Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7− Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of PICs from Skeletal Muscle
2.2. Cell Culture
2.3. Animals
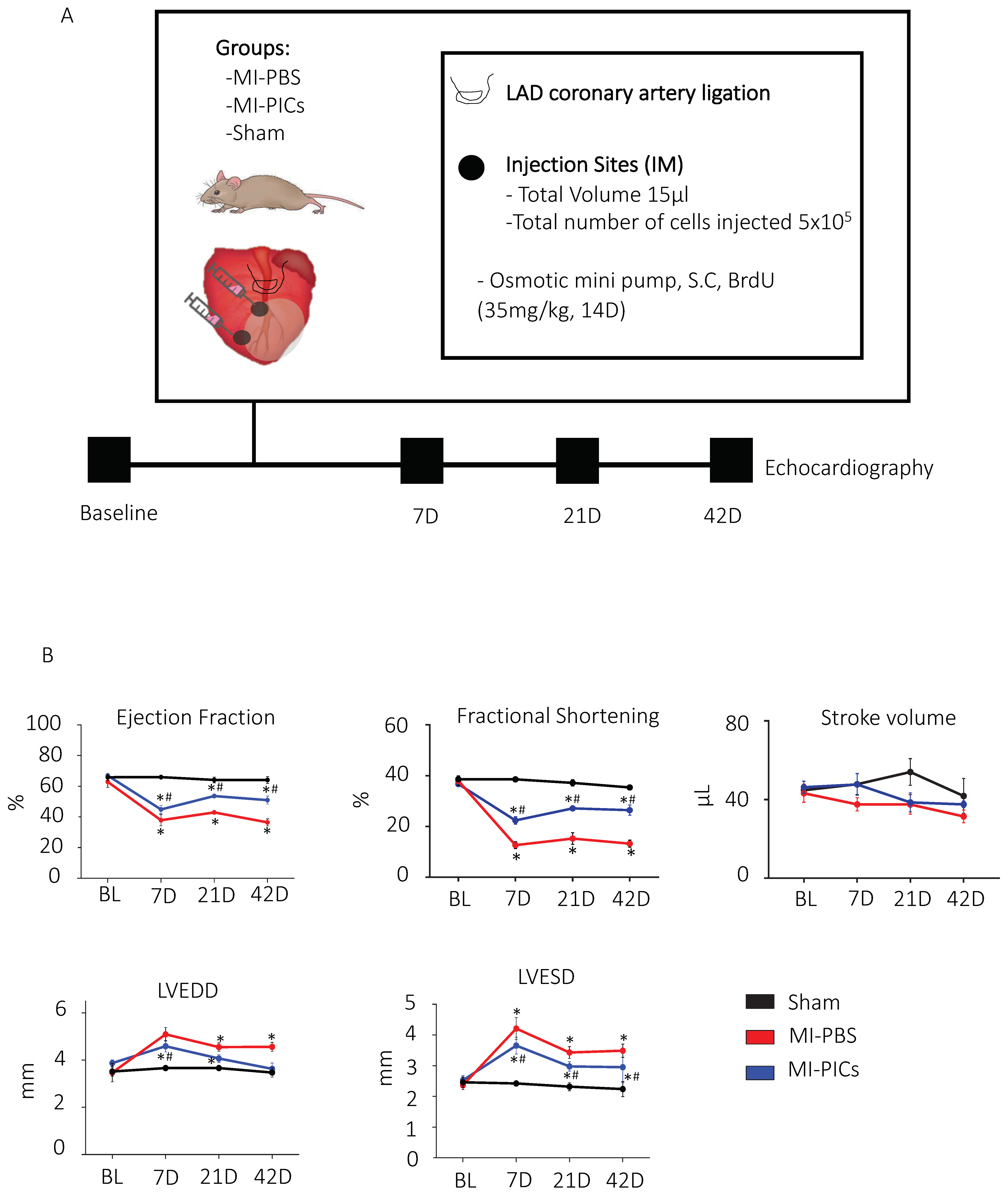
2.4. Echocardiography
2.5. Acute Myocardial Infarction Surgery
