Human In Vitro Models of Epilepsy Using Embryonic and Induced Pluripotent Stem Cells
Abstract
1. Introduction
2. Methods
2.1. Search Strategies
2.2. Eligibility Criteria
2.3. Publication Selection
2.4. Information Extraction
2.5. Data Analysis and Presentation
3. Results
3.1. Study Selection Process
3.2. Gene Editing Techniques for Disease Modelling and Isogenic Control
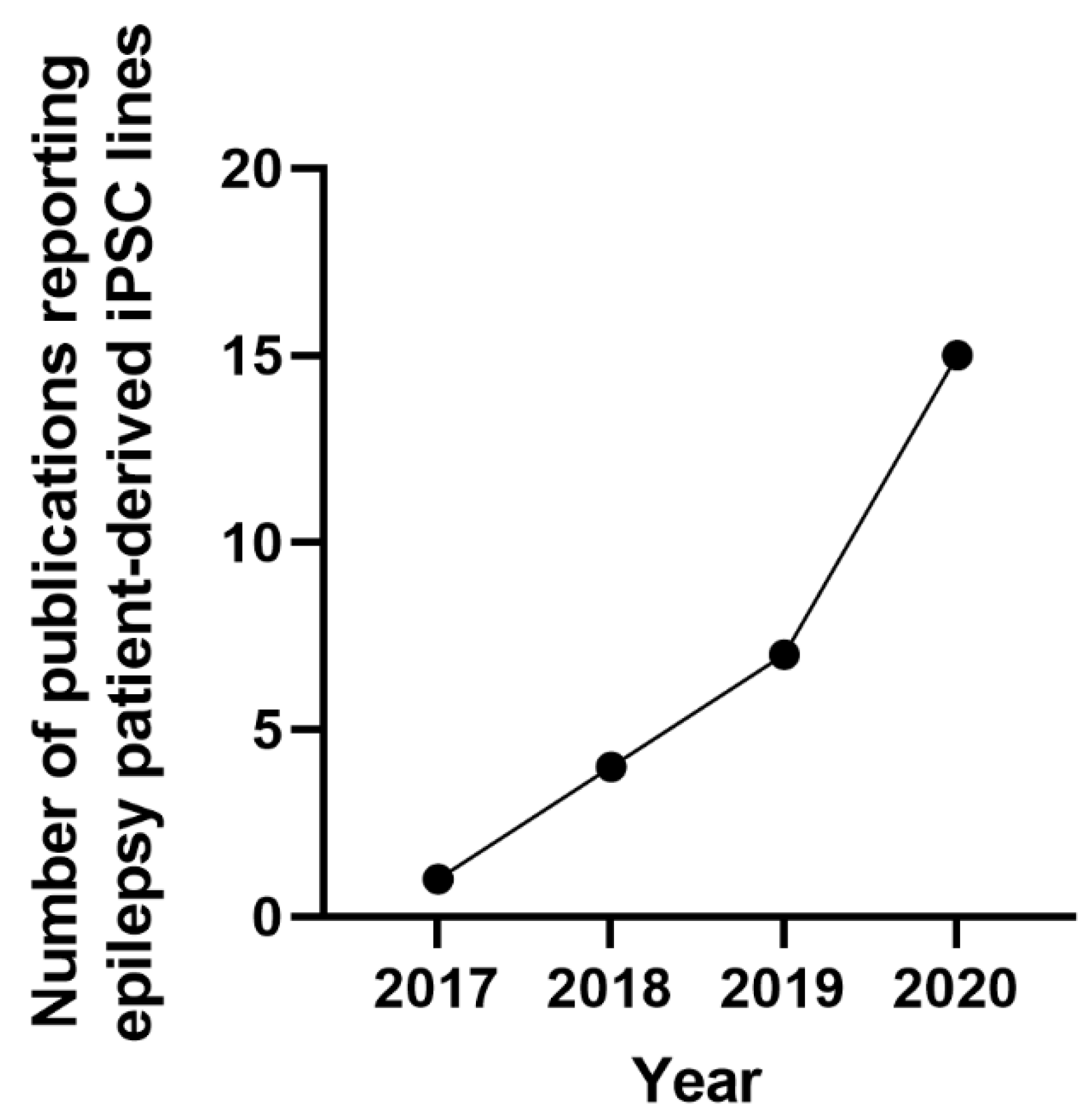
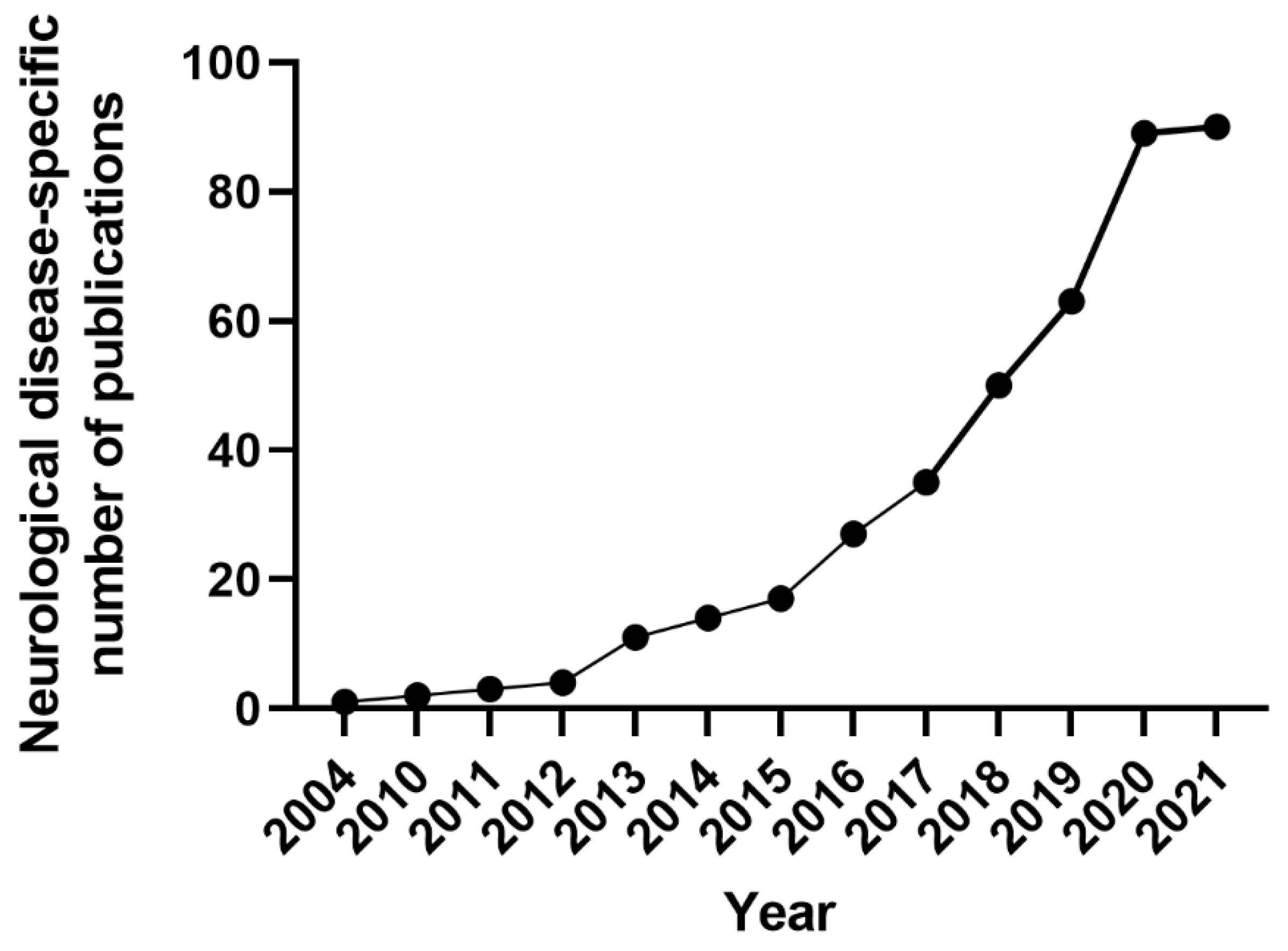
3.3. Epilepsy Patient-Specific iPSCs Derived Disease Models
3.4. Outcome Measures
4. Discussion
4.1. Limitations of Stem Cell-Derived Epilepsy Models
4.2. Limitations of Our Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Perucca, E. Identifying mutations in epilepsy genes: Impact on treatment selection. Epilepsy Res. 2019, 152, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Charlier, C.; Stauffer, D.; DuPont, B.R.; Leach, R.J.; Melis, R.; Ronen, G.M.; Bjerre, I.; Quattlebaum, T.; Murphy, J.V. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 1998, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Singh, N.A.; Ryan, S.G.; Lewis, T.B.; Reus, B.E.; Leach, R.J.; Leppert, M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 1998, 18, 53. [Google Scholar] [CrossRef]
- Claes, L.R.; Deprez, L.; Suls, A.; Baets, J.; Smets, K.; Van Dyck, T.; Deconinck, T.; Jordanova, A.; De Jonghe, P. The SCN1A variant database: A novel research and diagnostic tool. Hum. Mutat. 2009, 30, E904–E920. [Google Scholar] [CrossRef]
- Steinlein, O.K.; Mulley, J.C.; Propping, P.; Wallace, R.H.; Phillips, H.A.; Sutherland, G.R.; Scheffer, I.E.; Berkovic, S.F. A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 1995, 11, 201. [Google Scholar] [CrossRef]
- Baybis, M.; Yu, J.; Lee, A.; Golden, J.A.; Weiner, H.; McKhann, G.; Aronica, E.; Crino, P.B. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2004, 56, 478–487. [Google Scholar] [CrossRef]
- Baulac, S.; Ishida, S.; Marsan, E.; Miquel, C.; Biraben, A.; Nguyen, D.K.; Nordli, D.; Cossette, P.; Nguyen, S.; Lambrecq, V. Familial focal epilepsy with focal cortical dysplasia due to DEPDC 5 mutations. Ann. Neurol. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- D’Gama, A.M.; Geng, Y.; Couto, J.A.; Martin, B.; Boyle, E.A.; LaCoursiere, C.M.; Hossain, A.; Hatem, N.E.; Barry, B.J.; Kwiatkowski, D.J. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann. Neurol. 2015, 77, 720–725. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, W.; Kang, H.-C.; Kim, S.H.; Park, A.H.; Park, E.K.; Cho, Y.-W.; Kim, S.; Kim, H.M.; Kim, J.A. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat. Med. 2015, 21, 395. [Google Scholar] [CrossRef]
- Marsan, E.; Baulac, S. Mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy. Neuropathol. Appl. Neurobiol. 2018, 44, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lim, J.S.; Ramakrishina, S.; Kim, S.H.; Kim, W.K.; Lee, J.; Kang, H.-C.; Reiter, J.F.; Kim, D.S.; Kim, H.H. Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination. Neuron 2018, 99, 83–97.e87. [Google Scholar] [CrossRef] [PubMed]
- Scerri, T.; Riseley, J.R.; Gillies, G.; Pope, K.; Burgess, R.; Mandelstam, S.A.; Dibbens, L.; Chow, C.W.; Maixner, W.; Harvey, A.S. Familial cortical dysplasia type IIA caused by a germline mutation in DEPDC 5. Ann. Clin. Transl. Neurol. 2015, 2, 575–580. [Google Scholar] [CrossRef]
- Sim, J.C.; Scerri, T.; Fanjul-Fernández, M.; Riseley, J.R.; Gillies, G.; Pope, K.; Van Roozendaal, H.; Heng, J.I.; Mandelstam, S.A.; McGillivray, G. Familial cortical dysplasia caused by mutation in the mammalian target of rapamycin regulator NPRL3. Ann. Neurol. 2016, 79, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Van Kranenburg, M.; Hoogeveen-Westerveld, M.; Nellist, M. Preliminary Functional Assessment and Classification of DEPDC 5 Variants Associated with Focal Epilepsy. Hum. Mutat. 2015, 36, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-resistant epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, S.; Blume, W.T.; Girvin, J.P.; Eliasziw, M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 2001, 345, 311–318. [Google Scholar] [CrossRef]
- Dragunow, M. The adult human brain in preclinical drug development. Nat. Rev. Drug Discov. 2008, 7, 659. [Google Scholar] [CrossRef]
