Stem Cell-Based Therapeutic Strategies for Premature Ovarian Insufficiency and Infertility: A Focus on Aging
Abstract
1. Introduction
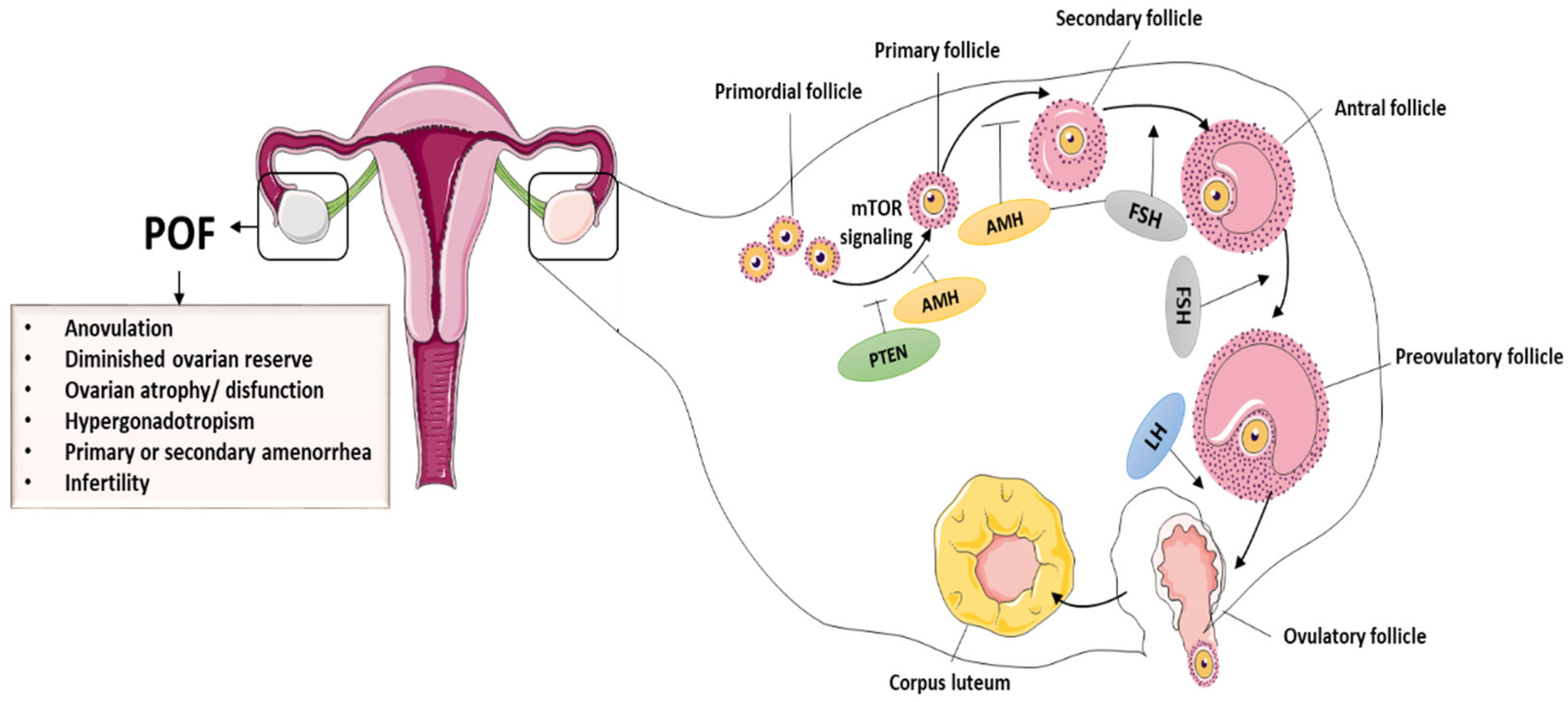
2. Premature Ovarian Insufficiency or Aging
2.1. Pathophysiology of POI
2.2. Causes of POI
2.3. Premature Ovarian Aging and Infertility
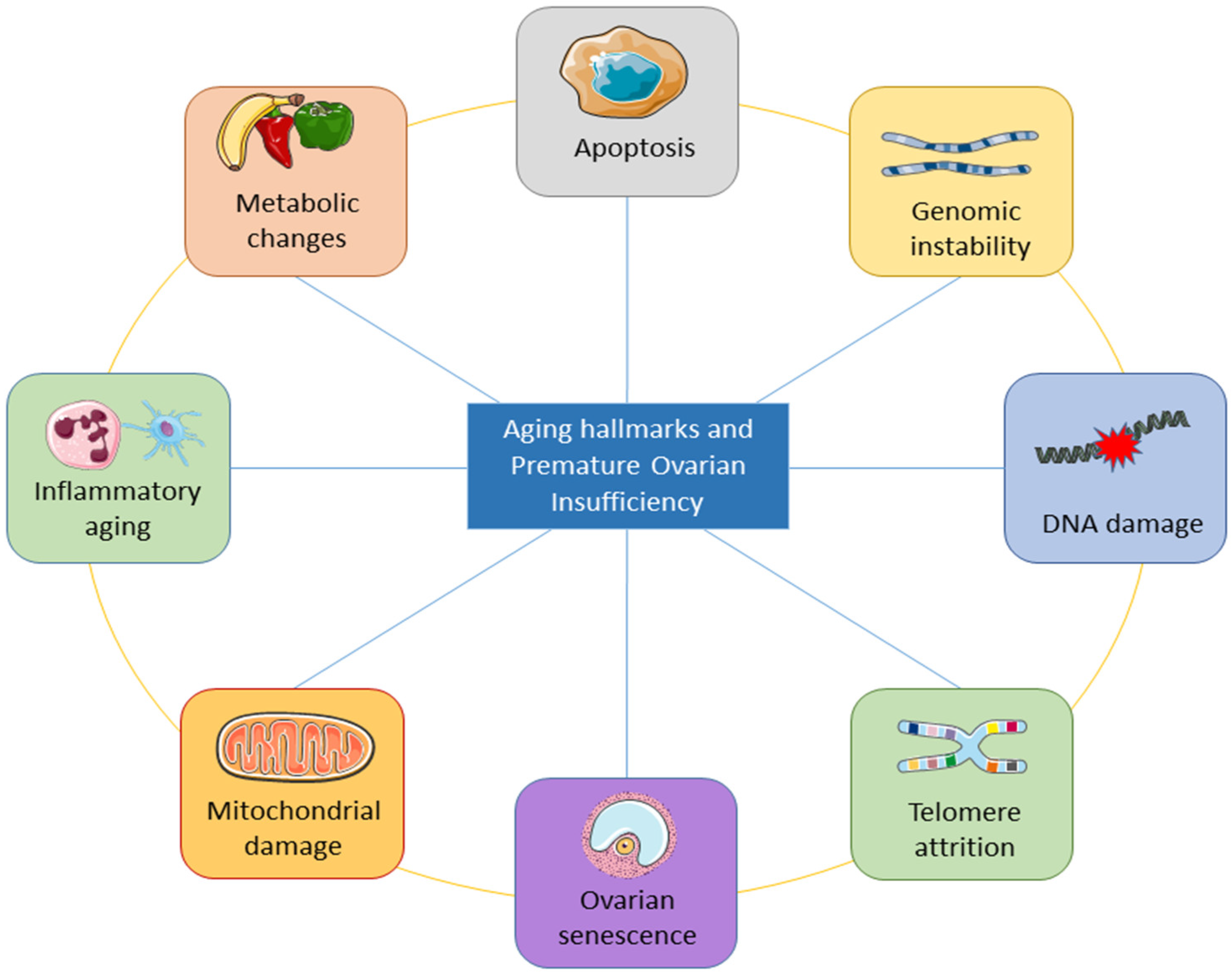
3. Aging Hallmarks of Premature Ovarian Insufficiency (POI)
3.1. Inflammatory Aging and POI
3.1.1. Oxidative Stress and Inflammatory Aging
3.1.2. Oxidative Stress and Inflammatory Aging
3.1.3. Inflammatory Aging Causes POI
3.2. Mitochondrial Damage and Ovarian Aging
3.3. Premature Ovarian Aging and Senescence
3.4. Telomere Shortening and DNA Damage in POI
3.5. Apoptosis
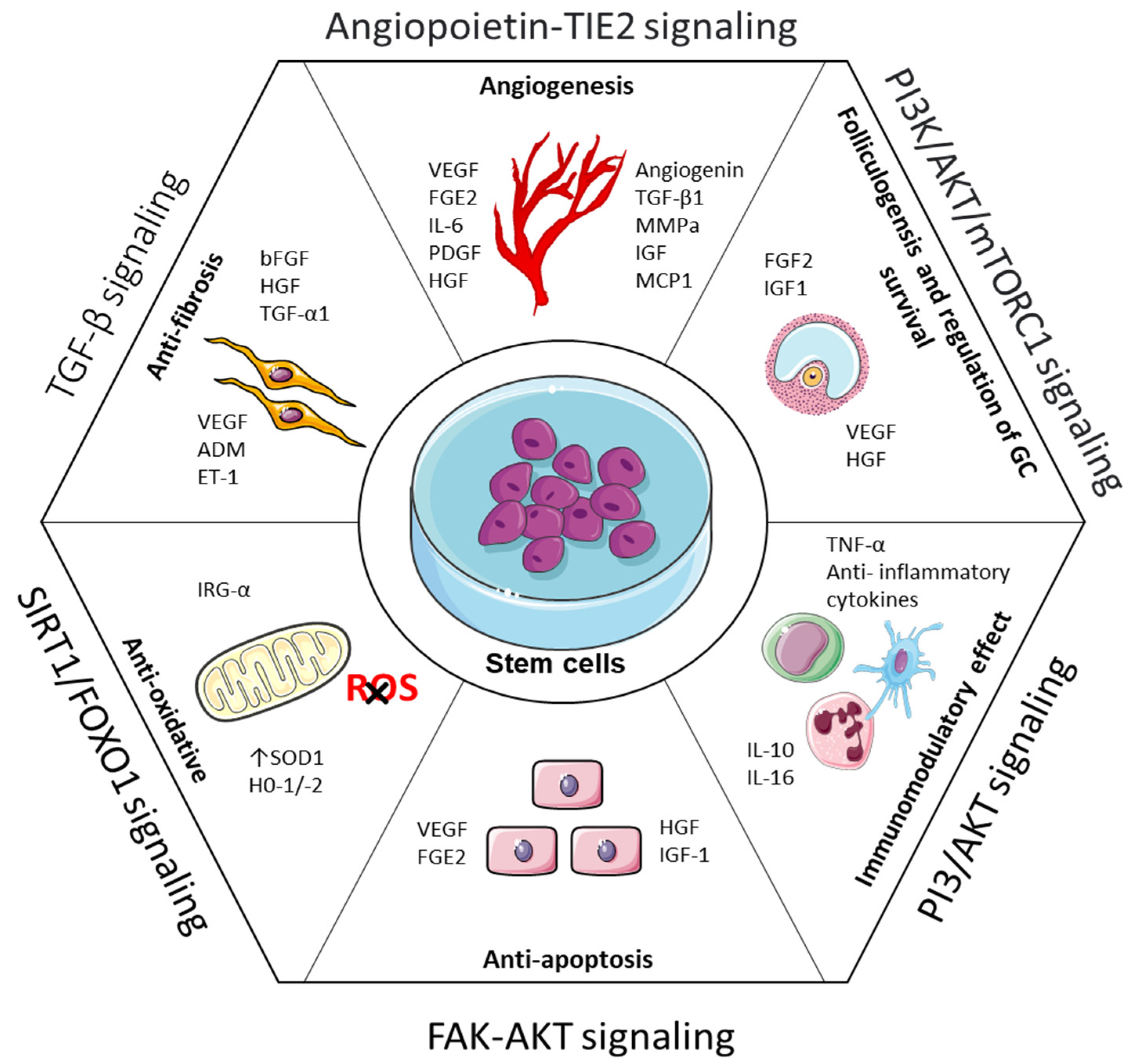
4. Stem Cell-Based Strategies for POI and Infertility Treatment
4.1. Mechanisms of Stem Cell Therapy in POI
4.2. Preclinical Studies of Stem Cell Therapy for POI
4.2.1. Mesenchymal Stem Cells
4.2.2. Bone Marrow Stromal Cells
4.2.3. Adipose-Derived Mesenchymal Stem Cells
4.2.4. Extraembryonic Stem Cells
4.2.5. Ovarian Stem Cells
4.2.6. Engineered Stem Cells
4.2.7. Induced Pluripotent Stem Cells
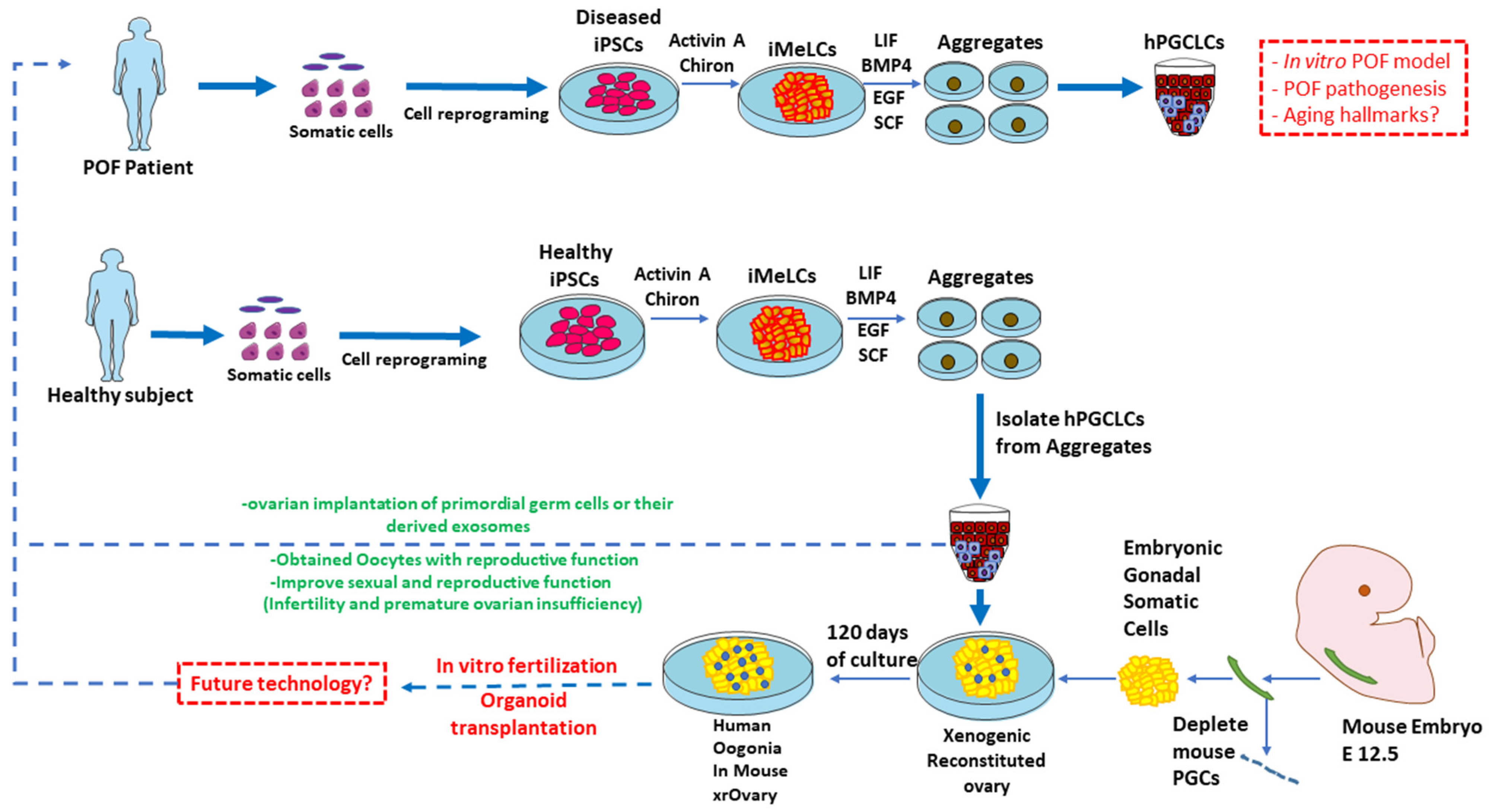
4.3. Recent Advancement in hiPSC Technology for Restoring Infertility
4.3.1. iPSC Differentiation towards Human Germline Cells
4.3.2. Derivation of PGCLCs from Pluripotent Stem Cells
4.3.3. In Vitro Gametogenesis for Clinical Application
5. Prospective of hiPSCs for POI and Infertility
5.1. HiPSC-Derived Germ Cells Application in the Field of Regenerative Medicine
5.2. Reconstitution of the In Vitro Oogenesis Niche
6. PGC-Derived Exosomes: Future Directions for POI Diagnosis and Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torrealday, S.; Kodaman, P.; Pal, L. Premature Ovarian Insufficiency—An update on recent advances in understanding and management. F1000Research 2017, 6, 2069. [Google Scholar] [CrossRef]
- Chon, S.J.; Umair, Z.; Yoon, M.-S. Premature ovarian insufficiency: Past, present, and future. Front. Cell Dev. Biol. 2021, 10, 672890. [Google Scholar] [CrossRef]
- Maclaren, N.; Chen, Q.-Y.; Kukreja, A.; Marker, J.; Zhang, C.H.; Sun, Z.S. Autoimmune hypogonadism as part of an autoimmune polyglandular syndrome. J. Soc. Gynecol. Investig. 2001, 8, S52–S54. [Google Scholar] [CrossRef] [PubMed]
- Makin, S. Cracking the genetic code of autoimmune disease. Nature 2021, 595, 57–59. [Google Scholar] [CrossRef]
- Woad, K.J.; Watkins, W.J.; Prendergast, D.; Shelling, A.N. The genetic basis of premature ovarian failure. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 242–244. [Google Scholar] [CrossRef]
- Jiao, X.; Ke, H.; Qin, Y.; Chen, Z.-J. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol. Metab. 2018, 29, 795–807. [Google Scholar] [CrossRef]
- Di-Battista, A.; Moysés-Oliveira, M.; Melaragno, M.I. Genetics of premature ovarian insufficiency and the association with X-autosome translocations. Reproduction 2020, 160, R55–R64. [Google Scholar] [CrossRef]
- Kirshenbaum, M.; Orvieto, R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J. Assist. Reprod. Genet. 2019, 36, 2207–2215. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, H.; Ke, H.; Zhang, J.; Cheng, L.; Liu, Y.; Qin, Y.; Chen, Z.-J. Premature ovarian insufficiency: Phenotypic characterization within different etiologies. J. Clin. Endocrinol. Metab. 2017, 102, 2281–2290. [Google Scholar] [CrossRef]
- Wesevich, V.; Kellen, A.N.; Pal, L. Recent advances in understanding primary ovarian insufficiency. F1000Research 2020, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Kruszewska, J.; Klicka, K.; Kowalczyk, J.; Grymowicz, M.; Skórska, J.; Pięta, W.; Smolarczyk, R. Premature ovarian insufficiency–aetiopathology, epidemiology, and diagnostic evaluation. Menopause Rev./Przegląd Menopauzalny 2018, 17, 105–108. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Fedintsev, A.; Moskalev, A. Stochastic non-enzymatic modification of long-lived macromolecules-A missing hallmark of aging. Ageing Res. Rev. 2020, 62, 101097. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A. Is aging a disease? Geneticist'point of view. Adv. Gerontol./Uspekhi Gerontol. 2017, 30, 843–844. [Google Scholar]
- Annelien, C.; Broekmans, F.J.; Lambalk, C.B. Role of AMH in Prediction of Menopause. Clinical Applications of Anti-Mullerian Hormone and its Measurement in Reproductive Medicine and Women’s Health. Front. Endocrinol. 2022, 12, 1664–2392. [Google Scholar]
- Yureneva, S.; Averkova, V.; Silachev, D.; Donnikov, A.; Gavisova, A.; Serov, V.; Sukhikh, G. Searching for female reproductive aging and longevity biomarkers. Aging 2021, 13, 16873. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-aging: A new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef]
- Mason, J.B.; Habermehl, T.L.; Underwood, K.B.; Schneider, A.; Brieño-Enriquez, M.A.; Masternak, M.M.; Parkinson, K.C. The interrelationship between female reproductive aging and survival. J. Gerontol. Ser. A 2022, 77, 75–83. [Google Scholar] [CrossRef]
- Sen, A.; Kushnir, V.A.; Barad, D.H.; Gleicher, N. Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol. 2014, 10, 37–50. [Google Scholar] [CrossRef]
- Reato, G.; Morlin, L.; Chen, S.; Furmaniak, J.; Smith, B.R.; Masiero, S.; Albergoni, M.; Cervato, S.; Zanchetta, R.; Betterle, C. Premature ovarian failure in patients with autoimmune Addison's disease: Clinical, genetic, and immunological evaluation. J. Clin. Endocrinol. Metab. 2011, 96, E1255–E1261. [Google Scholar] [CrossRef]
- Drukker, M.; Katchman, H.; Katz, G.; Even-Tov Friedman, S.; Shezen, E.; Hornstein, E.; Mandelboim, O.; Reisner, Y.; Benvenisty, N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 2006, 24, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.-L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Han, X.; Wang, M.; Duan, S.; Franco, P.J.; Kenty, J.H.-R.; Hedrick, P.; Xia, Y.; Allen, A.; Ferreira, L.M.; Strominger, J.L. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2019, 116, 10441–10446. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.-G.; Xu, Y. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, M.; Tian, Y.; Li, Q.; Huang, X. Mesenchymal stem cells in premature ovarian insufficiency: Mechanisms and prospects. Front. Cell Dev. Biol. 2021, 9, 718192. [Google Scholar] [CrossRef]
- Fu, Y.-X.; Ji, J.; Shan, F.; Li, J.; Hu, R. Human mesenchymal stem cell treatment of premature ovarian failure: New challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef]