2.6. Tissue Harvesting, Processing and Sectioning
2.7. Immunohistochemistry
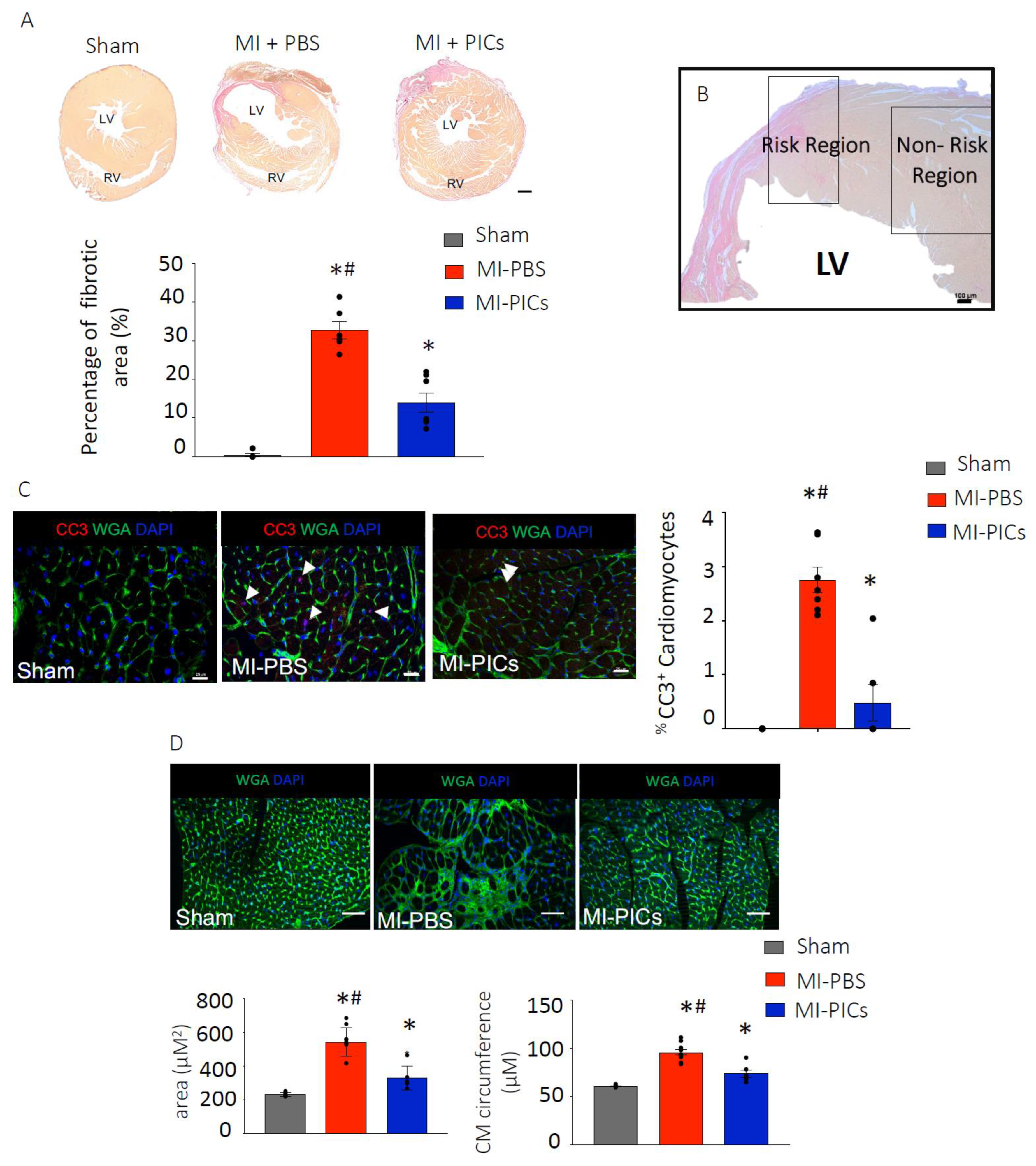
2.8. Hematoxylin VAN Gieson Staining for Fibrosis
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT–qPCR)
- 95 °C—5 min
- 40 cycles of:
- a.
- 95 °C—15 s
- b.
- 60 °C—30 s
- c.