- Grainger, A.I.; King, M.C.; Nagel, D.A.; Parri, H.R.; Coleman, M.D.; Hill, E.J. In vitro models for seizure-liability testing using induced pluripotent stem cells. Front. Neurosci. 2018, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Kandratavicius, L.; Balista, P.A.; Lopes-Aguiar, C.; Ruggiero, R.N.; Umeoka, E.H.; Garcia-Cairasco, N.; Bueno, L.S., Jr.; Leite, J.P. Animal models of epilepsy: Use and limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Easter, A.; Bell, M.E.; Damewood, J.R., Jr.; Redfern, W.S.; Valentin, J.-P.; Winter, M.J.; Fonck, C.; Bialecki, R.A. Approaches to seizure risk assessment in preclinical drug discovery. Drug Discov. Today 2009, 14, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Dolmetsch, R.; Geschwind, D.H. The human brain in a dish: The promise of iPSC-derived neurons. Cell 2011, 145, 831–834. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.-G.; Kim, K.-S.; Chung, S. Differentiation of human pluripotent stem cells into Medial Ganglionic Eminence vs. Caudal Ganglionic Eminence cells. Methods 2016, 101, 103–112. [Google Scholar] [CrossRef]
- Vazin, T.; Ashton, R.S.; Conway, A.; Rode, N.A.; Lee, S.M.; Bravo, V.; Healy, K.E.; Kane, R.S.; Schaffer, D.V. The effect of multivalent Sonic hedgehog on differentiation of human embryonic stem cells into dopaminergic and GABAergic neurons. Biomaterials 2014, 35, 941–948. [Google Scholar] [CrossRef]
- Hartley, B.J.; Watmuff, B.; Hunt, C.P.J.; Haynes, J.M.; Pouton, C.W.; Kaur, N.; Vemuri, M.C. In vitro Differentiation of Pluripotent Stem Cells towards either Forebrain GABAergic or Midbrain Dopaminergic Neurons. In Neural Stem Cell Assays; Wiley: Hoboken, NJ, USA, 2015; pp. 91–99. [Google Scholar]
- Germain, N.D.; Banda, E.C.; Becker, S.; Naegele, J.R.; Grabel, L.B. Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells. Stem Cells Dev. 2013, 22, 1477–1489. [Google Scholar] [CrossRef]
- Nicholas, C.R.; Chen, J.; Tang, Y.; Southwell, D.G.; Chalmers, N.; Vogt, D.; Arnold, C.M.; Chen, Y.-J.J.; Stanley, E.G.; Elefanty, A.G.; et al. Functional Maturation of hPSC-Derived Forebrain Interneurons Requires an Extended Timeline and Mimics Human Neural Development. Cell Stem Cell 2013, 12, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Meganathan, K.; Lewis, E.M.A.; Gontarz, P.; Liu, S.; Stanley, E.G.; Elefanty, A.G.; Huettner, J.E.; Zhang, B.; Kroll, K.L. Regulatory networks specifying cortical interneurons from human embryonic stem cells reveal roles for CHD2 in interneuron development. Proc. Natl. Acad. Sci. USA 2017, 114, E11180–E11189. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Shi, X.; Allen, A.; Baez-Nieto, D.; Nikish, A.; Sanjana, N.E.; Pan, J.Q. Overexpression of NEUROG2 and NEUROG1 in human embryonic stem cells produces a network of excitatory and inhibitory neurons. Faseb J. 2019, 33, 5287–5299. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Aigner, S.; Vukcevic, M.; Sauter, E.; Behr, K.; Ebeling, M.; Dunkley, T.; Friedlein, A.; Zoffmann, S.; Meyer, C.A. mTORC1 inhibition corrects neurodevelopmental and synaptic alterations in a human stem cell model of tuberous sclerosis. Cell Rep. 2016, 15, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Aoyama, T.; Shibata, S.; Sone, T.; Miyoshi, H.; Watanabe, H.; Nakamura, M.; Morota, S.; Uchino, H.; Yoo, A.S.; et al. miRNA-based rapid differentiation of purified neurons from hPSCs advancestowards quick screening for neuronal disease phenotypes in vitro. Cells 2020, 9, 532. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, B.; Li, M.; Mo, F.; Mi, T.; Wu, Y.; Teng, Z.; Zhou, Q.; Li, W.; Hu, B. Precisely controlling endogenous protein dosage in hPSCs and derivatives to model FOXG1 syndrome. Nat. Commun. 2019, 10, 928. [Google Scholar] [CrossRef]
- Rocktäschel, P.; Sen, A.; Cader, M.Z. High glucose concentrations mask cellular phenotypes in a stem cell model of tuberous sclerosis complex. Epilepsy Behav. 2019, 101, 106581. [Google Scholar] [CrossRef]
- Kardoost, M.; Hajizadeh-Saffar, E.; Ghorbanian, M.T.; Ghezelayagh, Z.; Bagheri, K.P.; Behdani, M.; Habibi-Anbouhi, M. Genotoxicity assessment of antiepileptic drugs (AEDs) in human embryonic stem cells. Epilepsy Res. 2019, 158, 106232. [Google Scholar] [CrossRef]
