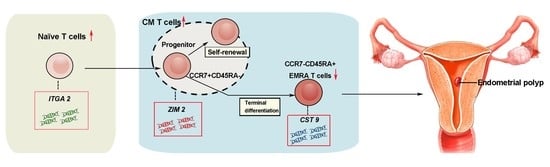
cfDNA Methylation Profiles and T-Cell Differentiation in Women with Endometrial Polyps
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Flow Cytometry Analysis
2.3. cfDNA Methylation Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Populations
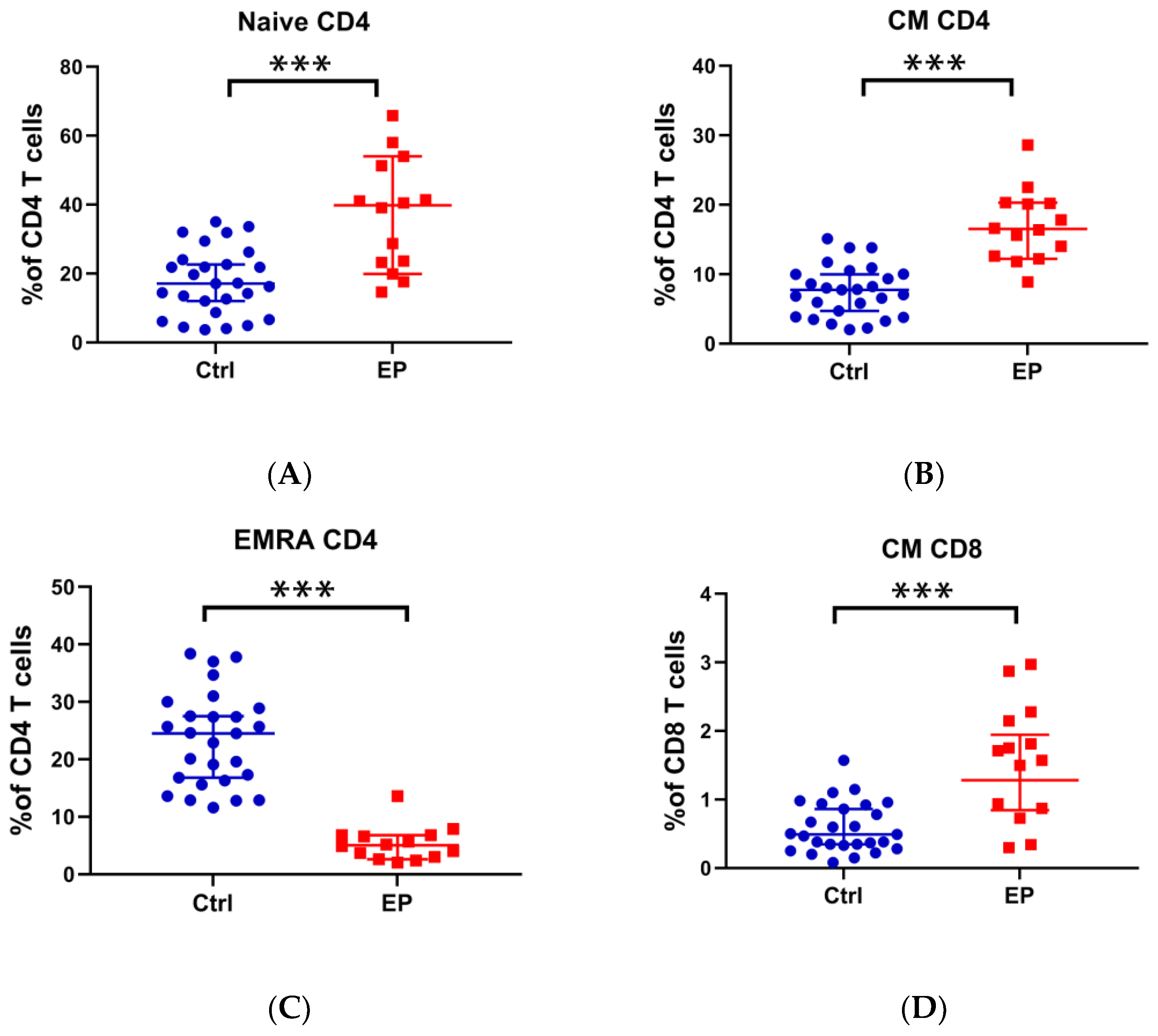
3.2. Analysis of T-Cell Immune Function in Patients with EPs and Controls
3.3. Differences in cfDNA Methylation between Patients with EP and Controls
3.4. Correlation Analysis between cfDNA Methylation and T-Cell Differentiation
3.5. Correlation Analysis of Clinical Indicators of EP with cfDNA Methylation and T-Cell Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansour, T.; Chowdhury, Y.S. Endometrial Polyp. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lasmar, B.P.; Lasmar, R.B. Endometrial polyp size and polyp hyperplasia. Int. J. Gynaecol. Obstet. 2013, 123, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Tanos, V.; Berry, K.E.; Seikkula, J.; Abi Raad, E.; Stavroulis, A.; Sleiman, Z.; Campo, R.; Gordts, S. The management of polyps in female reproductive organs. Int. J. Surg. 2017, 43, 7–16. [Google Scholar] [CrossRef]
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: Practice guidelines for the diagnosis and management of endometrial polyps. J. Minim. Invasive Gynecol. 2012, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vallve-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef]
- Li, X.H.; Lu, M.Y.; Li, Y.J.; Liu, Z.H.; Yin, Z.N.; Liu, B.; Wu, Y.Z. Circulating PD1+Vδ1+γδ T Cell Predicts Fertility in Endometrial Polyp Patients of Reproductive-Age. Front. Immunol. 2021, 12, 639221. [Google Scholar] [CrossRef] [PubMed]
- Borchiellini, M.; Ummarino, S.; Di Ruscio, A. The Bright and Dark Side of DNA Methylation: A Matter of Balance. Cells 2019, 8, 1243. [Google Scholar] [CrossRef]
- Feng, H.; Jin, P.; Wu, H. Disease prediction by cell-free DNA methylation. Brief. Bioinform. 2019, 20, 585–597. [Google Scholar] [CrossRef]
- Lio, C.J.; Rao, A. TET Enzymes and 5hmC in Adaptive and Innate Immune Systems. Front. Immunol. 2019, 10, 210. [Google Scholar] [CrossRef]