- Gell, J.J.; Clark, A.T. Restoring fertility with human induced pluripotent stem cells: Are we there yet? Cell Stem Cell 2018, 23, 777–779. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Suzuki, S.; Seita, Y.; Ito, J.; Sakata, Y.; Aso, H.; Sato, K.; Hermann, B.P.; Sasaki, K. Reconstitution of prospermatogonial specification in vitro from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 5656. [Google Scholar] [CrossRef]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell–like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-K.; Song, J.-H.; Lee, S.-B.; Do, J.-T. Germ Cell Derivation from Pluripotent Stem Cells for Understanding In Vitro Gametogenesis. Cells 2021, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Makar, K.; Sasaki, K. Roadmap of germline development and in vitro gametogenesis from pluripotent stem cells. Andrology 2020, 8, 842–851. [Google Scholar] [CrossRef]
- Yoshimatsu, S.; Kisu, I.; Qian, E.; Noce, T. A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”. Biology 2022, 11, 987. [Google Scholar] [CrossRef]
- Sükür, Y.E.; Kıvançlı, I.B.; Ozmen, B. Ovarian aging and premature ovarian failure. J. Turk. Ger. Gynecol. Assoc. 2014, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, X.; Dang, Y.; Zhang, X.; Zhao, S.; Lu, G.; Chan, W.-Y.; Leung, P.C.; Qin, Y. lncRNA GCAT1 is involved in premature ovarian insufficiency by regulating p27 translation in GCs via competitive binding to PTBP1. Mol. Ther.-Nucleic Acids 2021, 23, 132–141. [Google Scholar] [CrossRef]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte aging underlies female reproductive aging: Biological mechanisms and therapeutic strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Hussain, J.; Martínez-Marchal, A.; Aiken, C.E.; Azad, A.; Thompson, D.J.; Knoblochova, L.; Abe, H.; Tarry-Adkins, J.L. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 2021, 596, 393–397. [Google Scholar] [CrossRef]
- Saitou, M.; Hayashi, K. Mammalian in vitro gametogenesis. Science 2021, 374, eaaz6830. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [PubMed]
- Manku, G.; Culty, M. Mammalian gonocyte and spermatogonia differentiation: Recent advances and remaining challenges. Reproduction 2015, 149, R139–R157. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, W.; Lukianchikov, A.; Hancock, G.V.; Zimmerman, J.; Lowe, M.G.; Kim, R.; Galic, Z.; Irie, N.; Surani, M.A. Germline competency of human embryonic stem cells depends on eomesodermin. Biol. Reprod. 2017, 97, 850–861. [Google Scholar] [CrossRef]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef]
- Williams, C.J.; Erickson, G.F. Morphology and Physiology of the Ovary; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Dodson, W.C.; Whitesides, D.B.; Hughes, C.L., Jr.; Easley, H., III; Haney, A. Superovulation with intrauterine insemination in the treatment of infertility: A possible alternative to gamete intrafallopian transfer and in vitro fertilization. Fertil. Steril. 1987, 48, 441–445. [Google Scholar] [CrossRef]
- Domniz, N.; Meirow, D. Premature ovarian insufficiency and autoimmune diseases. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 42–55. [Google Scholar] [CrossRef]
- Fortuño, C.; Labarta, E. Genetics of primary ovarian insufficiency: A review. J. Assist. Reprod. Genet. 2014, 31, 1573–1585. [Google Scholar] [CrossRef]
- Vujovic, S. Aetiology of premature ovarian failure. Menopause Int. 2009, 15, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Cree, L.; Shelling, A.N. The genetics of premature ovarian failure: Current perspectives. Int. J. Women's Health 2015, 7, 799. [Google Scholar]
- Sheikhansari, G.; Aghebati-Maleki, L.; Nouri, M.; Jadidi-Niaragh, F.; Yousefi, M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed. Pharmacother. 2018, 102, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Mark-Kappeler, C.J.; Hoyer, P.B.; Devine, P.J. Xenobiotic effects on ovarian preantral follicles. Biol. Reprod. 2011, 85, 871–883. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef]
- Gelbaya, T.; Vitthala, S.; Nardo, L.; Seif, M. Optimizing hormone therapy for future reproductive performance in women with premature ovarian failure. Gynecol. Endocrinol. 2011, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haller-Kikkatalo, K.; Uibo, R.; Kurg, A.; Salumets, A. The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: A general population registry-based study. Hum. Reprod. 2015, 30, 1229–1238. [Google Scholar] [CrossRef]
- Vohra, B.; Sharma, S.; Kansal, V. Age-dependent variations in mitochondrial and cytosolic antioxidant enzymes and lipid peroxidation in different regions of central nervous system of guinea pigs. Indian J. Biochem. Biophys. 2001, 38, 321–326. [Google Scholar]
- Prattichizzo, F.; Micolucci, L.; Cricca, M.; De Carolis, S.; Mensà, E.; Ceriello, A.; Procopio, A.D.; Bonafè, M.; Olivieri, F. Exosome-based immunomodulation during aging: A nano-perspective on inflamm-aging. Mech. Ageing Dev. 2017, 168, 44–53. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466.e454. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gonzalez-Sanchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Landi, F.; Bernabei, R.; Marzetti, E. Fueling inflamm-aging through mitochondrial dysfunction: Mechanisms and molecular targets. Int. J. Mol. Sci. 2017, 18, 933. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Ottaviani, E.; Franceschi, C. The neuroimmunology of stress from invertebrates to man. Prog. Neurobiol. 1996, 48, 421–440. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Ali, I.; Li, L.; Wang, G. N-acetylcysteine modulates non-esterified fatty acid-induced pyroptosis and inflammation in granulosa cells. Mol. Immunol. 2020, 127, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sheng, L.; Xu, C.; Li, J.; Ali, I.; Li, H.; Cai, Y. Ufbp1, a Key Player of Ufm1 Conjugation System, Protects Against Ketosis-Induced Liver Injury via Suppressing Smad3 Activation. Front. Cell Dev. Biol. 2021, 9, 1665. [Google Scholar] [CrossRef]
- Mei, C.; Zheng, F. Chronic inflammation potentiates kidney aging. Semin. Nephrol. 2009, 29, 555–668. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Andersen-Ranberg, K.; vB Hjelmborg, J.; Pedersen, B.K.; Jeune, B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003, 115, 278–283. [Google Scholar] [CrossRef]
- Salvioli, S.; Capri, M.; Valensin, S.; Tieri, P.; Monti, D.; Ottaviani, E.; Franceschi, C. Inflamm-aging, cytokines and aging: State of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 2006, 12, 3161–3171. [Google Scholar] [CrossRef]