- Da Costa, R.F.M.; Kormann, M.L.; Galina, A.; Rehen, S.K. Valproate Disturbs Morphology and Mitochondrial Membrane Potential in Human Neural Cells. Appl. Vitr. Toxicol. 2015, 1, 254–261. [Google Scholar] [CrossRef]
- Poppe, D.; Doerr, J.; Schneider, M.; Wilkens, R.; Steinbeck, J.A.; Ladewig, J.; Tam, A.; Paschon, D.E.; Gregory, P.D.; Reik, A. Genome Editing in Neuroepithelial Stem Cells to Generate Human Neurons with High Adenosine-Releasing Capacity. Stem Cells Transl. Med. 2018, 7, 477–486. [Google Scholar] [CrossRef]
- Fedele, D.E.; Koch, P.; Scheurer, L.; Simpson, E.M.; Mohler, H.; Brustle, O.; Boison, D. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci. Lett. 2004, 370, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tanaka, Y.; Shirasu, N.; Yasunaga, S.; Higurashi, N.; Hirose, S. Establishment of human induced pluripotent stem cells derived from skin cells of a patient with Dravet syndrome. Stem Cell Res. 2020, 47, 101857. [Google Scholar] [CrossRef] [PubMed]
- Malerba, N.; Benzoni, P.; Squeo, G.M.; Milanesi, R.; Giannetti, F.; Sadleir, L.G.; Poke, G.; Augello, B.; Croce, A.I.; Barbuti, A.; et al. Generation of the induced human pluripotent stem cell lines CSSi009-A from a patient with a GNB5 pathogenic variant, and CSSi010-A from a CRISPR/Cas9 engineered GNB5 knock-out human cell line. Stem Cell Res. 2019, 40, 101547. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.; Uysal, B.; Rosa, F.; Löffler, H.; Mau-Holzmann, U.A.; Liebau, S.; Lerche, H. Generation of an induced pluripotent stem cell (iPSC) line from a patient with developmental and epileptic encephalopathy carrying a KCNA2 (p. Leu328Val) mutation. Stem Cell Res. 2018, 33, 6–9. [Google Scholar] [CrossRef]
- Sun, C.; Yang, M.; Qin, F.; Guo, R.; Liang, S.; Hu, H. Generation of an induced pluripotent stem cell line SYSUi-003-A from a child with epilepsy carrying GRIN2A mutation. Stem Cell Res. 2020, 43, 101706. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Peng, J.; Hao, Y.; Guan, Y. Generation of a human induced pluripotent stem cell line from an epilepsy patient carrying mutations in the PIK3R2 gene. Stem Cell Res. 2020, 44, 101711. [Google Scholar] [CrossRef]
- Zhao, H.; He, L.; Li, S.; Huang, H.; Tang, F.; Han, X.; Lin, Z.; Tian, C.; Huang, R.; Zhou, P.; et al. Generation of corrected-hiPSC (USTCi001-A-1) from epilepsy patient iPSCs using TALEN-mediated editing of the SCN1A gene. Stem Cell Res. 2020, 46, 101864. [Google Scholar] [CrossRef]
- Arbini, A.; Gilmore, J.; King, M.D.; Gorman, K.M.; Krawczyk, J.; McInerney, V.; O’Brien, T.; Shen, S.; Allen, N.M. Generation of three induced pluripotent stem cell (iPSC) lines from a patient with developmental epileptic encephalopathy due to the pathogenic KCNA2 variant c.869T>G; p.Leu290Arg (NUIGi052-A, NUIGi052-B, NUIGi052-C). Stem Cell Res. 2020, 46, 101853. [Google Scholar] [CrossRef]
- Gong, P.; Jiao, X.; Zhang, Y.; Yang, Z. Generation of a human iPSC line from an epileptic encephalopathy patient with electrical status epilepticus during sleep carrying KCNA2 (p.P405L) mutation. Stem Cell Res. 2020, 49, 102080. [Google Scholar] [CrossRef]
- Nengqing, L.; Dian, L.; Yingjun, X.; Yi, C.; Lina, H.; Diyu, C.; Yinghong, Y.; Bing, S.; Xiaofang, S. Generation of induced pluripotent stem cell GZHMCi001-A and GZHMCi001-B derived from peripheral blood mononuclear cells of epileptic patients with KCNC1 mutation. Stem Cell Res. 2020, 47, 101897. [Google Scholar] [CrossRef]
- Schwarz, N.; Uysal, B.; Rosa, F.; Loffler, H.; Mau-Holzmann, U.A.; Liebau, S.; Lerche, H. Establishment of a human induced pluripotent stem cell (iPSC)line (HIHDNEi002-A) from a patient with developmental and epileptic encephalopathy carrying a KCNA2 (p.Arg297Gln)mutation. Stem Cell Res. 2019, 37, 101445. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.W.; Kondo, T.; Murakami, N.; Imamura, K.; Enami, T.; Tsukita, K.; Shibukawa, R.; Funayama, M.; Matsumoto, R.; Ikeda, A. Induced pluripotent stem cells derived from an autosomal dominant lateral temporal epilepsy (ADLTE) patient carrying S473L mutation in leucine-rich glioma inactivated 1 (LGI1). Stem Cell Res. 2017, 24, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Higurashi, N.; Shirasu, N.; Yasunaga, S.i.; Moreira, K.M.; Okano, H.; Hirose, S. Establishment of a human induced stem cell line (FUi002-A) from Dravet syndrome patient carrying heterozygous R1525X mutation in SCN1A gene. Stem Cell Res. 2018, 31, 11–15. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sone, T.; Higurashi, N.; Sakuma, T.; Suzuki, S.; Ishikawa, M.; Yamamoto, T.; Mitsui, J.; Tsuji, H.; Okano, H. Generation of D1-1 TALEN isogenic control cell line from Dravet syndrome patient iPSCs using TALEN-mediated editing of the SCN1A gene. Stem Cell Res. 2018, 28, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, S.; He, L.; Han, X.; Huang, H.; Tang, F.; Lin, Z.; Deng, S.; Tian, C.; Huang, R. Generation of iPSC line (USTCi001-A) from human skin fibroblasts of a patient with epilepsy. Stem Cell Res. 2020, 45, 101785. [Google Scholar] [CrossRef]
- Alsaqati, M.; Heine, V.M.; Harwood, A.J. Pharmacological intervention to restore connectivity deficits of neuronal networks derived from ASD patient iPSC with a TSC2 mutation. Mol. Autism 2020, 11, 80. [Google Scholar] [CrossRef]
- Ishii, M.N.; Yamamoto, K.; Shoji, M.; Asami, A.; Kawamata, Y. Human induced pluripotent stem cell (hiPSC)-derived neurons respond to convulsant drugs when co-cultured with hiPSC-derived astrocytes. Toxicology 2017, 389, 130–138. [Google Scholar] [CrossRef]
- Odawara, A.; Katoh, H.; Matsuda, N.; Suzuki, I. Physiological maturation and drug responses of human induced pluripotent stem cell-derived cortical neuronal networks in long-term culture. Sci. Rep. 2016, 6, 26181. [Google Scholar] [CrossRef]
- Jiao, J.; Yang, Y.; Shi, Y.; Chen, J.; Gao, R.; Fan, Y.; Yao, H.; Liao, W.; Sun, X.F.; Gao, S. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum. Mol. Genet. 2013, 22, 4241–4252. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Zhang, L.; Xu, H.; Guo, X.; Deng, S.; Liu, L.; Yu, D.; Chen, Y.; Li, Z. Generation of the SCN1A epilepsy mutation in hiPS cells using the TALEN technique. Sci. Rep. 2014, 4, 5404. [Google Scholar] [CrossRef]
- Higurashi, N.; Uchida, T.; Lossin, C.; Misumi, Y.; Okada, Y.; Akamatsu, W.; Imaizumi, Y.; Zhang, B.; Nabeshima, K.; Mori, M.X. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol. Brain 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Quan, Z.; Kim, Y.-B.; Cheong, E.; Kim, H.D.; Cho, M.; Jang, J.; Yoo, Y.R.; Lee, J.S.; Kim, J.H. Differential effects on sodium current impairments by distinct SCN1A mutations in GABAergic neurons derived from Dravet syndrome patients. Brain Dev. 2018, 40, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lopez-Santiago, L.F.; Yuan, Y.; Jones, J.M.; Zhang, H.; O’Malley, H.A.; Patino, G.A.; O’Brien, J.E.; Rusconi, R.; Gupta, A. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann. Neurol. 2013, 74, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Chiyonobu, T.; Yoshida, M.; Yamashita, S.; Zuiki, M.; Kidowaki, S.; Isoda, K.; Yamakawa, K.; Morimoto, M.; Nakahata, T. Establishment of isogenic iPSCs from an individual with SCN1A mutation mosaicism as a model for investigating neurocognitive impairment in Dravet syndrome. J. Hum. Genet. 2016, 61, 565. [Google Scholar] [CrossRef]
- Schuster, J.; Fatima, A.; Sobol, M.; Norradin, F.H.; Laan, L.; Dahl, N. Generation of three human induced pluripotent stem cell (iPSC) lines from three patients with Dravet syndrome carrying distinct SCN1A gene mutations. Stem Cell Res. 2019, 39, 101523. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dolmetsch, R.E. Investigating the therapeutic mechanism of cannabidiol in a human induced pluripotent stem cell (iPSC)-based model of Dravet syndrome. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ng, N.N.; Safrina, O.S.; Ramos, C.M.; Ess, K.C.; Schwartz, P.H.; Smith, M.A.; O’Dowd, D.K. Comparisons of dual isogenic human iPSC pairs identify functional alterations directly caused by an epilepsy associated SCN1A mutation. Neurobiol. Dis. 2020, 134, 104627. [Google Scholar] [CrossRef]
- Fruscione, F.; Valente, P.; Sterlini, B.; Romei, A.; Baldassari, S.; Fadda, M.; Prestigio, C.; Giansante, G.; Sartorelli, J.; Rossi, P.; et al. PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. Brain 2018, 141, 1000–1016. [Google Scholar] [CrossRef]
