a rat model of neuropathic pain with unique characteristics in spinal monoamine turnover. J. Pharmacol. Exp. Ther. 2014, 348, 372–382. [Google Scholar] [CrossRef]
- Hoshino, H.; Obata, H.; Saito, S. Antihyperalgesic effect of duloxetine and amitriptyline in rats after peripheral nerve injury: Influence of descending noradrenergic plasticity. Neurosci. Lett. 2015, 602, 62–67. [Google Scholar] [CrossRef]
- Ito, S.; Suto, T.; Saito, S.; Obata, H. Repeated Administration of Duloxetine Suppresses Neuropathic Pain by Accumulating Effects of Noradrenaline in the Spinal Cord. Anesth. Analg. 2018, 126, 298–307. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Kumamoto, E.; Furue, H.; Yoshimura, M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology 2003, 98, 682–689. [Google Scholar] [CrossRef]
- Doxey, J.C.; Lane, A.C.; Roach, A.G.; Virdee, N.K. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 325, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Chung, Y.; Choi, S.; Min, B.I.; Kim, S.K. Duloxetine Protects against Oxaliplatin-Induced Neuropathic Pain and Spinal Neuron Hyperexcitability in Rodents. Int. J. Mol. Sci. 2017, 18, 2626. [Google Scholar] [CrossRef] [PubMed]
- North, R.A.; Yoshimura, M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J. Physiol. 1984, 349, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Goldstein, P.A.; Okamoto, M.; Kohno, T.; Ataka, T.; Yoshimura, M.; Shimoji, K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): Effects on somatodendritic sites of GABAergic neurons. Anesthesiology 2000, 92, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Shimoji, K.; Yoshimura, M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): Effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology 2000, 92, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yoshihara, K.; Kawanabe, R.; Hatada, I.; Koga, K.; Tsuda, M. Stress-induced antinociception to noxious heat requires α(1A)-adrenaline receptors of spinal inhibitory neurons in mice. Mol. Brain 2022, 15, 6. [Google Scholar] [CrossRef]
- Häring, M.; Zeisel, A.; Hochgerner, H.; Rinwa, P.; Jakobsson, J.E.T.; Lönnerberg, P.; La Manno, G.; Sharma, N.; Borgius, L.; Kiehn, O.; et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018, 21, 869–880. [Google Scholar] [CrossRef]
- Koch, S.C.; Acton, D.; Goulding, M. Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol. 2018, 80, 189–217. [Google Scholar] [CrossRef]
- Tomita, H.; Sugano, E.; Fukazawa, Y.; Isago, H.; Sugiyama, Y.; Hiroi, T.; Ishizuka, T.; Mushiake, H.; Kato, M.; Hirabayashi, M.; et al. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS ONE 2009, 4, e7679. [Google Scholar] [CrossRef]
- Ji, Z.G.; Ito, S.; Honjoh, T.; Ohta, H.; Ishizuka, T.; Fukazawa, Y.; Yawo, H. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. PLoS ONE 2012, 7, e32699. [Google Scholar] [CrossRef]
- Kohro, Y.; Sakaguchi, E.; Tashima, R.; Tozaki-Saitoh, H.; Okano, H.; Inoue, K.; Tsuda, M. A new minimally-invasive method for microinjection into the mouse spinal dorsal horn. Sci. Rep. 2015, 5, 14306. [Google Scholar] [CrossRef]
- Tsuda, M.; Kohro, Y.; Yano, T.; Tsujikawa, T.; Kitano, J.; Tozaki-Saitoh, H.; Koyanagi, S.; Ohdo, S.; Ji, R.R.; Salter, M.W.; et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain: J. Neurol. 2011, 134, 1127–1139. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Georgiev, S.K.; Wakai, A.; Kohno, T.; Yamakura, T.; Baba, H. Actions of norepinephrine and isoflurane on inhibitory synaptic transmission in adult rat spinal cord substantia gelatinosa neurons. Anesth. Analg. 2006, 102, 124–128. [Google Scholar] [CrossRef]
- Gassner, M.; Ruscheweyh, R.; Sandkühler, J. Direct excitation of spinal GABAergic interneurons by noradrenaline. Pain 2009, 145, 204–210. [Google Scholar] [CrossRef]
- Koga, K.; Shiraishi, Y.; Yamagata, R.; Tozaki-Saitoh, H.; Shiratori-Hayashi, M.; Tsuda, M. Intrinsic braking role of descending locus coeruleus noradrenergic neurons in acute and chronic itch in mice. Mol. Brain 2020, 13, 144. [Google Scholar] [CrossRef]
- Pertovaara, A. Noradrenergic pain modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef]
- Sun, Y.H.; Li, H.S.; Zhu, C.; Hu, W.; Yang, J.; Zhao, G.L.; Lu, G.J.; Wu, S.X.; Dong, Y.L. The analgesia effect of duloxetine on post-operative pain via intrathecal or intraperitoneal administration. Neurosci. Lett. 2014, 568, 6–11. [Google Scholar] [CrossRef]
- Kohro, Y.; Matsuda, T.; Yoshihara, K.; Kohno, K.; Koga, K.; Katsuragi, R.; Oka, T.; Tashima, R.; Muneta, S.; Yamane, T.; et al. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat. Neurosci. 2020, 23, 1376–1387. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, T.; Sueto, D.; Yoshikawa, Y.; Koga, K.; Yamaura, K.; Tsuda, M. Identification of Spinal Inhibitory Interneurons Required for Attenuating Effect of Duloxetine on Neuropathic Allodynia-like Signs in Rats. Cells 2022, 11, 4051. https://doi.org/10.3390/cells11244051
Ishibashi T, Sueto D, Yoshikawa Y, Koga K, Yamaura K, Tsuda M. Identification of Spinal Inhibitory Interneurons Required for Attenuating Effect of Duloxetine on Neuropathic Allodynia-like Signs in Rats. Cells. 2022; 11(24):4051. https://doi.org/10.3390/cells11244051
Chicago/Turabian StyleIshibashi, Tadayuki, Daichi Sueto, Yu Yoshikawa, Keisuke Koga, Ken Yamaura, and Makoto Tsuda. 2022. "Identification of Spinal Inhibitory Interneurons Required for Attenuating Effect of Duloxetine on Neuropathic Allodynia-like Signs in Rats" Cells 11, no. 24: 4051. https://doi.org/10.3390/cells11244051
APA StyleIshibashi, T., Sueto, D., Yoshikawa, Y., Koga, K., Yamaura, K., & Tsuda, M. (2022). Identification of Spinal Inhibitory Interneurons Required for Attenuating Effect of Duloxetine on Neuropathic Allodynia-like Signs in Rats. Cells, 11(24), 4051. https://doi.org/10.3390/cells11244051