- Naz, R.K.; Thurston, D.; Santoro, N. Circulating tumor necrosis factor (TNF)-α in normally cycling women and patients with premature ovarian failure and polycystic ovaries. Am. J. Reprod. Immunol. 1995, 34, 170–175. [Google Scholar] [CrossRef]
- Yan, H.; Xu, J.J.; Ali, I.; Zhang, W.; Jiang, M.; Li, G.; Teng, Y.; Zhu, G.; Cai, Y. CDK5RAP3, an essential regulator of checkpoint, interacts with RPL26 and maintains the stability of cell growth. Cell Prolif. 2022, 4, e13240. [Google Scholar] [CrossRef]
- Yan, H.; Wen, J.; Zhang, T.; Zheng, W.; He, M.; Huang, K.; Guo, Q.; Chen, Q.; Yang, Y.; Deng, G. Oocyte-derived E-cadherin acts as a multiple functional factor maintaining the primordial follicle pool in mice. Cell Death Dis. 2019, 10, 160. [Google Scholar] [CrossRef]
- Rossetti, R.; Ferrari, I.; Bonomi, M.; Persani, L. Genetics of primary ovarian insufficiency. Clin. Genet. 2017, 91, 183–198. [Google Scholar] [CrossRef]
- Ye, H.; Li, X.; Zheng, T.; Liang, X.; Li, J.; Huang, J.; Pan, Z.; Zheng, Y. The effect of the immune system on ovarian function and features of ovarian germline stem cells. Springerplus 2016, 5, 990. [Google Scholar] [CrossRef]
- Wang, X.-F.; Zhang, L.; Wu, Q.-H.; Min, J.-X.; Ma, N.; Luo, L.-C. Biological mechanisms of premature ovarian failure caused by psychological stress based on support vector regression. Int. J. Clin. Exp. Med. 2015, 8, 21393. [Google Scholar]
- Bouali, N.; Francou, B.; Bouligand, J.; Imanci, D.; Dimassi, S.; Tosca, L.; Zaouali, M.; Mougou, S.; Young, J.; Saad, A. New MCM8 mutation associated with premature ovarian insufficiency and chromosomal instability in a highly consanguineous Tunisian family. Fertil. Steril. 2017, 108, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dadhwal, V.; Sharma, K.A.; Mittal, S. Xanthogranulomatous inflammation: A rare cause of premature ovarian failure. Arch. Gynecol. Obstet. 2009, 279, 729–731. [Google Scholar] [CrossRef]
- Altuntas, C.Z.; Johnson, J.M.; Tuohy, V.K. Autoimmune targeted disruption of the pituitary-ovarian axis causes premature ovarian failure. J. Immunol. 2006, 177, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Said, R.S.; El-Demerdash, E.; Nada, A.S.; Kamal, M.M. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly (ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 2016, 103, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, L.; Ou, R. Analysis of anti-zona pellucida antibody and tumor necrosis factor-α, γ-interferon and interleukin-2 in sera from patients with premature ovarian failure. J. Reprod. Med. 2003, 12, 47–50. [Google Scholar]
- Sundaresan, N.; Saxena, V.; Sastry, K.; Nagarajan, K.; Jain, P.; Singh, R.; Anish, D.; Ravindra, P.; Saxena, M.; Ahmed, K. Cytokines and chemokines in postovulatory follicle regression of domestic chicken (Gallus gallus domesticus). Dev. Comp. Immunol. 2008, 32, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Wang, F.; Dong, Z.; Zhang, Q. Skin-derived mesenchymal stem cells help restore function to ovaries in a premature ovarian failure mouse model. PLoS ONE 2014, 9, e98749. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in female fertility: A role in oxidative stress and aging. Antioxid. Redox Signal. 2020, 32, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.R.; Green, M.P.; Shelling, A.N.; Berg, M.C.; Peek, J.C.; Cree, L.M. Oocyte mitochondrial deletions and heteroplasmy in a bovine model of ageing and ovarian stimulation. Mol. Hum. Reprod. 2016, 22, 261–271. [Google Scholar] [CrossRef]
- Zhen, X.; Wu, B.; Wang, J.; Lu, C.; Gao, H.; Qiao, J. Increased incidence of mitochondrial cytochrome c oxidase 1 gene mutations in patients with primary ovarian insufficiency. PLoS ONE 2015, 10, e0132610. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, M.; Jiang, Z.; Seli, E. Mitochondrial dysfunction and ovarian aging. Am. J. Reprod. Immunol. 2017, 77, e12651. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chretien, M.; Jacques, C.; Vasseur, C.; Malthiery, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Casper, R.F.; Diez-Juan, A.; Simon, C.; Domar, A.D.; Frydman, R. Aging and the environment affect gamete and embryo potential: Can we intervene? Fertil. Steril. 2016, 105, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Pellicer, A.; Moreno-Loshuertos, R.; Lechuga-Vieco, A.V.; Sánchez-Cabo, F.; Torroja, C.; Acín-Pérez, R.; Calvo, E.; Aix, E.; González-Guerra, A.; Logan, A. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 2016, 535, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, M.; Alfarawati, S.; Hurd, D.; Paolucci, M.; Shovelton, J.; Fragouli, E.; Wells, D. Simultaneous assessment of aneuploidy, polymorphisms, and mitochondrial DNA content in human polar bodies and embryos with the use of a novel microarray platform. Fertil. Steril. 2014, 102, 1385–1392. [Google Scholar] [CrossRef]
- Kumar, M.; Pathak, D.; Kriplani, A.; Ammini, A.; Talwar, P.; Dada, R. Nucleotide variations in mitochondrial DNA and supra-physiological ROS levels in cytogenetically normal cases of premature ovarian insufficiency. Arch. Gynecol. Obstet. 2010, 282, 695–705. [Google Scholar] [CrossRef]
- Kasapoğlu, I.; Seli, E. Mitochondrial dysfunction and ovarian aging. Endocrinology 2020, 63, 101168. [Google Scholar] [CrossRef]
- He, Q.; Gu, L.; Lin, Q.; Ma, Y.; Liu, C.; Pei, X.; Li, P.A.; Yang, Y. The Immp2l mutation causes ovarian aging through ROS-Wnt/β-catenin-estrogen pathway: Preventive effect of melatonin. Endocrinology 2020, 161, bqaa119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bener, M.B.; Jiang, Z.; Wang, T.; Esencan, E.; Scott III, R.; Horvath, T.; Seli, E. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging 2019, 11, 3919. [Google Scholar] [CrossRef]
- Udagawa, O.; Ishihara, T.; Maeda, M.; Matsunaga, Y.; Tsukamoto, S.; Kawano, N.; Miyado, K.; Shitara, H.; Yokota, S.; Nomura, M. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr. Biol. 2014, 24, 2451–2458. [Google Scholar] [CrossRef]
- Gispert, S.; Parganlija, D.; Klinkenberg, M.; Dröse, S.; Wittig, I.; Mittelbronn, M.; Grzmil, P.; Koob, S.; Hamann, A.; Walter, M. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum. Mol. Genet. 2013, 22, 4871–4887. [Google Scholar] [CrossRef]
- Luoma, P.; Melberg, A.; Rinne, J.O.; Kaukonen, J.A.; Nupponen, N.N.; Chalmers, R.M.; Oldfors, A.; Rautakorpi, I.; Peltonen, L.; Majamaa, K. Parkinsonism, premature menopause, and mitochondrial DNA polymerase γ mutations: Clinical and molecular genetic study. Lancet 2004, 364, 875–882. [Google Scholar] [CrossRef]