- Martin, P.; Wagh, V.; Reis, S.A.; Erdin, S.; Beauchamp, R.L.; Shaikh, G.; Talkowski, M.; Thiele, E.; Sheridan, S.D.; Haggarty, S.J. TSC patient-derived isogenic neural progenitor cells reveal altered early neurodevelopmental phenotypes and rapamycin-induced MNK-eIF4E signaling. Mol. Autism 2020, 11, 2. [Google Scholar] [CrossRef]
- Winden, K.D.; Sundberg, M.; Yang, C.; Wafa, S.M.; Dwyer, S.; Chen, P.-F.; Buttermore, E.D.; Sahin, M. Biallelic mutations in TSC2 lead to abnormalities associated with cortical tubers in human iPSC-derived neurons. J. Neurosci. 2019, 39, 9294–9305. [Google Scholar] [CrossRef]
- Zucco, A.J.; Dal Pozzo, V.; Afinogenova, A.; Hart, R.P.; Devinsky, O.; D’Arcangelo, G. Neural progenitors derived from tuberous sclerosis complex patients exhibit attenuated PI3K/AKT signaling and delayed neuronal differentiation. Mol. Cell. Neurosci. 2018, 92, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 2017, 20, 435–449.e4. [Google Scholar] [CrossRef] [PubMed]
- Burnight, E.R.; Bohrer, L.R.; Giacalone, J.C.; Klaahsen, D.L.; Daggett, H.T.; East, J.S.; Madumba, R.A.; Worthington, K.S.; Mullins, R.F.; Stone, E.M. CRISPR-Cas9-Mediated correction of the 1.02 kb common deletion in CLN3 in induced pluripotent stem cells from patients with batten disease. CRISPR J. 2018, 1, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.H.; Zhong, F.L.; Niwa, S.; Bonnard, C.; Utami, K.H.; Zeng, R.; Lee, H.; Eskin, A.; Nelson, S.F.; Xie, W.H.; et al. A homozygous loss-of-function camk2a mutation causes growth delay, frequent seizures and severe intellectual disability. Elife 2018, 7, e32451. [Google Scholar] [CrossRef]
- Chou, S.-J.; Tseng, W.-L.; Chen, C.-T.; Lai, Y.-F.; Chien, C.-S.; Chang, Y.-L.; Lee, H.-C.; Wei, Y.-H.; Chiou, S.-H. Impaired ROS scavenging system in human induced pluripotent stem cells generated from patients with MERRF syndrome. Sci. Rep. 2016, 6, 23661. [Google Scholar] [CrossRef]
- Fink, J.J.; Robinson, T.M.; Germain, N.D.; Sirois, C.L.; Bolduc, K.A.; Ward, A.J.; Rigo, F.; Chamberlain, S.J.; Levine, E.S. Disrupted neuronal maturation in Angelman syndrome-derived induced pluripotent stem cells. Nat. Commun. 2017, 8, 15038. [Google Scholar] [CrossRef]
- Germain, N.D.; Chen, P.-F.; Plocik, A.M.; Glatt-Deeley, H.; Brown, J.; Fink, J.J.; Bolduc, K.A.; Robinson, T.M.; Levine, E.S.; Reiter, L.T. Gene expression analysis of human induced pluripotent stem cell-derived neurons carrying copy number variants of chromosome 15q11-q13. 1. Mol. Autism 2014, 5, 44. [Google Scholar] [CrossRef]
- Gillentine, M.A.; Yin, J.; Bajic, A.; Zhang, P.; Cummock, S.; Kim, J.J.; Schaaf, C.P. Functional consequences of CHRNA7 copy-number alterations in induced pluripotent stem cells and neural progenitor cells. Am. J. Hum. Genet. 2017, 101, 874–887. [Google Scholar] [CrossRef]
- Guemez-Gamboa, A.; Çağlayan, A.O.; Stanley, V.; Gregor, A.; Zaki, M.S.; Saleem, S.N.; Musaev, D.; McEvoy-Venneri, J.; Belandres, D.; Akizu, N. Loss of Protocadherin-12 L eads to D iencephalic-M esencephalic J unction D ysplasia S yndrome. Ann. Neurol. 2018, 84, 638–647. [Google Scholar] [CrossRef]
- Homan, C.C.; Pederson, S.; To, T.H.; Tan, C.; Piltz, S.; Corbett, M.A.; Wolvetang, E.; Thomas, P.Q.; Jolly, L.A.; Gecz, J. PCDH19 regulation of neural progenitor cell differentiation suggests asynchrony of neurogenesis as a mechanism contributing to PCDH19 Girls Clustering Epilepsy. Neurobiol. Dis. 2018, 116, 106–119. [Google Scholar] [CrossRef]
- Majolo, F.; Marinowic, D.; Machado, D.; Da Costa, J.C. Notch signaling in human iPS-derived neuronal progenitor lines from Focal Cortical Dysplasia patients. Int. J. Dev. Neurosci. 2018, 69, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Majolo, F.; Marinowic, D.R.; Palmini, A.L.F.; DaCosta, J.C.; Machado, D.C. Migration and synaptic aspects of neurons derived from human induced pluripotent stem cells from patients with focal cortical dysplasia II. Neuroscience 2019, 408, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Marinowic, D.R.; Majolo, F.; Sebben, A.D.; Da Silva, V.D.; Lopes, T.G.; Paglioli, E.; Palmini, A.; Machado, D.C.; Da Costa, J.C. Induced pluripotent stem cells from patients with focal cortical dysplasia and refractory epilepsy. Mol. Med. Rep. 2017, 15, 2049–2056. [Google Scholar] [CrossRef]