- Hurtado, C.; Acevedo Saenz, L.Y.; Vasquez Trespalacios, E.M.; Urrego, R.; Jenks, S.; Sanz, I.; Vasquez, G. DNA methylation changes on immune cells in Systemic Lupus Erythematosus. Autoimmunity 2020, 53, 114–121. [Google Scholar] [CrossRef]
- Mittelstaedt, N.N.; Becker, A.L.; de Freitas, D.N.; Zanin, R.F.; Stein, R.T.; Duarte de Souza, A.P. DNA Methylation and Immune Memory Response. Cells 2021, 10, 2943. [Google Scholar] [CrossRef]
- Calle-Fabregat, C.; Morante-Palacios, O.; Ballestar, E. Understanding the Relevance of DNA Methylation Changes in Immune Differentiation and Disease. Genes 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Hao, X.; Keith, J.; Feng, Y. DNA Methylation in Regulatory T Cell Differentiation and Function: Challenges and Opportunities. Biomolecules 2022, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet 2018, 392, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Lu, M.Y.; Sun, R.L.; Yin, Z.N.; Liu, B.; Wu, Y.Z. Characteristics of Peripheral Immune Function in Reproductive Females with Uterine Leiomyoma. J. Oncol. 2019, 2019, 5935640. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Xia, Y.; Yang, X.; Lv, Q.; Fang, F.; Wang, Q.; Bu, W.; Wang, Y.; Zhang, K.; et al. UVB induces cutaneous squamous cell carcinoma progression by de novo ID4 methylation via methylation regulating enzymes. eBioMedicine 2020, 57, 102835. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Pan, X.; Xu, D.; Ji, G.; Wang, Y.; Tian, Y.; Cai, J.; Li, J.; Zhang, Z.; Yuan, X. Benchmarking DNA methylation analysis of 14 alignment algorithms for whole genome bisulfite sequencing in mammals. Comput. Struct. Biotechnol. J. 2022, 20, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Beckstette, M.; Lu, C.W.; Herppich, S.; Diem, E.C.; Ntalli, A.; Ochel, A.; Kruse, F.; Pietzsch, B.; Neumann, K.; Huehn, J.; et al. Profiling of epigenetic marker regions in murine ILCs under homeostatic and inflammatory conditions. J. Exp. Med. 2022, 219, e20210663. [Google Scholar] [CrossRef] [PubMed]
- Asomaning, N.; Archer, K.J. High-throughput DNA methylation datasets for evaluating false discovery rate methodologies. Comput. Stat. Data Anal. 2012, 56, 1748–1756. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Ding, X.; Li, W. Identification of lncRNA/circRNA-miRNA-mRNA ceRNA Network as Biomarkers for Hepatocellular Carcinoma. Front. Genet. 