- Pierce, S.B.; Chisholm, K.M.; Lynch, E.D.; Lee, M.K.; Walsh, T.; Opitz, J.M.; Li, W.; Klevit, R.E.; King, M.-C. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 6543–6548. [Google Scholar] [CrossRef] [PubMed]
- Stolk, L.; Zhai, G.; van Meurs, J.B.; Verbiest, M.M.; Visser, J.A.; Estrada, K.; Rivadeneira, F.; Williams, F.M.; Cherkas, L.; Deloukas, P. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat. Genet. 2009, 41, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T. Ovarian ageing and the general biology of senescence. Maturitas 1998, 30, 105–111. [Google Scholar] [CrossRef]
- Guigon, C.J.; Cohen-Tannoudji, M. Reconsidering the roles of female germ cells in ovarian development and folliculogenesis. Biol. Aujourd'hui 2011, 205, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ansere, V.A.; Ali-Mondal, S.; Sathiaseelan, R.; Garcia, D.N.; Isola, J.V.; Henseb, J.D.; Saccon, T.D.; Ocañas, S.R.; Tooley, K.B.; Stout, M.B. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech. Ageing Dev. 2021, 194, 111425. [Google Scholar] [CrossRef]
- Stolk, L.; Perry, J.R.; Chasman, D.I.; He, C.; Mangino, M.; Sulem, P.; Barbalic, M.; Broer, L.; Byrne, E.M.; Ernst, F. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet. 2012, 44, 260–268. [Google Scholar] [CrossRef]
- Perry, J.R.; Hsu, Y.-H.; Chasman, D.I.; Johnson, A.D.; Elks, C.; Albrecht, E.; Andrulis, I.L.; Beesley, J.; Berenson, G.S.; Bergmann, S. DNA mismatch repair gene MSH6 implicated in determining age at natural menopause. Hum. Mol. Genet. 2014, 23, 2490–2497. [Google Scholar] [CrossRef]
- He, C.; Kraft, P.; Chen, C.; Buring, J.E.; Paré, G.; Hankinson, S.E.; Chanock, S.J.; Ridker, P.M.; Hunter, D.J.; Chasman, D.I. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat. Genet. 2009, 41, 724–728. [Google Scholar] [CrossRef]
- He, C.; Kraft, P.; Chasman, D.I.; Buring, J.E.; Chen, C.; Hankinson, S.E.; Paré, G.; Chanock, S.; Ridker, P.M.; Hunter, D.J. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum. Genet. 2010, 128, 515–527. [Google Scholar] [CrossRef]
- Bonafe, M.; Storci, G.; Franceschi, C. Inflamm-aging of the stem cell niche: Breast cancer as a paradigmatic example: Breakdown of the multi-shell cytokine network fuels cancer in aged people. Bioessays 2012, 34, 40–49. [Google Scholar] [CrossRef]
- Yamada-Fukunaga, T.; Yamada, M.; Hamatani, T.; Chikazawa, N.; Ogawa, S.; Akutsu, H.; Miura, T.; Miyado, K.; Tarín, J.J.; Kuji, N. Age-associated telomere shortening in mouse oocytes. Reprod. Biol. Endocrinol. 2013, 11, 108. [Google Scholar] [CrossRef]
- Liu, L.; Franco, S.; Spyropoulos, B.; Moens, P.B.; Blasco, M.A.; Keefe, D.L. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 6496–6501. [Google Scholar] [CrossRef]
- Butts, S.; Riethman, H.; Ratcliffe, S.; Shaunik, A.; Coutifaris, C.; Barnhart, K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J. Clin. Endocrinol. Metab. 2009, 94, 4835–4843. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Zhang, X.; Liu, Y.; Wang, Z.; Wang, P.; Du, Y.; Qin, Y.; Chen, Z.-J. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum. Reprod. 2017, 32, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Feingold, E.; Chakraborty, S.; Dey, S.K. Telomere length is associated with types of chromosome 21 nondisjunction: A new insight into the maternal age effect on Down syndrome birth. Hum. Genet. 2010, 127, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, C.; Harada, M.; Takahashi, N.; Azhary, J.M.; Kusamoto, A.; Nose, E.; Oi, N.; Takeuchi, A.; Wada-Hiraike, O.; Hirata, T. Activation of endoplasmic reticulum stress mediates oxidative stress–induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol. Hum. Reprod. 2020, 26, 40–52. [Google Scholar] [CrossRef]
- Regan, S.L.; Knight, P.G.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Granulosa cell apoptosis in the ovarian follicle—A changing view. Front. Endocrinol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Daokuan, Z.; Guoqing, T.; Li, L. Apoptotic mechanism of premature ovarian failure and rescue effect of Traditional Chinese Medicine: A review. J. Tradit. Chin. Med. 2021, 41, 491. [Google Scholar]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Acuña-Hernández, D.G.; Arreola-Mendoza, L.; Santacruz-Márquez, R.; García-Zepeda, S.P.; Parra-Forero, L.Y.; Olivares-Reyes, J.A.; Hernández-Ochoa, I. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol. Appl. Pharmacol. 2018, 344, 13–22. [Google Scholar] [CrossRef]
- Thomas, P. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes. J. Steroid Biochem. Mol. Biol. 2017, 167, 153–161. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef]
- Dupont, J.; Scaramuzzi, R.J. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem. J. 2016, 473, 1483–1501. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Sheng, Y.; Fu, X.; Cheng, H.; Zhou, R. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy 2015, 11, 1081–1098. [Google Scholar] [CrossRef]
- Wang, W.; Craig, Z.R.; Basavarajappa, M.S.; Hafner, K.S.; Flaws, J.A. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol. Reprod. 2012, 87, 152. [Google Scholar]
- Sobinoff, A.P.; Beckett, E.L.; Jarnicki, A.G.; Sutherland, J.M.; McCluskey, A.; Hansbro, P.M.; McLaughlin, E.A. Scrambled and fried: Cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol. Appl. Pharmacol. 2013, 271, 156–167. [Google Scholar] [CrossRef]
- Ma, M.; Chen, X.-Y.; Gu, C.; Xiao, X.-R.; Guo, T.; Li, B. Biochemical changes of oxidative stress in premature ovarian insufficiency induced by tripterygium glycosides. Int. J. Clin. Exp. Pathol. 2014, 7, 8855. [Google Scholar]
- Tokmak, A.; Yıldırım, G.; Sarıkaya, E.; Çınar, M.; Boğdaycıoğlu, N.; Yılmaz, F.M.; Yılmaz, N. Increased oxidative stress markers may be a promising indicator of risk for primary ovarian insufficiency: A cross-sectional case control study. Rev. Bras. Ginecol. Obs. 2015, 37, 411–416. [Google Scholar]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed interplay between autophagy and inflammasomes. Aging 2012, 4, 166. [Google Scholar] [CrossRef]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014, 56, 154–163. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, M.; Cao, Z.; Du, X.; Ji, D.; Liang, D.; Cao, Y.; Liu, Y. Melatonin protects against excessive autophagy-induced mitochondrial and ovarian reserve function deficiency though ERK signaling pathway in Chinese hamster ovary (CHO) cells. Mitochondrion 2021, 61, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Bojic, S.; Nurkovic, J.; Volarevic, A.; Ljujic, B.; Arsenijevic, N.; Lako, M.; Stojkovic, M. Stem cells as new agents for the treatment of infertility: Current and future perspectives and challenges. BioMed Res. Int. 2014, 2014, 507234. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Cantore, A.; Chaimov, D.; Orlando, G. Regenerative medicine: The red planet for clinicians. Intern. Emerg. Med. 2019, 14, 911–921. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Sabouni, R.; Cayton Vaught, K.C.; Owen, C.M.; Albertini, D.F.; Segars, J.H. Biomechanics and mechanical signaling in the ovary: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, P.; Tan, J. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell Rev. Rep. 2019, 15, 241–255. [Google Scholar] [CrossRef]