- Marinowic, D.R.; Majolo, F.; Zanirati, G.G.; Plentz, I.; Neto, E.P.; Palmini, A.L.F.; Machado, D.C.; Da Costa, J.C. Analysis of genes involved in cell proliferation, adhesion, and control of apoptosis during embryonic neurogenesis in Induced Pluripotent Stem Cells (iPSCs) from patients with Focal Cortical Dysplasia. Brain Res. Bull. 2020, 155, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, A.; Mzezewa, R.; Sukki, L.; Ryynanen, T.; Kreutzer, J.; Hyvarinen, T.; Vinogradov, A.; Aarnos, L.; Lekkala, J.; Kallio, P.; et al. A modular brain-on-a-chip for modelling epileptic seizures with functionally connected human neuronal networks. Biosens. Bioelectron. 2020, 168, 112553. [Google Scholar] [CrossRef] [PubMed]
- Shahsavani, M.; Pronk, R.J.; Falk, R.; Lam, M.; Moslem, M.; Linker, S.B.; Salma, J.; Day, K.; Schuster, J.; Anderlid, B.M.; et al. An in vitro model of lissencephaly: Expanding the role of DCX during neurogenesis. Mol. Psychiatry 2018, 23, 1674–1684. [Google Scholar] [CrossRef]
- Yamashita, S.; Chiyonobu, T.; Yoshida, M.; Maeda, H.; Zuiki, M.; Kidowaki, S.; Isoda, K.; Morimoto, M.; Kato, M.; Saitsu, H.; et al. Mislocalization of syntaxin-1 and impaired neurite growth observed in a human iPSC model for STXBP1-related epileptic encephalopathy. Epilepsia 2016, 57, e81–e86. [Google Scholar] [CrossRef]
- Khan, T.; Pasca, S. Neuronal Defects in a Human cellular Model of 22q11.2 Deletion Syndrome. Gene Expr. Omn. 2020, 26, 1888–1898. [Google Scholar] [CrossRef]
- Dang, L.T.; Glanowska, K.M.; Iffland Ii, P.H.; Barnes, A.E.; Baybis, M.; Liu, Y.; Patino, G.; Vaid, S.; Streicher, A.M.; Parker, W.E.; et al. Multimodal Analysis of STRADA Function in Brain Development. Front. Cell Neurosci. 2020, 14, 122. [Google Scholar] [CrossRef]
- Di Matteo, F.; Pipicelli, F.; Kyrousi, C.; Tovecci, I.; Penna, E.; Crispino, M.; Chambery, A.; Russo, R.; Ayo-Martin, A.C.; Giordano, M.; et al. Cystatin B is essential for proliferation and interneuron migration in individuals with EPM1 epilepsy. EMBO Mol. Med. 2020, 12, e11419. [Google Scholar] [CrossRef]
- Glaß, H.; Neumann, P.; Pal, A.; Reinhardt, P.; Storch, A.; Sterneckert, J.; Hermann, A. Combined Dendritic and Axonal Deterioration Are Responsible for Motoneuronopathy in Patient-Derived Neuronal Cell Models of Chorea-Acanthocytosis. Int. J. Mol. Sci. 2020, 21, 1797. [Google Scholar] [CrossRef] [PubMed]
- Gunnewiek, T.M.K.; Van Hugte, E.J.H.; Frega, M.; Guardia, G.S.; Foreman, K.; Panneman, D.; Mossink, B.; Linda, K.; Keller, J.M.; Schubert, D.; et al. m.3243A > G-Induced Mitochondrial Dysfunction Impairs Human Neuronal Development and Reduces Neuronal Network Activity and Synchronicity. Cell Rep. 2020, 31, 107538. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Patzke, C.; Südhof, T.C. The conditional KO approach: Cre/Lox technology in human neurons. Rare Dis. 2016, 4, 3560–3571. [Google Scholar] [CrossRef]
- Quraishi, I.H.; Stern, S.; Mangan, K.P.; Zhang, Y.; Ali, S.R.; Mercier, M.R.; Marchetto, M.C.; McLachlan, M.J.; Jones, E.M.; Gage, F.H.; et al. An epilepsy-associated KCNT1 mutation enhances excitability of human iPSC-derived neurons by increasing slack KNa currents. J. Neurosci. 2019, 39, 7438–7449. [Google Scholar] [CrossRef]
- Simkin, D.; Marshall, K.A.; Vanoye, C.G.; Desai, R.R.; Bustos, B.I.; Piyevsky, B.N.; Ortega, J.A.; Forrest, M.; Robertson, G.L.; Penzes, P. Dyshomeostatic modulation of Ca2+-activated K+ channels in a human neuronal model of KCNQ2 encephalopathy. Elife 2021, 10, e64434. [Google Scholar] [CrossRef]
- Tidball, A.M.; Lopez-Santiago, L.F.; Yuan, Y.; Glenn, T.W.; Margolis, J.L.; Clayton Walker, J.; Kilbane, E.G.; Miller, C.A.; Martina Bebin, E.; Scott Perry, M. Variant-specific changes in persistent or resurgent sodium current in SCN8A-related epilepsy patient-derived neurons. Brain 2020, 143, 3025–3040. [Google Scholar] [CrossRef]
- Van Diepen, L.; Buettner, F.F.R.; Hoffmann, D.; Thiesler, C.T.; von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V.; Jensen, L.R.; Steinemann, D.; Edvardson, S.; Elpeleg, O.; et al. A patient-specific induced pluripotent stem cell model for West syndrome caused by ST3GAL3 deficiency. Eur. J. Hum. Genet. 2018, 26, 1773–1783. [Google Scholar] [CrossRef]