- 72 °C—30 s
2.10. Preparation of PIC Conditioned Media
2.11. ELISA
2.12. Angiogenesis Assay
2.13. Statistical Analysis
3. Results
3.1. Improvement in Cardiac Function after PICs Transplantation in MI Mice
3.2. PIC Transplantation Attenuates Cardiac Remodelling Post-MI
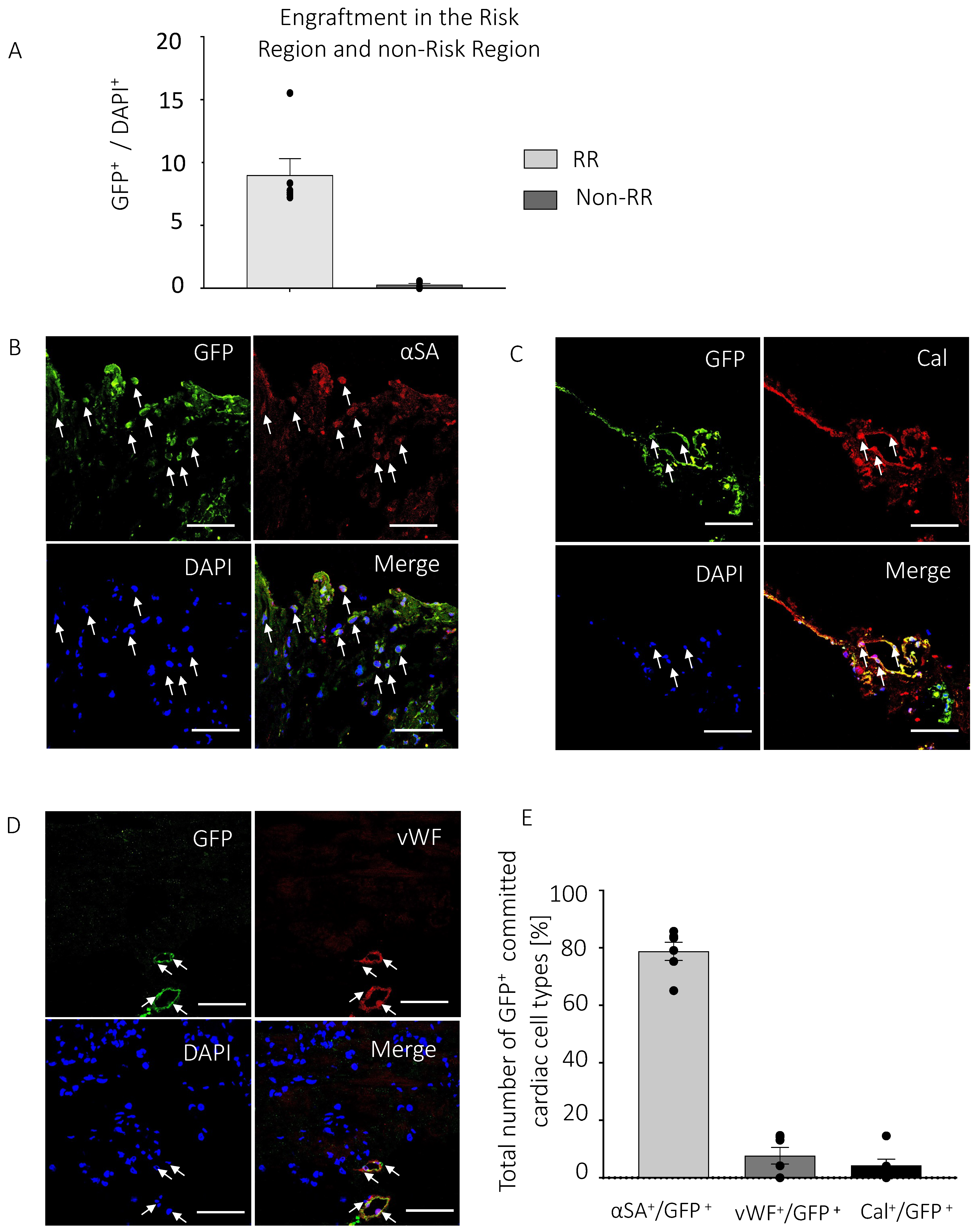
3.3. PICs Engraft in the Myocardial Infarcted Heart
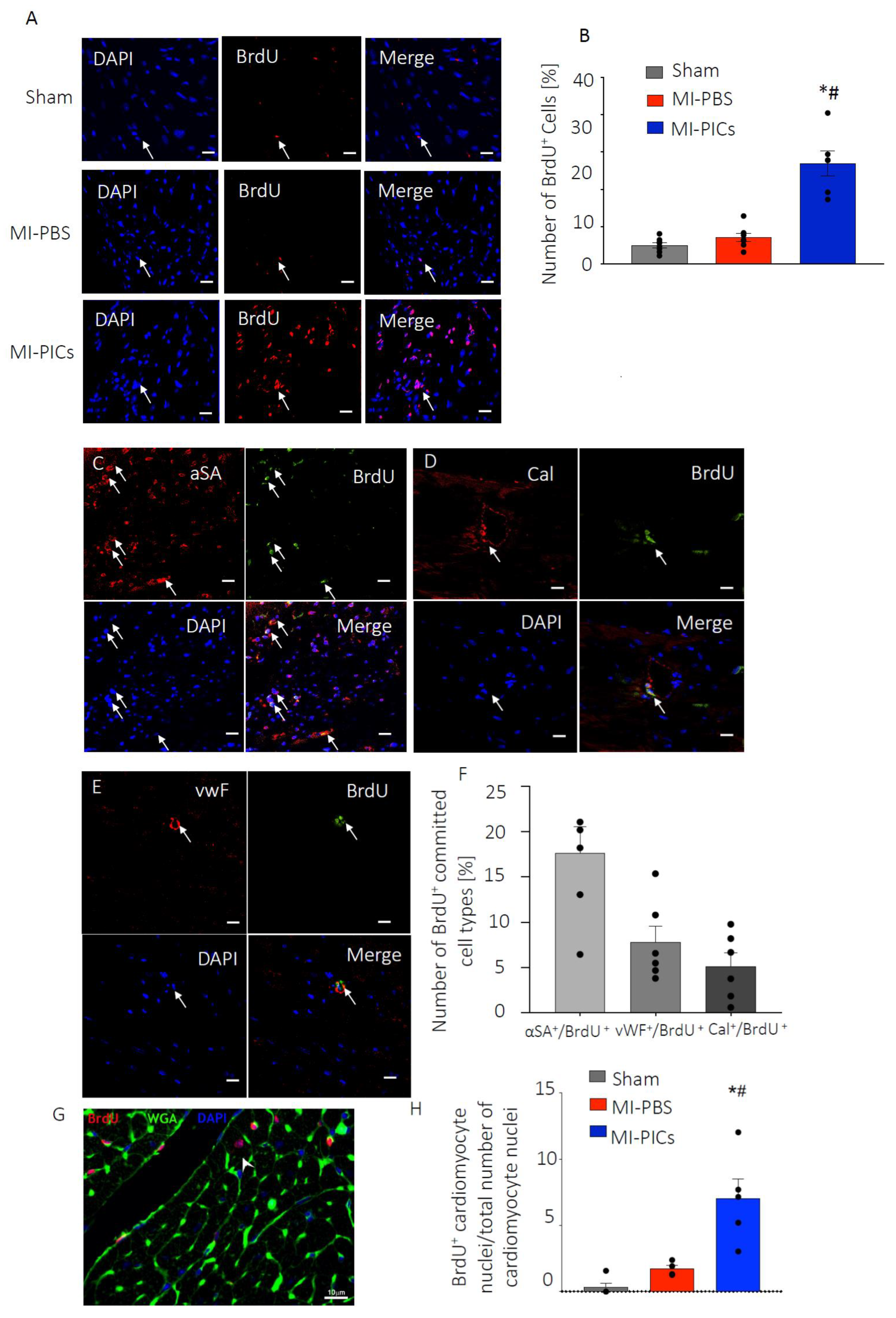
3.4. PICs Transplantation Increases Cell Proliferation in the MI-Heart
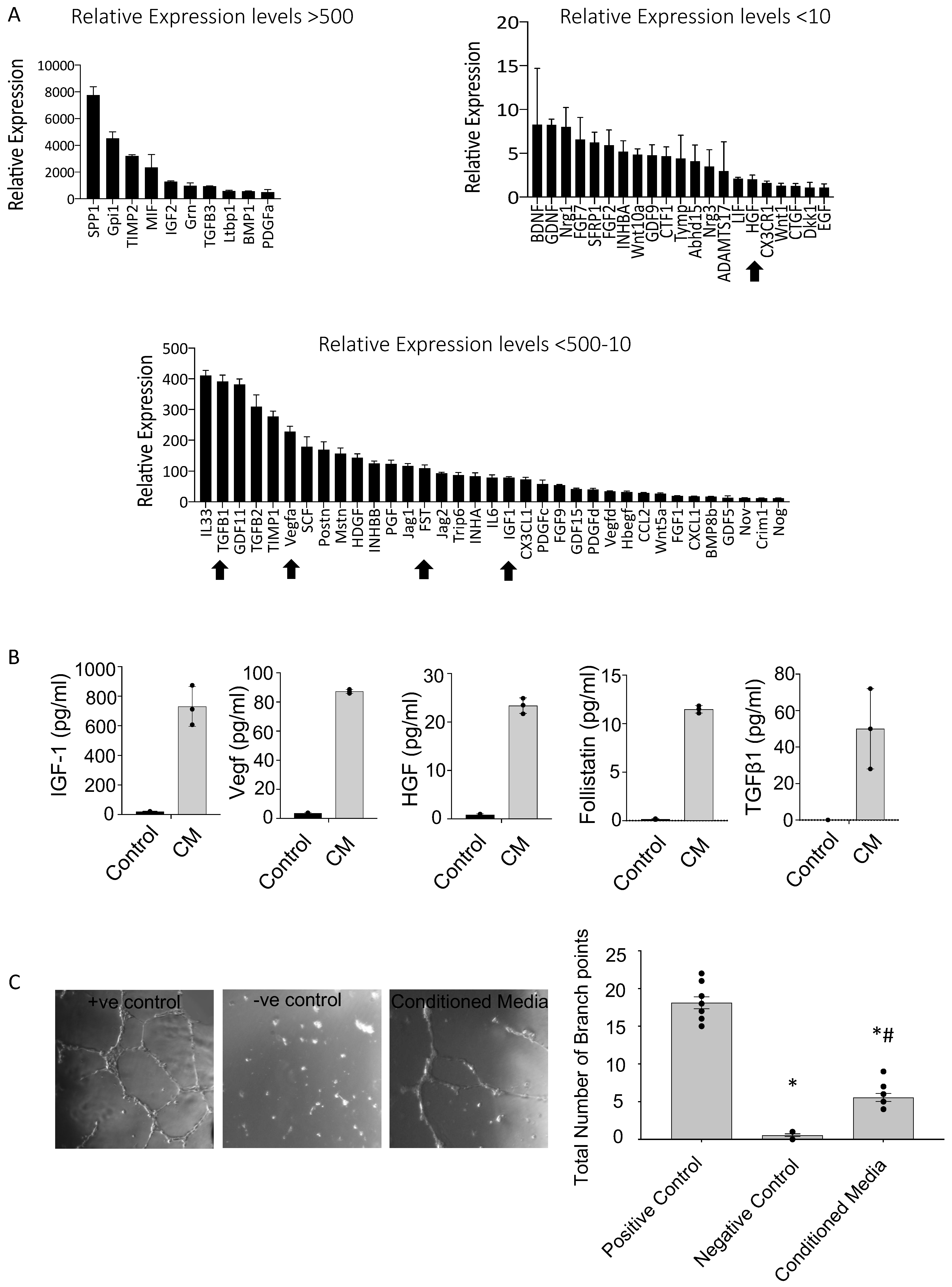
3.5. PICs Secrete an Array of Pro-Survival and Reparative Paracrine Factors
4. Discussion
4.1. Improvement in Cardiac Function after PICs Transplantation in MI Mice
4.2. Improved Cardiac Remodelling after PICs Transplantation in MI Mice
4.3. Increased Proliferation of Cardiac Cells after PICs Transplantation in MI Mice
4.4. Transplanted PICs Engraft but Do Not Significantly Differentiate into Mature Cardiomyocytes
4.5. PICs Express and Secrete Pro-Survival and Regenerative Factors That Stimulate Angiogenesis
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, W.J.; Clarke, S.A.; Quinn, T.A.; Holmes, J.W. Physiological Implications of Myocardial Scar Structure. Compr. Physiol. 2015, 5, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Hippenmeyer, S.; Saadat, L.V.; Luo, L.; Weissman, I.L.; Ardehali, R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 8850–8855. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Zhang, L.; Yan, J.; Chen, J.; Cai, W.; Razzaque, S.; Jeong, D.; Sheng, W.; Bu, L.; Xu, M.; et al. Resident c-kit+ cells in the heart are not cardiac stem cells. Nat. Commun. 2015, 6, 8701. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Kim, H.; Yoo, J.; Kwon, S.P.; Park, B.W.; Kim, J.; Ban, K.; Char, K.; Park, H.; Kim, B. Prevascularized, multiple-layered cell sheets of direct cardiac reprogrammed cells for cardiac repair. Biomater. Sci. 2020, 8, 4508–4520. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Honda, A.; Nagai, T.; Fukushima, N.; Iwanaga, K.; Tokunaga, M.; Shimizu, T.; Kasanuki, H.; Hagiwara, N.; Komuro, I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J. Clin. Investig. 2009, 119, 2204–2217. [Google Scholar] [CrossRef]
- Liew, L.C.; Ho, B.X.; Soh, B.S. Mending a broken heart: Current strategies and limitations of cell-based therapy. Stem Cell Res. Ther. 2020, 11, 138. [Google Scholar] [CrossRef]
- Tachibana, A.; Santoso, M.R.; Mahmoudi, M.; Shukla, P.; Wang, L.; Bennett, M.; Goldstone, A.; Wang, M.; Fukushi, M.; Ebert, A.; et al. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ. Res. 2017, 121, e22–e36. [Google Scholar] [CrossRef]