- Fazeli, Z.; Abedindo, A.; Omrani, M.D.; Ghaderian, S.M.H. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: A systematic review. Stem Cell Rev. Rep. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Chen, S.-R.; Su, P.-P.; Huang, F.-H.; Shi, Y.-C.; Shi, Q.-Y.; Lin, S. Using mesenchymal stem cells to treat female infertility: An update on female reproductive diseases. Stem Cells Int. 2019, 2019, 9071720. [Google Scholar] [CrossRef]
- Davis, M.E. Exosomes: What do we love so much about them? Circ. Res. 2016, 119, 1280–1282. [Google Scholar] [CrossRef]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv. Sci. 2020, 7, 2001334. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Hao, J.-X.; Meng, T.-G.; Guo, L.; Dong, M.-Z.; Fan, L.-H.; Ouyang, Y.-C.; Wang, G.; Sun, Q.-Y.; Ou, X.-H. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging 2017, 9, 2480. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Chugh, R.M.; Park, H.-s.; Hobeika, E.; Ulin, M.; Al-Hendy, A. Mesenchymal stem cells as a bio organ for treatment of female infertility. Cells 2020, 9, 2253. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Feng, X.; Wei, T.; Wang, Y.; Wang, Y.; Wang, Z.; Tang, D.; Luo, Y.; Xiong, Z. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res. Ther. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, B.; Li, X.; Ma, W.; Zhang, P.; Chen, X.; Wu, J. Tracing and characterizing the development of transplanted female germline stem cells in vivo. Mol. Ther. 2017, 25, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Vural, B.; Duruksu, G.; Vural, F.; Gorguc, M.; Karaoz, E. Effects of VEGF+ mesenchymal stem cells and platelet-rich plasma on inbred rat ovarian functions in cyclophosphamide-induced premature ovarian insufficiency model. Stem Cell Rev. Rep. 2019, 15, 558–573. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, B.; Jiang, R.; Liao, S.; Wei, Z.; Bi, Y.; Liu, X.; Deng, R.; Jin, Y.; Tan, Y. G-CSF-mobilized peripheral blood mononuclear cells combined with platelet-rich plasma accelerate restoration of ovarian function in cyclophosphamide-induced POI rats. Biol. Reprod. 2019, 101, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lee, Y.; Kim, H.J.; Oh, H.J.; Kang, S.K.; Ra, J.C.; Lee, B.C. Intravenous human endothelial progenitor cell administration into aged mice enhances embryo development and oocyte quality by reducing inflammation, endoplasmic reticulum stress and apoptosis. J. Vet. Med. Sci. 2018, 80, 1905–1913. [Google Scholar] [CrossRef]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cell Mater. 2010, 20, e33. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Sun, M.; Mu, S.; Wang, C.; Wang, D.; Yao, Y. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res. Int. 2013, 2013, 690491. [Google Scholar] [CrossRef]
- Li, J.; Mao, Q.; He, J.; She, H.; Zhang, Z.; Yin, C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res. Ther. 2017, 8, 55. [Google Scholar] [CrossRef]
- Abd-Allah, S.H.; Shalaby, S.M.; Pasha, H.F.; Amal, S.; Raafat, N.; Shabrawy, S.M.; Awad, H.A.; Amer, M.G.; Gharib, M.A.; El Gendy, E.A. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy 2013, 15, 64–75. [Google Scholar] [CrossRef]
- Lai, D.; Wang, F.; Yao, X.; Zhang, Q.; Wu, X.; Xiang, C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J. Transl. Med. 2015, 13, 155. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Shalaby, S.M.; Abdelaziz, M.; Brakta, S.; Hill, W.D.; Ismail, N.; Al-Hendy, A. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod. Sci. 2018, 25, 51–63. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Li, Y.; Yu, L.; Gu, F.; Wang, C.; Yao, Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res. Ther. 2013, 4, 80. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Zhang, J.; Qin, W.; Chi, H.; Chen, J.; Yu, Z.; Chen, C. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014, 23, 1548–1557. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yang, T.; Li, J.; Yang, X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res. Ther. 2017, 8, 11. [Google Scholar] [CrossRef]
- Castilho-Fernandes, A.; Lopes, T.G.; Ferreira, F.U.; Rezende, N.; Silva, V.F.; Primo, F.L.; Fontes, A.M.; Ribeiro-Silva, A.; Tedesco, A.C. Adipogenic differentiation of murine bone marrow mesenchymal stem cells induced by visible light via photo-induced biomodulation. Photodiagn. Photodyn. Ther. 2019, 25, 119–127. [Google Scholar] [CrossRef]
- Rimola, A.; Londoño, M.-C.; Guevara, G.; Bruguera, M.; Navasa, M.; Forns, X.; García-Retortillo, M.; García-Valdecasas, J.-C.; Rodes, J. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation 2004, 78, 686–691. [Google Scholar] [CrossRef]
- Ringe, J.; Strassburg, S.; Neumann, K.; Endres, M.; Notter, M.; Burmester, G.R.; Kaps, C.; Sittinger, M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007, 101, 135–146. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Badawy, A.; Sobh, M.A.; Ahdy, M.; Abdelhafez, M.S. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int. J. Women's Health 2017, 9, 441. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemotherapy 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Wang, K.; Yu, L.-Y.; Jiang, L.-Y.; Wang, H.-B.; Wang, C.-Y.; Luo, Y. The paracrine effects of adipose-derived stem cells on neovascularization and biocompatibility of a macroencapsulation device. Acta Biomater. 2015, 15, 65–76. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Xiao, G.-Y.; Liu, I.-H.; Cheng, C.-C.; Chang, C.-C.; Lee, Y.-H.; Cheng, W.T.-K.; Wu, S.-C. Amniotic fluid stem cells prevent follicle atresia and rescue fertility of mice with premature ovarian failure induced by chemotherapy. PLoS ONE 2014, 9, e106538. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Yao, X.; Lai, D.; Guo, L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Res. Ther. 2013, 4, 124. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017, 8, 187. [Google Scholar] [CrossRef]