- Bell, S.; Hettige, N.C.; Silveira, H.; Peng, H.; Wu, H.; Jefri, M.; Antonyan, L.; Zhang, Y.; Zhang, X.; Ernst, C. Differentiation of Human Induced Pluripotent Stem Cells (iPSCs) into an Effective Model of Forebrain Neural Progenitor Cells and Mature Neurons. Bio. Protoc. 2019, 9, e3188. [Google Scholar] [CrossRef]
- Chi, L.; Fan, B.; Zhang, K.; Du, Y.; Liu, Z.; Fang, Y.; Chen, Z.; Ren, X.; Xu, X.; Jiang, C.; et al. Targeted Differentiation of Regional Ventral Neuroprogenitors and Related Neuronal Subtypes from Human Pluripotent Stem Cells. Stem Cell Rep. 2016, 7, 941–954. [Google Scholar] [CrossRef]
- DeRosa, B.A.; Belle, K.C.; Thomas, B.J.; Cukier, H.N.; Pericak-Vance, M.A.; Vance, J.M.; Dykxhoorn, D.M. hVGAT-mCherry: A novel molecular tool for analysis of GABAergic neurons derived from human pluripotent stem cells. Mol. Cell. Neurosci. 2015, 68, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.A.S.; Zhou, Y.; Patterson, D.G.; Scharer, C.D.; Han, Y.; Boss, J.M.; Wen, Z.; Escayg, A. Transcriptomic and epigenomic dynamics associated with development of human iPSC-derived GABAergic interneurons. Hum. Mol. Genet. 2020, 29, 2579–2595. [Google Scholar] [CrossRef] [PubMed]
- Klofas, L.K.; Short, B.P.; Snow, J.P.; Sinnaeve, J.; Rushing, G.V.; Westlake, G.; Weinstein, W.; Ihrie, R.A.; Ess, K.C.; Carson, R.P. DEPDC5 haploinsufficiency drives increased mTORC1 signaling and abnormal morphology in human iPSC-derived cortical neurons. Neurobiol. Dis. 2020, 143, 104975. [Google Scholar] [CrossRef] [PubMed]
- Kreir, M.; De Bondt, A.; Van den Wyngaert, I.; Teuns, G.; Lu, H.R.; Gallacher, D.J. Role of Kv7.2/Kv7.3 and M(1) muscarinic receptors in the regulation of neuronal excitability in hiPSC-derived neurons. Eur. J. Pharm. 2019, 858, 172474. [Google Scholar] [CrossRef] [PubMed]
- Nebel, R.; Zhao, D.; Pedrosa, E.; Kirschen, J.; Lachman, H.; Zheng, D.; Abrahams, B. Reduced CYFIP1 in iPSC Derived Human Neural Progenitors Results in Donor Specific Dysregulation of Schizophrenia and Epilepsy Genes. Neuropsychopharmacology 2015, 40, S379. [Google Scholar]
- Ricciardi, S.; Ungaro, F.; Hambrock, M.; Rademacher, N.; Stefanelli, G.; Brambilla, D.; Sessa, A.; Magagnotti, C.; Bachi, A.; Giarda, E. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1–PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat. Cell Biol. 2012, 14, 911–923. [Google Scholar] [CrossRef]
- Tukker, A.M.; Van Kleef, R.; Wijnolts, F.M.J.; De Groot, A.; Westerink, R.H.S. Towards animal-free neurotoxicity screening: Applicability of hiPSC-derived neuronal models for in vitro seizure liability assessment. Altex 2020, 37, 121–135. [Google Scholar] [CrossRef]
- Amenduni, M.; De Filippis, R.; Cheung, A.Y.; Disciglio, V.; Epistolato, M.C.; Ariani, F.; Mari, F.; Mencarelli, M.A.; Hayek, Y.; Renieri, A.; et al. iPS cells to model CDKL5-related disorders. Eur. J. Hum. Genet. 2011, 19, 1246–1255. [Google Scholar] [CrossRef]
- Miskinyte, G.; Devaraju, K.; Grønning Hansen, M.; Monni, E.; Tornero, D.; Woods, N.B.; Bengzon, J.; Ahlenius, H.; Lindvall, O.; Kokaia, Z. Direct conversion of human fibroblasts to functional excitatory cortical neurons integrating into human neural networks. Stem Cell Res. Ther. 2017, 8, 207. [Google Scholar] [CrossRef]
- Sakauchi, M.; Oguni, H.; Kato, I.; Osawa, M.; Hirose, S.; Kaneko, S.; Takahashi, Y.; Takayama, R.; Fujiwara, T. Retrospective multiinstitutional study of the prevalence of early death in Dravet syndrome. Epilepsia 2011, 52, 1144–1149. [Google Scholar] [CrossRef]
- Fatima, A.; Schuster, J.; Akram, T.; Sobol, M.; Hoeber, J.; Dahl, N. Generation of a human Neurochondrin deficient iPSC line KICRi002-A-3 using CRISPR/Cas9. Stem Cell Res. 2020, 44, 101758. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, M.; Zhang, F. Applications of CRISPR–Cas systems in neuroscience. Nat. Rev. Neurosci. 2016, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, C.; Chen, W.; Ma, W.; Li, X.; Shi, Y.; Zhang, H.; Zhang, L.; Long, Y.; Xu, H. CRISPR/Cas9 facilitates investigation of neural circuit disease using human iPSCs: Mechanism of epilepsy caused by an SCN1A loss-of-function mutation. Transl. Psychiatry 2016, 6, e703. [Google Scholar] [CrossRef]
- Gamboa, A.G.; Rakotomamonjy, J.; Rylaarsdaam, L.; Thomas, D. Loss of PCDH12 causes cell migration and differentiation defects in human embryonic stem cell-derived neuroprogenitors. J. Neurochem. 2019, 150, 191. [Google Scholar] [CrossRef]