2022, 13, 838869. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Z.; Du, M.; Yi, L.; Gong, G.; Tang, X. Macrophages in patients with recurrent endometrial polyps could exacerbate Th17 responses. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, M.; Yi, L.; Liu, Z.; Gong, G.; Tang, X. CD4+ T cell imbalance is associated with recurrent endometrial polyps. Clin. Exp. Pharmacol. Physiol. 2018, 45, 507–513. [Google Scholar] [CrossRef]
- Kulp, J.L.; Mamillapalli, R.; Taylor, H.S. Aberrant HOXA10 Methylation in Patients with Common Gynecologic Disorders: Implications for Reproductive Outcomes. Reprod. Sci. 2016, 23, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Li, C.H.; Huang, S.C.; Chang, D.Y.; Chou, L.Y.; Sheu, B.C. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer 2010, 116, 5777–5788. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhao, J.; Sun, Y.; Li, D.; Meng, Z.; Wang, B.; Fan, P.; Liu, Z.; Jin, X.; Wu, H. Overexpressed ITGA2 promotes malignant tumor aggression by up-regulating PD-L1 expression through the activation of the STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 485. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Liang, C.Y.; Labitzky, V.; Ritz, D.; Oliveira, T.; Cumin, C.; Estermann, M.; Lange, T.; Everest-Dass, A.V.; Jacob, F. Site-specific N-glycosylation of integrin alpha2 mediates collagen-dependent cell survival. iScience 2021, 24, 103168. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yan, L.; Zou, C.; Wang, K.; Chen, M.; Xu, B.; Zhou, Z.; Zhang, D. Integrins regulate stemness in solid tumor: An emerging therapeutic target. J. Hematol. Oncol. 2021, 14, 177. [Google Scholar] [CrossRef]
- LaFlamme, S.E.; Auer, K.L. Integrin signaling. Semin. Cancer Biol. 1996, 7, 111–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H. Integrin signalling and function in immune cells. Immunology 2012, 135, 268–275. [Google Scholar] [CrossRef]