- Xiao, G.-Y.; Cheng, C.-C.; Chiang, Y.-S.; Cheng, W.T.-K.; Liu, I.; Wu, S.-C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016, 6, 23120. [Google Scholar] [CrossRef]
- Zhang, Q.; Bu, S.; Sun, J.; Xu, M.; Yao, X.; He, K.; Lai, D. Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res. Ther. 2017, 8, 270. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, L.; Zhang, X.; Hu, C.; Liu, R. Biological and biomechanical analysis of two types of mesenchymal stem cells for intervention in chemotherapy-induced ovarian dysfunction. Arch. Gynecol. Obstet. 2017, 295, 247–252. [Google Scholar] [CrossRef]
- Na, J.; Kim, G.J. Recent trends in stem cell therapy for premature ovarian insufficiency and its therapeutic potential: A review. J. Ovarian Res. 2020, 13, 74. [Google Scholar] [CrossRef]
- Qiu, P.-L.; Liu, S.-Y.; Bradshaw, M.; Rooney-Latham, S.; Takamatsu, S.; Bulgakov, T.S.; Tang, S.-R.; Feng, J.; Jin, D.-N.; Aroge, T. Multi-locus phylogeny and taxonomy of an unresolved, heterogeneous species complex within the genus Golovinomyces (Ascomycota, Erysiphales), including G. ambrosiae, G. circumfusus and G. spadiceus. BMC Microbiol. 2020, 20, 51. [Google Scholar] [CrossRef]
- Song, D.; Zhong, Y.; Qian, C.; Zou, Q.; Ou, J.; Shi, Y.; Gao, L.; Wang, G.; Liu, Z.; Li, H. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. BioMed Res. Int. 2016, 10, 2314–6133. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, J.; Liu, J.; Liu, Y.; Wang, L.; Jiang, R.; Diao, Z.; Yan, G.; Pèault, B.; Sun, H. Different angiogenic potentials of mesenchymal stem cells derived from umbilical artery, umbilical vein, and Wharton’s jelly. Stem Cells Int. 2017, 2017, 3175748. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Liu, J.; Shang, D.; Xiao, Y.; Zhong, P.; Cheng, H.; Zhou, R. Isolation and characterization of string-forming female germline stem cells from ovaries of neonatal mice. J. Biol. Chem. 2017, 292, 16003–16013. [Google Scholar] [CrossRef]
- Stimpfel, M.; Cerkovnik, P.; Novakovic, S.; Maver, A.; Virant-Klun, I. Putative mesenchymal stem cells isolated from adult human ovaries. J. Assist. Reprod. Genet. 2014, 31, 959–974. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, Z.-B.; Feng, H.-L.; Miao, Y.-L.; Wang, Q.; Yu, Y.; Wei, Y.-C.; Yan, J.; Wang, W.-H.; Shen, W. The root of reduced fertility in aged women and possible therapentic options: Current status and future perspects. Mol. Asp. Med. 2014, 38, 54–85. [Google Scholar] [CrossRef]
- Eggan, K.; Jurga, S.; Gosden, R.; Min, I.M.; Wagers, A.J. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature 2006, 441, 1109–1114. [Google Scholar] [CrossRef]
- Silvestris, E.; Cafforio, P.; Felici, C.; Cormio, G.; D’Oronzo, S. Ddx4+ oogonial stem cells in postmenopausal women’s ovaries: A controversial, undefined role. Cells 2019, 8, 650. [Google Scholar] [CrossRef]
- Silvestris, E.; Cafforio, P.; D’Oronzo, S.; Felici, C.; Silvestris, F.; Loverro, G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: Single-cell isolation and molecular characterization. Hum. Reprod. 2018, 33, 464–473. [Google Scholar] [CrossRef]
- Silvestris, E.; D’oronzo, S.; Cafforio, P.; Kardhashi, A.; Dellino, M.; Cormio, G. In vitro generation of oocytes from ovarian stem cells (OSCs): In search of major evidence. Int. J. Mol. Sci. 2019, 20, 6225. [Google Scholar] [CrossRef]
- Kimbrel, E.A.; Lanza, R. Next-generation stem cells—Ushering in a new era of cell-based therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef]
- Gornalusse, G.G.; Hirata, R.K.; Funk, S.E.; Riolobos, L.; Lopes, V.S.; Manske, G.; Prunkard, D.; Colunga, A.G.; Hanafi, L.-A.; Clegg, D.O. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017, 35, 765–772. [Google Scholar] [CrossRef]
- Cheng, Z.; Ou, L.; Zhou, X.; Li, F.; Jia, X.; Zhang, Y.; Liu, X.; Li, Y.; Ward, C.A.; Melo, L.G. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008, 16, 571–579. [Google Scholar] [CrossRef]
- Sun, S.; White, R.R.; Fischer, K.E.; Zhang, Z.; Austad, S.N.; Vijg, J. Inducible aging in Hydra oligactis implicates sexual reproduction, loss of stem cells, and genome maintenance as major pathways. GeroScience 2020, 42, 1119–1132. [Google Scholar] [CrossRef]
- Gore, A.; Li, Z.; Fung, H.-L.; Young, J.E.; Agarwal, S.; Antosiewicz-Bourget, J.; Canto, I.; Giorgetti, A.; Israel, M.A.; Kiskinis, E. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011, 471, 63–67. [Google Scholar] [CrossRef]
- Hussein, S.M.; Batada, N.N.; Vuoristo, S.; Ching, R.W.; Autio, R.; Närvä, E.; Ng, S.; Sourour, M.; Hämäläinen, R.; Olsson, C. Copy number variation and selection during reprogramming to pluripotency. Nature 2011, 471, 58–62. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Kida, Y.S.; Hawkins, R.D.; Nery, J.R.; Hon, G.; Antosiewicz-Bourget, J.; O’Malley, R.; Castanon, R.; Klugman, S. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature 2011, 471, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Su, G.-h.; Sun, Y.-f.; Lu, Y.-x.; Shuai, X.-x.; Liao, Y.-h.; Liu, Q.-y.; Han, J.; Luo, P. Hepatocyte growth factor gene-modified bone marrow-derived mesenchymal stem cells transplantation promotes angiogenesis in a rat model of hindlimb ischemia. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2013, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Q.; Wang, S.; Chen, C.; Zheng, J. Transplantation of ovarian granulosa-like cells derived from human induced pluripotent stem cells for the treatment of murine premature ovarian failure. Mol. Med. Rep. 2016, 13, 5053–5058. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qin, W.; Huang, Y.; Zhao, Y.; Wang, J. Induction of estrogen-sensitive epithelial cells derived from human-induced pluripotent stem cells to repair ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. DNA Cell Biol. 2013, 32, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cheng, M.J.; Xu, C.J. Secretion of oestrogen from murine-induced pluripotent stem cells co-cultured with ovarian granulosa cells in vitro. Cell Biol. Int. 2011, 35, 871–874. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wu, Z.; Tan, X.; Liu, F.; Huang, X.; Fang, X. Differentiation of rat iPS cells and ES cells into granulosa cell-like cells in vitro. Acta Biochim. Biophys Sin 2013, 45, 289–295. [Google Scholar] [CrossRef][Green Version]
- Leng, L.; Tan, Y.; Gong, F.; Hu, L.; Ouyang, Q.; Zhao, Y.; Lu, G.; Lin, G. Differentiation of primordial germ cells from induced pluripotent stem cells of primary ovarian insufficiency. Hum. Reprod. 2015, 30, 737–748. [Google Scholar] [CrossRef]