- Rafatian, G.; Davis, D.R. Review: Heart-Derived Cell Therapy 2.0: Paracrine Strategies to Increase Therapeutic Repair of Injured Myocardium. Stem Cells 2018, 36, 1794–1803. [Google Scholar] [CrossRef]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef]
- Ellison, G.M.; Nadal-Ginard, B.; Torella, D. Optimizing Cardiac Repair and Regeneration Through Activation of the Endogenous Cardiac Stem Cell Compartment. J. Cardiovasc. Transl. Res. 2012, 5, 667–677. [Google Scholar] [CrossRef]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.; Fransioli, J.; Gude, N.A.; Alvarez, R., Jr.; Zhang, X.; Gustafsson, Å.B.; Sussman, M. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ. Res. 2012/07/16 2012, 111, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Yutoku, M.; Grossberg, A.L.; Pressman, D. A Cell Surface Antigenic Determinant Present on Mouse Plasmacytes and Only about Half of Mouse Thymocytes. J. Immunol. 1974, 112, 1774–1781. [Google Scholar] [PubMed]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michel, L.; Berhringer, R.; Garry, D.; et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Q.; Nakamura, Y.; Lee, J.; Zhang, G. The Role of the Sca-1+/CD31− Cardiac Progenitor Cell Population in Postinfarction Left Ventricular Remodeling. Stem Cells 2006, 24, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Ashihara, E.; Takehara, N.; Nomura, T.; Honsho, S.; Nakagami, T.; Morikawa, S.; Takahashi, T.; Ueyama, T.; Matsubara, H.; et al. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J. Cell Sci. 2007, 120, 1791–1800. [Google Scholar] [CrossRef]
- Noseda, M.; Harada, M.; McSweeney, S.; Leja, T.; Belian, E.; Stuckey, D.J.; Abreu Paiva, M.; Habib, J.; Macaulay, I.; de Smith, A.; et al. PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat. Commun. 2015, 6, 6930. [Google Scholar] [CrossRef]
- Lewis, F.C.; Henning, B.J.; Marazzi, G.; Sassoon, D.; Ellison, G.M.; Nadal-Ginard, B. Porcine Skeletal Muscle-Derived Multipotent PW1pos/Pax7neg Interstitial Cells: Isolation, Characterization, and Long-Term Culture. Stem Cells Transl. Med. 2014, 3, 702–712. [Google Scholar] [CrossRef]
- Cottle, B.J.; Lewis, F.C.; Shone, V.; Ellison-Hughes, G.M. Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 158. [Google Scholar] [CrossRef]
- Lewis, F.C.; Cottle, B.J.; Shone, V.; Marazzi, G.; Sassoon, D.; Tseng, C.C.S.; Dankers, P.; Chamuleau, S.; Nadal-Ginard, B.; Ellison-Hughes, G. Transplantation of Allogeneic PW1(pos)/Pax7(neg) Interstitial Cells Enhance Endogenous Repair of Injured Porcine Skeletal Muscle. JACC Basic Transl. Sci. 2017, 2, 717–736. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pannérec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ruchaya, P.J.; Paton, J.F.R.; Murphy, D.; Yao, S.T. A cardiovascular role for fractalkine and its cognate receptor, CX3CR1, in the rat nucleus of the solitary tract. Neuroscience 2012, 209, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ruchaya, P.J.; Antunes, V.R.; Paton, J.F.R.; Murphy, D.; Yao, S.T. The cardiovascular actions of fractalkine/CX3CL1 in the hypothalamic paraventricular nucleus are attenuated in rats with heart failure. Exp. Physiol. 2014, 99, 111–122. [Google Scholar] [CrossRef]
- Tang, X.L.; Nakamura, S.; Li, Q.; Wysoczynski, M.; Gumpert, A.M.; Wu, W.J.; Hunt, G.; Stower, H.; Ou, Q.; Bolli, R. Repeated Administrations of Cardiac Progenitor Cells Are Superior to a Single Administration of an Equivalent Cumulative Dose. J. Am. Heart Assoc. 2018, 7, e007400. Available online: https://pubmed.ncbi.nlm.nih.gov/29440036 (accessed on 30 September 2022). [CrossRef] [PubMed]
- Tang, X.L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-Term Outcome of Administration of c-kitPOS Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at L. Circ. Res. 2016, 118, 1091–1105. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Dassanayaka, S.; Zafir, A.; Ghafghazi, S.; Long, B.W.; Noble, C.; Demartino, A.; Birttain, K.; Bolli, R. A New Method to Stabilize C-Kit Expression in Reparative Cardiac Mesenchymal Cells. Front. Cell Dev. Biol. 2016, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Haider, K.H.; Ashraf, M. Identification and Characterization of a Novel Multipotent Sub-Population of Sca-1+ Cardiac Progenitor Cells for Myocardial Regeneration. PLoS ONE 2011, 6, e25265. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt-Velin, N.; Ogay, S.; Felley, A.; Stanford, W.L.; Pedrazzini, T. Cardiac dysfunction and impaired compensatory response to pressure overload in mice deficient in stem cell antigen-1. FASEB J. 2012, 26, 229–239. [Google Scholar] [CrossRef]
- Zhou, H.; Bian, Z.Y.; Zong, J.; Deng, W.; Yan, L.; Shen, D.F.; Guo, H.; Dai, J.; Yuan, Y.; Zhang, R.; et al. Stem Cell Antigen 1 Protects Against Cardiac Hypertrophy and Fibrosis After Pressure Overload. Hypertension 2012, 60, 802–809. [Google Scholar] [CrossRef][Green Version]
- Malliaras, K.; Zhang, Y.; Seinfeld, J.; Galang, G.; Tseliou, E.; Cheng, K.; Sun, B.; Aminzadeh, M.; Marban, E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol. Med. 2013, 5, 191–209. Available online: https://pubmed.ncbi.nlm.nih.gov/23255322 (accessed on 11 November 2020). [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maratta, A.; Sergi, D.; Muscolli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, R.; Campbell, K.F.; Carvalho, J.L.; Ruiz, E.C.; Kim, G.C.; Schmuck, E.; Raval, A.; da Rocha, A.; Herron, T.; et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 2019, 10, 2238. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Tsuchihashi, T.; Ivey, K.N.; Ross, R.S.; Hong, T.T.; Shaw, R.M.; Srivastava, D. Cardiac Fibroblasts Regulate Myocardial Proliferation through β1 Integrin Signaling. Dev. Cell 2009, 16, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Simões, F.C.; Cahill, T.J.; Kenyon, A.; Gavriouchkina, D.; Vieira, J.M.; Sun, X.; Pezzolla, D.; Ravaud, D.; Masmanian, E.; Weinberger, M.; et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020, 11, 600. [Google Scholar] [CrossRef]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.; Striparo, G.; Papait, R.; Scarfo, M.; et al. Adult c-kitpos Cardiac Stem Cells Are Necessary and Sufficient for Functional Cardiac Regeneration and Repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Feng, B.; Wang, X.; He, X.; Hu, R.; Yin, M.; Wang, W.; Fu, W.; Xu, Z. Isolation and characterization of a Sca-1+/CD31-progenitor cell lineage derived from mouse heart tissue. BMC Biotechnol. 2014, 14, 75. [Google Scholar] [CrossRef]
- Uchida, S.; de Gaspari, P.; Kostin, S.; Jenniches, K.; Kilic, A.; Izumiya, Y.; Shiojima, I.; Grosse Kreymborg, K.; Renz, H.; Walsh, K.; et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Rep. 2013, 1, 397–410. [Google Scholar] [CrossRef]
- Hassan, N.; Tchao, J.; Tobita, K. Concise Review: Skeletal Muscle Stem Cells and Cardiac Lineage: Potential for Heart Repair. Stem Cells Transl. Med. 2014, 3, 183–193. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Cai, M.; Shen, R.; Song, L.; Lu, M.; Wang, J.; Zhao, S.; Tang, Y.; Meng, X.; Li, Z.; He, Z. Bone Marrow Mesenchymal Stem Cells (BM-MSCs) Improve Heart Function in Swine Myocardial Infarction Model through Paracrine Effects. Sci. Rep. 2016, 6, 28250. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, R.Y.; Park, B.W.; Lee, S.; Choi, S.W.; Park, J.H.; Choi, J.; Kim, S.; Jang, J.; Cho, D.; et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019, 10, 3123. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Smith, A.J.; Waring, C.D.; Hasan, M.K.; Miyamoto, S.; Matsuoka, R.; Ellison, G. c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS ONE 2010, 5, e14297. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G.M.; Torella, D.; Dellegrottaglie, S.; Perez-Martinez, C.; Perez de Prado, A.; Vicinanza, C.; Purushothaman, S.; Galuppo, V.; Iaconetti, C.; Waring, C.; et al. Endogenous Cardiac Stem Cell Activation by Insulin-Like Growth Factor-1/Hepatocyte Growth Factor Intracoronary Injection Fosters Survival and Regeneration of the Infarcted Pig Heart. J. Am. Coll. Cardiol. 2011, 58, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Santini, M.P.; Claycomb, W.C.; Ladurner, A.G.; Rosenthal, N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging 2010, 2, 43–62. [Google Scholar] [CrossRef]