- He, P.; Wang, B.H.; Cao, R.R.; Zhu, D.C.; Ge, B.; Zhou, X.; Wu, L.F.; Lei, S.F.; Deng, F.Y. ITGA2 protein is associated with rheumatoid arthritis in Chinese and affects cellular function of T cells. Clin. Chim. Acta 2021, 523, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Adorno-Cruz, V.; Liu, H. Regulation and functions of integrin alpha2 in cell adhesion and disease. Genes Dis. 2019, 6, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, A.; Ozaksit, G.; Sarikaya, E.; Kuru-Pekcan, M.; Kosem, A. Decreased ADAMTS-1, -9 and -20 levels in women with endometrial polyps: A possible link between extracellular matrix proteases and endometrial pathologies. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2019, 39, 845–850. [Google Scholar] [CrossRef]
- Nair, V.; Nigam, J.S.; Bharti, J.N.; Dey, B.; Singh, A. Giant Endometrial Polyp in a Postmenopausal Woman. Cureus 2021, 13, e12789. [Google Scholar] [CrossRef]
- Cea Garcia, J.; Jimenez Caraballo, A.; Rios Vallejo, M.D.M.; Zapardiel, I. Retrospective Cohort Study on the Symptomatic Recurrence Pattern after Hysteroscopic Polypectomy. Gynecol. Minim. Invasive Ther. 2020, 9, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bergmann, A.; Stubbs, L. Exon sharing of a novel human zinc-finger gene, ZIM2, and paternally expressed gene 3 (PEG3). Genomics 2000, 64, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Thiaville, M.M.; Kim, H.; Frey, W.D.; Kim, J. Identification of an evolutionarily conserved cis-regulatory element controlling the Peg3 imprinted domain. PLoS ONE 2013, 8, e75417. [Google Scholar] [CrossRef]
- Moon, S.; Hwang, S.; Kim, B.; Lee, S.; Kim, H.; Lee, G.; Hong, K.; Song, H.; Choi, Y. Hippo Signaling in the Endometrium. Int. J. Mol. Sci. 2022, 23, 3852. [Google Scholar] [CrossRef]
- Moon, S.; Lee, O.H.; Kim, B.; Park, J.; Hwang, S.; Lee, S.; Lee, G.; Kim, H.; Song, H.; Hong, K.; et al. Estrogen Regulates the Expression and Localization of YAP in the Uterus of Mice. Int. J. Mol. Sci. 2022, 23, 9772. [Google Scholar] [CrossRef]
- Moon, S.; Lee, O.H.; Lee, S.; Lee, J.; Park, H.; Park, M.; Chang, E.M.; Park, K.H.; Choi, Y. STK3/4 Expression Is Regulated in Uterine Endometrial Cells during the Estrous Cycle. Cells 2019, 8, 1643. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Tan, H.; Li, Y.; Nguyen, T.M.; Dhungana, Y.; Guy, C.; Vogel, P.; Neale, G.; Rankin, S.; et al. Hippo Kinases Mst1 and Mst2 Sense and Amplify IL-2R-STAT5 Signaling in Regulatory T Cells to Establish Stable Regulatory Activity. Immunity 2018, 49, 899–914.e6. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Kondo, N.; Kinashi, T. MST1/2 Balance Immune Activation and Tolerance by Orchestrating Adhesion, Transcription, and Organelle Dynamics in Lymphocytes. Front. Immunol. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wen, J.; Wang, Y.; Karmaus, P.W.F.; Khatamian, A.; Tan, H.; Li, Y.; Guy, C.; Nguyen, T.M.; Dhungana, Y.; et al. Hippo/Mst signalling couples metabolic state and immune function of CD8α+ dendritic cells. Nature 2018, 558, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, F.; Zhang, Z.G.; Yang, X.M.; Zhang, R. STK3 Suppresses Ovarian Cancer Progression by Activating NF-kappaB Signaling to Recruit CD8+ T-Cells. J. Immunol. Res. 2020, 2020, 7263602. [Google Scholar] [CrossRef] [PubMed]
- Holloway, A.J.; Yu, J.; Arulanandam, B.P.; Hoskinson, S.M.; Eaves-Pyles, T. Cystatins 9 and C as a Novel Immunotherapy Treatment That Protects against Multidrug-Resistant New Delhi Metallo-Beta-Lactamase-1-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Graham, N.; Eisenhauer, P.; Diehl, S.A.; Pierce, K.K.; Whitehead, S.S.; Durbin, A.P.; Kirkpatrick, B.D.; Sette, A.; Weiskopf, D.; Boyson, J.E.; et al. Rapid Induction and Maintenance of Virus-Specific CD8(+) TEMRA and CD4(+) TEM Cells Following Protective Vaccination Against Dengue Virus Challenge in Humans. Front. Immunol. 2020, 11, 479. [Google Scholar] [CrossRef]


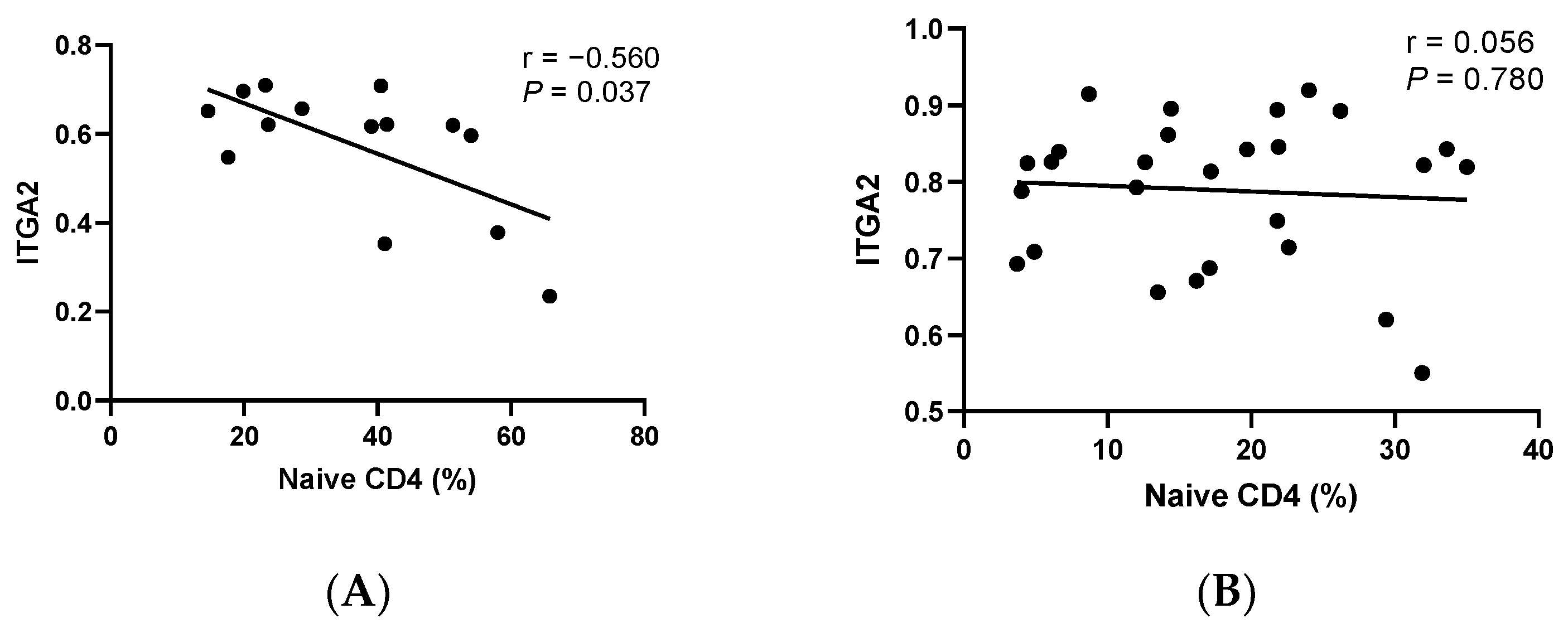

| Variables | Controls, n (%) | Cases, n (%) | pa |
|---|---|---|---|
| All subjects | 27(100.0) | 14(100.0) | |
| Age, years | 0.269 | ||
| <30 | 20(74.1) | 7(50.0) | |
| ≥30 | 7(25.9) | 7(50.0) | |
| Menstrual cycle, days | 0.714 | ||
| 24–35 | 27(100.0) | 12(85.7) | |
| >35 | 0(0.0) | 2(14.3) | |
| Menstrual duration, days | 0.042 | ||
| ≤7 | 27(100.0) | 14(100.0) | |
| >7 | 0(0.0) | 0(0.0) | |
| Without dysmenorrhea | 26(96.3) | 12(85.7) | 0.548 |
| AUB | - | 2(14.3) | |
| Gravidities | 0.674 | ||
| 0 | 15(55.6) | 8(57.1) | |
| 1 | 6(22.2) | 5(35.8) | |
| ≥2 | 6(22.2) | 1(7.1) | |
| Abortion | 0.471 | ||
| 0 | 18(66.7) | 11(78.6) | |
| 1 | 6(22.2) | 3(21.4) | |
| ≥2 | 3(11.1) | 0(11.0) | |
| Deliveries | 0.968 | ||
| 0 | 19(70.4) | 10(71.4) | |
| 1 | 8(29.6) | 4(28.6) | |
| Red blood cells (×1012/L) | 0.714 | ||
| <4.57 | 13(48.1) | 9(64.3) | |