- Sun, B.; Ma, Y.; Wang, F.; Hu, L.; Sun, Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 360. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Investig. 2020, 100, 342–352. [Google Scholar] [CrossRef]
- Terraciano, P.; Garcez, T.; Ayres, L.; Durli, I.; Baggio, M.; Kuhl, C.P.; Laurino, C.; Passos, E.; Paz, A.H.; Cirne-Lima, E. Cell therapy for chemically induced ovarian failure in mice. Stem Cells Int. 2014, 2014. [Google Scholar] [CrossRef]
- Su, J.; Ding, L.; Cheng, J.; Yang, J.; Li, X.a.; Yan, G.; Sun, H.; Dai, J.; Hu, Y. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum. Reprod. 2016, 31, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Noory, P.; Navid, S.; Zanganeh, B.M.; Talebi, A.; Borhani-Haghighi, M.; Gholami, K.; Manshadi, M.D.; Abbasi, M. Human menstrual blood stem cell-derived granulosa cells participate in ovarian follicle formation in a rat model of premature ovarian failure in vivo. Cell. Reprogram. 2019, 21, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Patel, A.N.; Ichim, T.E.; Riordan, N.H.; Wang, H.; Min, W.-P.; Woods, E.J.; Reid, M.; Mansilla, E.; Marin, G.H. Feasibility investigation of allogeneic endometrial regenerative cells. J. Transl. Med. 2009, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Shalaby, S.; Brakta, S.; Elam, L.; Elsharoud, A.; Al-Hendy, A. Umbilical cord blood mesenchymal stem cells as an infertility treatment for chemotherapy induced premature ovarian insufficiency. Biomedicines 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.F.; Hu, H.B.; Xu, H.Y.; Fu, X.F.; Peng, D.X.; Su, W.Y.; He, Y.L. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J. Cell. Mol. Med. 2015, 19, 2108–2117. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, L.; Wang, S.; Sheng, X.; Yan, G.; Xu, L.; Liu, J.; Liu, M.; Zhen, X.; Ding, L. Transplantation of umbilical cord–derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. Vitr. Cell. Dev. Biol.-Anim. 2019, 55, 302–311. [Google Scholar] [CrossRef]
- Elfayomy, A.K.; Almasry, S.M.; El-Tarhouny, S.A.; Eldomiaty, M.A. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: Possible direct and indirect effects. Tissue Cell 2016, 48, 370–382. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Guo, L.; Cheng, W.; Zou, G. CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive and proliferate in the ovary long-term in a mouse model of chemotherapy-induced premature ovarian failure. Int. J. Med. Sci. 2012, 9, 592. [Google Scholar] [CrossRef]
- Ling, L.; Feng, X.; Wei, T.; Wang, Y.; Wang, Y.; Zhang, W.; He, L.; Wang, Z.; Zeng, Q.; Xiong, Z. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. Stem Cell Res. Ther. 2017, 8, 283. [Google Scholar] [CrossRef]
- Yin, N.; Zhao, W.; Luo, Q.; Yuan, W.; Luan, X.; Zhang, H. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by Treg cells and associated cytokines. Reprod. Sci. 2018, 25, 1073–1082. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Q.; Lu, X.; Yin, N.; Zhou, D.; Zhang, L.; Zhao, W.; Wang, D.; Du, P.; Hou, Y. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. Stem Cell Res. Ther. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, Y.; Lu, X.; Liu, R.; Zhang, L.; Zhao, W.; Yuan, W.; Luo, Q.; Wu, H.; Luan, X. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res. Ther. 2018, 9, 37. [Google Scholar] [CrossRef]
- Li, H.; Zhao, W.; Wang, L.; Luo, Q.; Yin, N.; Lu, X.; Hou, Y.; Cui, J.; Zhang, H. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. Cell Biol. Int. 2019, 43, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, C.; Zhu, S.; Li, S.; Zhang, Q.; Song, L.; Liang, X. The therapeutic effect of stem cells on chemotherapy-induced premature ovarian failure. Curr. Mol. Med. 2021, 21, 376. [Google Scholar] [CrossRef] [PubMed]
- Eddy, C.A.; Pauerstein, C.J. Anatomy and physiology of the fallopian tube. Clin. Obstet. Gynecol. 1980, 23, 1177–1194. [Google Scholar] [CrossRef]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Kahler, D.J.; Ahmad, F.S.; Ritz, A.; Hua, H.; Moroziewicz, D.N.; Sproul, A.A.; Dusenberry, C.R.; Shang, L.; Paull, D.; Zimmer, M. Improved methods for reprogramming human dermal fibroblasts using fluorescence activated cell sorting. PLoS ONE 2013, 8, e59867. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, C.; Sasaki, K.; Yokobayashi, S.; Kojima, Y.; Saitou, M. Generation of human oogonia from induced pluripotent stem cells in culture. Nat. Protoc. 2020, 15, 1560–1583. [Google Scholar] [CrossRef]
- Saitou, M.; Miyauchi, H. Gametogenesis from pluripotent stem cells. Cell Stem Cell 2016, 18, 721–735. [Google Scholar] [CrossRef]
- Tang, W.W.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef]
- Seki, Y.; Yamaji, M.; Yabuta, Y.; Sano, M.; Shigeta, M.; Matsui, Y.; Saga, Y.; Tachibana, M.; Shinkai, Y.; Saitou, M. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 2007, 134, 2627–2638. [Google Scholar] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Surani, M.A.; Hayashi, K.; Hajkova, P. Genetic and epigenetic regulators of pluripotency. Cell 2007, 128, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, Y.; Payer, B.; O'Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Kurimoto, K.; Ohinata, Y.; Seki, Y.; Saitou, M. Gene expression dynamics during germline specification in mice identified by quantitative single-cell gene expression profiling. Biol. Reprod. 2006, 75, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Sasaki, K.; Yokobayashi, S.; Sakai, Y.; Nakamura, T.; Yabuta, Y.; Nakaki, F.; Nagaoka, S.; Woltjen, K.; Hotta, A. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 2017, 21, 517–532.e515. [Google Scholar] [CrossRef]
- Nakamura, T.; Okamoto, I.; Sasaki, K.; Yabuta, Y.; Iwatani, C.; Tsuchiya, H.; Seita, Y.; Nakamura, S.; Yamamoto, T.; Saitou, M. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 2016, 537, 57–62. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Morohaku, K.; Tanimoto, R.; Sasaki, K.; Kawahara-Miki, R.; Kono, T.; Hayashi, K.; Hirao, Y.; Obata, Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9021–9026. [Google Scholar] [CrossRef]
- Eguizabal, C.; Montserrat, N.; Vassena, R.; Barragan, M.; Garreta, E.; Garcia-Quevedo, L.; Vidal, F.; Giorgetti, A.; Veiga, A.; Belmonte, J.I. Complete meiosis from human induced pluripotent stem cells. Stem Cells 2011, 29, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Ramathal, C.; Durruthy-Durruthy, J.; Sukhwani, M.; Arakaki, J.E.; Turek, P.J.; Orwig, K.E.; Pera, R.A.R. Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Rep. 2014, 7, 1284–1297. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Easley IV, C.A.; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012, 2, 440–446. [Google Scholar]