- Touvron, M.; Escoubet, B.; Mericskay, M.; Angelini, A.; Lamotte, L.; Santini, M.P.; Rosenthal, N.; Daegelen, D.; Tuil, D.; Decaux, J. Locally expressed IGF1 propeptide improves mouse heart function in induced dilated cardiomyopathy by blocking myocardial fibrosis and SRF-dependent CTGF induction. Dis. Model Mech. 2012, 5, 481–491. [Google Scholar] [CrossRef]
- Koudstaal, S.; Bastings, M.M.C.; Feyen, D.A.M.; Waring, C.D.; van Slochteren, F.J.; Dankers, P.Y.W.; Torella, D.; Slujter, J.; Nadal-Ginard, B.; Doevendans, P.; et al. Sustained Delivery of Insulin-Like Growth Factor-1/Hepatocyte Growth Factor Stimulates Endogenous Cardiac Repair in the Chronic Infarcted Pig Heart. J. Cardiovasc. Transl. Res. 2014, 7, 232–241. [Google Scholar] [CrossRef]
- Gómez-Mauricio, G.; Moscoso, I.; Martín-Cancho, M.F.; Crisóstomo, V.; Prat-Vidal, C.; Báez-Díaz, C.; Sanchez-Margallo, F.; Bernad, A. Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res. Ther. 2016, 7, 94. [Google Scholar] [CrossRef]
- Chen, H.; Xia, R.; Li, Z.; Zhang, L.; Xia, C.; Ai, H.; Yang, Z.; Guo, Y. Mesenchymal Stem Cells Combined with Hepatocyte Growth Factor Therapy for Attenuating Ischaemic Myocardial Fibrosis: Assessment using Multimodal Molecular Imaging. Sci. Rep. 2016, 6, 33700. [Google Scholar] [CrossRef]
- Okayama, K.; Azuma, J.; Dosaka, N.; Iekushi, K.; Sanada, F.; Kusunoki, H.; Iwabayashi, M.; Rakugi, H.; Taniyama, Y.; Morishita, R. Hepatocyte Growth Factor Reduces Cardiac Fibrosis by Inhibiting Endothelial-Mesenchymal Transition. Hypertension 2012, 59, 958–965. [Google Scholar] [CrossRef]
- Deasy, B.M.; Feduska, J.M.; Payne, T.R.; Li, Y.; Ambrosio, F.; Huard, J. Effect of VEGF on the Regenerative Capacity of Muscle Stem Cells in Dystrophic Skeletal Muscle. Mol. Ther. 2009, 17, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.B.; Wu, H.Y.; Lai, B.L.; Zheng, L.; Zhou, D.C.; Chang, Z.S.; Mao, C.; Liu, G.; Park, K.; Zhao, H.; et al. Gene delivery of hypoxia-inducible VEGF targeting collagen effectively improves cardiac function after myocardial infarction. Sci. Rep. 2017, 7, 13273. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Kivelä, R. Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Ren, G.; Dewald, O.; Zymek, P.; Haudek, S.; Koerting, A.; Winklemann, K.; Micheal, L.; Lawlar, J.; Entman, M. Critical Role of Endogenous Thrombospondin-1 in Preventing Expansion of Healing Myocardial Infarcts. Circulation 2005, 111, 2935–2942. [Google Scholar] [CrossRef]