| ≥4.57 | 14(51.9) | 5(35.7) | |
| Hemoglobin (g/L) | 0.115 | ||
| <136 | 13(48.1) | 9(64.3) | |
| ≥136 | 14(51.9) | 5(35.7) | |
| White blood cells (×109/L) | 0.796 | ||
| <6.15 | 15(55.6) | 7(50.0) | |
| ≥6.15 | 12(44.4) | 7(50.0) | |
| FSH (IU/L) | |||
| <8.01 | - | 10(71.4) | |
| ≥8.01 | - | 4(28.6) | |
| LH (IU/L) | |||
| <11.11 | - | 11(78.6) | |
| ≥11.11 | - | 3(21.4) | |
| Testosterone (nmol/L) | |||
| <1.54 | - | 10(71.4) | |
| ≥1.54 | - | 10(71.4) | |
| E2 (pmol/L) | |||
| <582 | - | 11(78.6) | |
| ≥582 | - | 3(21.4) | |
| Progesterone (nmol/L) | |||
| <8.50 | - | 13(92.9) | |
| ≥8.50 | - | 1(7.1) | |
| Prolactin (μg/L) | |||
| <11.88 | - | 9(64.3) | |
| ≥11.88 | - | 5(35.7) | |
| Endometrial thickness, cm | 0.9 ± 0.3 | ||
| <0.9 | - | 6(42.9) | |
| ≥0.9 | - | 8(57.1) | |
| Single polyp | - | 10(71.4) | |
| Multiple polyps | - | 4(28.6) | |
| Diameter of polyp, cm | - | 1.1 ± 0.6 | |
| >1 | - | 6(42.9) | |
| ≤1 | - | 8(57.1) |
| a. Correlation analysis of Hormone level with T-cell subsets and DNA methylation level | ||||
|---|---|---|---|---|
| r a | p a | |||
| E2 | ||||
| Naïve CD4 | −0.589 | 0.027 | ||
| Testosterone | ||||
| ZIM2 | −0.656 | 0.011 | ||
| b. Correlation analysis between methylation level and clinical indexes | ||||
| r a | p a | r b | p b | |
| ITGA2 | ||||
| Diameter of EP | 0.562 | 0.036 | 0.038 | 0.903 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-H.; Lu, M.-Y.; Niu, J.-L.; Zhu, D.-Y.; Liu, B. cfDNA Methylation Profiles and T-Cell Differentiation in Women with Endometrial Polyps. Cells 2022, 11, 3989. https://doi.org/10.3390/cells11243989
Li X-H, Lu M-Y, Niu J-L, Zhu D-Y, Liu B. cfDNA Methylation Profiles and T-Cell Differentiation in Women with Endometrial Polyps. Cells. 2022; 11(24):3989. https://doi.org/10.3390/cells11243989
Chicago/Turabian StyleLi, Xiao-Hong, Mei-Yin Lu, Jia-Li Niu, Dong-Yan Zhu, and Bin Liu. 2022. "cfDNA Methylation Profiles and T-Cell Differentiation in Women with Endometrial Polyps" Cells 11, no. 24: 3989. https://doi.org/10.3390/cells11243989
APA StyleLi, X.-H., Lu, M.-Y., Niu, J.-L., Zhu, D.-Y., & Liu, B. (2022). cfDNA Methylation Profiles and T-Cell Differentiation in Women with Endometrial Polyps. Cells, 11(24), 3989. https://doi.org/10.3390/cells11243989