- Durruthy-Durruthy, R.; Gottlieb, A.; Hartman, B.H.; Waldhaus, J.; Laske, R.D.; Altman, R.; Heller, S. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell 2014, 157, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yu, Y. Research Advances in Gametogenesis and Embryogenesis Using Pluripotent Stem Cells. Front. Cell Dev. Biol. 2021, 9, 801468. [Google Scholar] [PubMed]
- Yang, S.; Liu, Z.; Wu, S.; Zou, L.; Cao, Y.; Xu, H.; Huang, J.; Tian, Q.; Wu, F.; Li, P. Meiosis resumption in human primordial germ cells from induced pluripotent stem cells by in vitro activation and reconstruction of ovarian nests. Stem Cell Res. Ther. 2022, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Popp, B.; Krumbiegel, M.; Grosch, J.; Sommer, A.; Uebe, S.; Kohl, Z.; Plötz, S.; Farrell, M.; Trautmann, U.; Kraus, C. Need for high-resolution genetic analysis in iPSC: Results and lessons from the ForIPS consortium. Sci. Rep. 2018, 8, 17201. [Google Scholar] [CrossRef]
- Cyranoski, D. Japan Set to Allow Gene Editing in Human Embryos: Draft Guidelines Permit Gene-Editing Tools for Research into Early Human Development. 10 May 2018. Available online: https://www.nature.com/articles/d41586-018-06847-7 (accessed on 1 October 2021).
- Grive, K.J. Pathways coordinating oocyte attrition and abundance during mammalian ovarian reserve establishment. Mol. Reprod. Dev. 2020, 87, 843–856. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef]
- De Almeida, P.E.; Ransohoff, J.D.; Nahid, A.; Wu, J.C. Immunogenicity of pluripotent stem cells and their derivatives. Circ. Res. 2013, 112, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Polonio, A.M.; García-Velasco, J.A.; Herraiz, S. Stem cell paracrine signaling for treatment of premature ovarian insufficiency. Front. Endocrinol. 2021, 11, 626322. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Szwedowicz, U.; Łapińska, Z.; Gajewska-Naryniecka, A.; Choromańska, A. Exosomes and Other Extracellular Vesicles with High Therapeutic Potential: Their Applications in Oncology, Neurology, and Dermatology. Molecules 2022, 27, 1303. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.; Song, K.; Wei, J.; Yao, S.; Li, Z.; Su, X.; Ju, X.; Chao, L.; Deng, X. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci. Rep. 2017, 7, 2552. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T. HucMSC-derived exosomes mitigate the age-related retardation of fertility in female mice. Mol. Ther. 2020, 28, 1200–1213. [Google Scholar] [CrossRef]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef]

| Stem Cells | Model | Effect | Reference |
|---|---|---|---|
| Bone Marrow Stromal Cell | Rabbit | Increasing VEGF secretion | [152] |
| Mice | The development of fresh primordial follicles | [162] | |
| Rat | Raising the E2 level, follicle numbers, and ovarian weight | [170] | |
| Rat | Stopping the apoptosis of GCs | [199] | |
| Rat | Follicle counts, E2, and AMH levels rising | [200] | |
| Adipose-Derived Stem Cell | Mice | Increasing the proportion of follicles with a healthy structure | [201] |
| Mice | Growth of follicles at various phases and ovulation | [155] | |
| Rat | Rising E2 levels, follicle numbers, and pregnancy rates | [202] | |
| Menstrual Blood-Derived Mesenchymal Stem Cell | Mice | Boost the E2 level, follicle count, and ovarian weight | [156] |
| Rat | Boost levels of AMH, E2, and progesterone | [203] | |
| Mice | Stopping the apoptosis of GCs | [204] | |
| Umbilical Cord Mesenchymal Stem Cell | Murine | Rising ovarian weight, follicle numbers, and AMH levels; increasing follicular expression of Inhibin A and FSHR | [205] |
| Rat | Improvement of the endocrine secretion system, folliculogenesis, and inhibition of GCs apoptosis | [176] | |
| Rat | Estrus cycle recovery, sex hormone levels, and fertility | [206] | |
| Mice | Decrease in GC apoptosis, rise in sex hormone levels, and increase in follicle count | [150] | |
| Mice | AMH and E2 levels, ovarian angiogenesis, follicle count, and volume are all increasing | [207] | |
| Rat | Follicular numbers and E2 levels rising | [208] | |
| Amniotic Fluid Stem Cell | Mice | Follicular atresia prevention while maintaining healthy follicles | [168] |
| Mice | Development of new ovarian cells | [209] | |
| Mice | GC apoptosis and follicular atresia inhibition | [171] | |
| Amnion-Derived Mesenchymal Stem Cell | Rat | GCs’ apoptosis is inhibited, ovarian angiogenesis is increased, and follicular growth is accelerated | [142] |
| Rat | Reduction of GC apoptosis, growth of follicles, and elevation of AMH levels | [144] | |
| Rat | Reducing inflammation | [210] | |
| Placenta Mesenchymal Stem Cell | Mice | GC apoptotic inhibition and elevated E2 levels | [211] |
| Mice | GCs’ apoptosis is inhibited, and ovarian function is improved | [212] | |
| Mice | Rising follicular counts, E2 levels, and AMH levels | [213] | |
| Mice | Stopping the apoptosis of GCs | [214] | |
| Induced pluripotent stem cells | Mice | Vimentin and fibronectin expression in ovarian tissue was decreased, while ovarian weight and E2 levels were both enhanced | [195] |
| Murine | Ovarian tissue expanded, ovarian granulosa cell markers were expressed, estradiol levels rose, and the number of atretic follicles decreased | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Padhiar, A.A.; Wang, T.; He, L.; Chen, M.; Wu, S.; Zhou, Y.; Zhou, G. Stem Cell-Based Therapeutic Strategies for Premature Ovarian Insufficiency and Infertility: A Focus on Aging. Cells 2022, 11, 3713. https://doi.org/10.3390/cells11233713
Ali I, Padhiar AA, Wang T, He L, Chen M, Wu S, Zhou Y, Zhou G. Stem Cell-Based Therapeutic Strategies for Premature Ovarian Insufficiency and Infertility: A Focus on Aging. Cells. 2022; 11(23):3713. https://doi.org/10.3390/cells11233713
Chicago/Turabian StyleAli, Ilyas, Arshad Ahmed Padhiar, Ting Wang, Liangge He, Mingzhuang Chen, Shengda Wu, Yan Zhou, and Guangqian Zhou. 2022. "Stem Cell-Based Therapeutic Strategies for Premature Ovarian Insufficiency and Infertility: A Focus on Aging" Cells 11, no. 23: 3713. https://doi.org/10.3390/cells11233713
APA StyleAli, I., Padhiar, A. A., Wang, T., He, L., Chen, M., Wu, S., Zhou, Y., & Zhou, G. (2022). Stem Cell-Based Therapeutic Strategies for Premature Ovarian Insufficiency and Infertility: A Focus on Aging. Cells, 11(23), 3713. https://doi.org/10.3390/cells11233713