- Giacomoni, J.; Bruzelius, A.; Stamouli, C.A.; Ottosson, D.R. Direct conversion of human stem cell-derived glial progenitor cells into GABAergic interneurons. Cells 2020, 9, 2451. [Google Scholar] [CrossRef] [PubMed]
- Assad, J.A.; Berdondini, L.; Cancedda, L.; De Angelis, F.; Diaspro, A.; Dipalo, M.; Fellin, T.; Maccione, A.; Panzeri, S.; Sileo, L. Brain function: Novel technologies driving novel understanding. In Bioinspired Approaches for Human-Centric Technologies; Springer: Berlin/Heidelberg, Germany, 2014; pp. 299–334. [Google Scholar]
- Contreras, D. Electrophysiological classes of neocortical neurons. Neural Netw. 2004, 17, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science 1988, 242, 1654–1664. [Google Scholar] [CrossRef]
- Wood, C.; Williams, C.; Waldron, G.J. Patch clamping by numbers. Drug Discov. Today 2004, 9, 434–441. [Google Scholar] [CrossRef]
- Volpato, V.; Webber, C. Addressing variability in iPSC-derived models of human disease: Guidelines to promote reproducibility. Dis. Model. Mech. 2020, 13, dmm042317. [Google Scholar] [CrossRef]
- Chambers, S.M.; Qi, Y.; Mica, Y.; Lee, G.; Zhang, X.J.; Niu, L.; Bilsland, J.; Cao, L.; Stevens, E.; Whiting, P.; et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012, 30, 715–720. [Google Scholar] [CrossRef]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; Marro, S.; Patzke, C.; Acuna, C.; Covy, J.; et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013, 78, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013, 14, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Cader, Z.; Graf, M.; Burcin, M.; Mandenius, C.-F.; Ross, J.A. Cell-based assays using differentiated human induced pluripotent cells. In Cell-Based Assays Using iPSCs for Drug Development and Testing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–14. [Google Scholar]
- Leha, A.; Moens, N.; Meleckyte, R.; Culley, O.J.; Gervasio, M.K.; Kerz, M.; Reimer, A.; Cain, S.A.; Streeter, I.; Folarin, A. A high-content platform to characterise human induced pluripotent stem cell lines. Methods 2016, 96, 85–96. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, P.A.; Steeg, R.; Wachter, E.; Bruce, K.; King, J.; Hoeve, M.; Khadun, S.; McConnachie, G.; Holder, J.; Kurtz, A. Rapid establishment of the European Bank for Induced Pluripotent Stem Cells (EBiSC)-the hot start experience. Stem Cell Res. 2017, 20, 105–114. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; D’Antonio, M.; Benaglio, P.; Williams, R.; Hashem, S.I.; Schuldt, B.M.; DeBoever, C.; Arias, A.D.; Garcia, M.; Nelson, B.C. iPSCORE: A resource of 222 iPSC lines enabling functional characterization of genetic variation across a variety of cell types. Stem Cell Rep. 2017, 8, 1086–1100. [Google Scholar] [CrossRef]
- Tran, L.; Tam, D.N.H.; Elshafay, A.; Dang, T.; Hirayama, K.; Huy, N.T. Quality assessment tools used in systematic reviews of in vitro studies: A systematic review. BMC Med. Res. Methodol. 2021, 21, 101. [Google Scholar] [CrossRef]
- Engle, S.J.; Blaha, L.; Kleiman, R.J. Best practices for translational disease modeling using human iPSC-derived neurons. Neuron 2018, 100, 783–797. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javaid, M.S.; Tan, T.; Dvir, N.; Anderson, A.; J. O’Brien, T.; Kwan, P.; Antonic-Baker, A. Human In Vitro Models of Epilepsy Using Embryonic and Induced Pluripotent Stem Cells. Cells 2022, 11, 3957. https://doi.org/10.3390/cells11243957
Javaid MS, Tan T, Dvir N, Anderson A, J. O’Brien T, Kwan P, Antonic-Baker A. Human In Vitro Models of Epilepsy Using Embryonic and Induced Pluripotent Stem Cells. Cells. 2022; 11(24):3957. https://doi.org/10.3390/cells11243957
Chicago/Turabian StyleJavaid, Muhammad Shahid, Tracie Tan, Naomi Dvir, Alison Anderson, Terence J. O’Brien, Patrick Kwan, and Ana Antonic-Baker. 2022. "Human In Vitro Models of Epilepsy Using Embryonic and Induced Pluripotent Stem Cells" Cells 11, no. 24: 3957. https://doi.org/10.3390/cells11243957
APA StyleJavaid, M. S., Tan, T., Dvir, N., Anderson, A., J. O’Brien, T., Kwan, P., & Antonic-Baker, A. (2022). Human In Vitro Models of Epilepsy Using Embryonic and Induced Pluripotent Stem Cells. Cells, 11(24), 3957. https://doi.org/10.3390/cells11243957