- Bujak, M.; Ren, G.; Kweon, H.J.; Dobaczewski, M.; Reddy, A.; Taffet, G.; Wang, X.; Frangogiannis, N. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation 2007, 116, 2127–2138. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Xu, Z.; Alloush, J.; Beck, E.; Weisleder, N. A murine model of myocardial ischemia-reperfusion injury through ligation of the left anterior descending artery. J. Vis. Exp. 2014, 86, e51329. [Google Scholar] [CrossRef]
- De Villiers, C.; Riley, P.R. Mouse models of myocardial infarction: Comparing permanent ligation and ischaemia-reperfusion. Dis. Model Mech. 2020, 13, dmm046565. [Google Scholar] [CrossRef]
- Huang, K.; Hu, S.; Cheng, K. A New Era of Cardiac Cell Therapy: Opportunities and Challenges. Adv. Healthc. Mater. 2021, 8, e1801011. [Google Scholar] [CrossRef]
- Isotta, C.; Ruckdeschel, S.R.; Tao-Sheng, L.; Gary, G.; Elisa, M.; Alessandro, G.; Eduardo, M. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted into Infarcted Mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Stem cell therapy in heart failure: Where do we stand today? Biochim. Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165489. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruchaya, P.J.; Lewis-McDougall, F.C.; Sornkarn, N.; Amin, S.; Grimsdell, B.; Shaalan, A.; Gritti, G.; Soe, K.T.; Clark, J.E.; Ellison-Hughes, G.M. Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7− Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction. Cells 2022, 11, 4050. https://doi.org/10.3390/cells11244050
Ruchaya PJ, Lewis-McDougall FC, Sornkarn N, Amin S, Grimsdell B, Shaalan A, Gritti G, Soe KT, Clark JE, Ellison-Hughes GM. Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7− Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction. Cells. 2022; 11(24):4050. https://doi.org/10.3390/cells11244050
Chicago/Turabian StyleRuchaya, Prashant J, Fiona C. Lewis-McDougall, Nitiphat Sornkarn, Sachin Amin, Benjamin Grimsdell, Abeer Shaalan, Guilia Gritti, Kyi Thar Soe, James E. Clark, and Georgina M. Ellison-Hughes. 2022. "Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7− Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction" Cells 11, no. 24: 4050. https://doi.org/10.3390/cells11244050
APA StyleRuchaya, P. J., Lewis-McDougall, F. C., Sornkarn, N., Amin, S., Grimsdell, B., Shaalan, A., Gritti, G., Soe, K. T., Clark, J. E., & Ellison-Hughes, G. M. (2022). Transplantation of Skeletal Muscle-Derived Sca-1+/PW1+/Pax7− Interstitial Cells (PICs) Improves Cardiac Function and Attenuates Remodeling in Mice Subjected to Myocardial Infarction. Cells, 11(24), 4050. https://doi.org/10.3390/cells11244050





