Manganese Deficiency Suppresses Growth and Photosynthetic Processes but Causes an Increase in the Expression of Photosynthetic Genes in Scots Pine Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determining the Fresh Weight and Water Content
2.3. Determining the Morphometric Parameters
2.4. Determining the Contents of Essential Elements
2.5. Determination of Photosynthetic Pigments
2.6. Evaluating the Level of Lipid Peroxidation
2.7. Determination of Chlorophyll Fluorescence
2.8. RNA Extraction and Quantitative RT-PCR
2.9. Statistical Analysis
3. Results
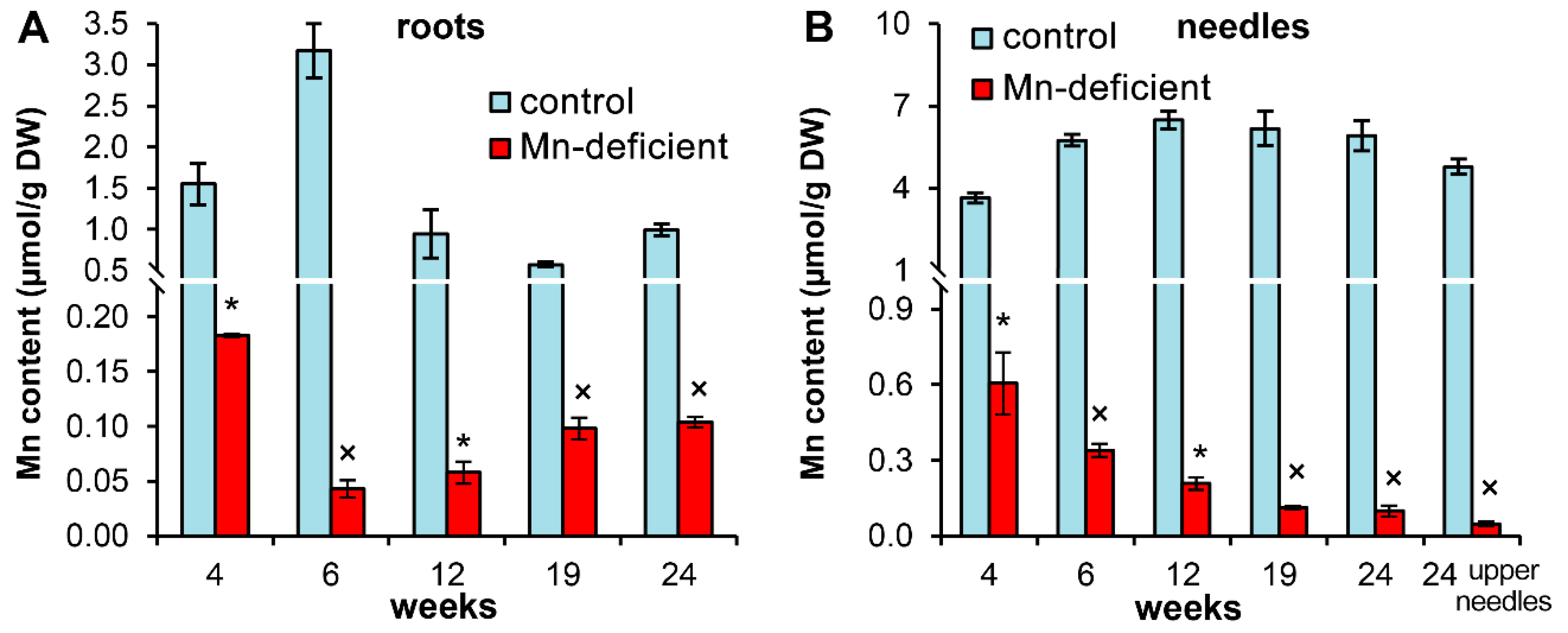
3.1. The Mn Content in the Nutrient Solutions, Seeds and Seedling Organs
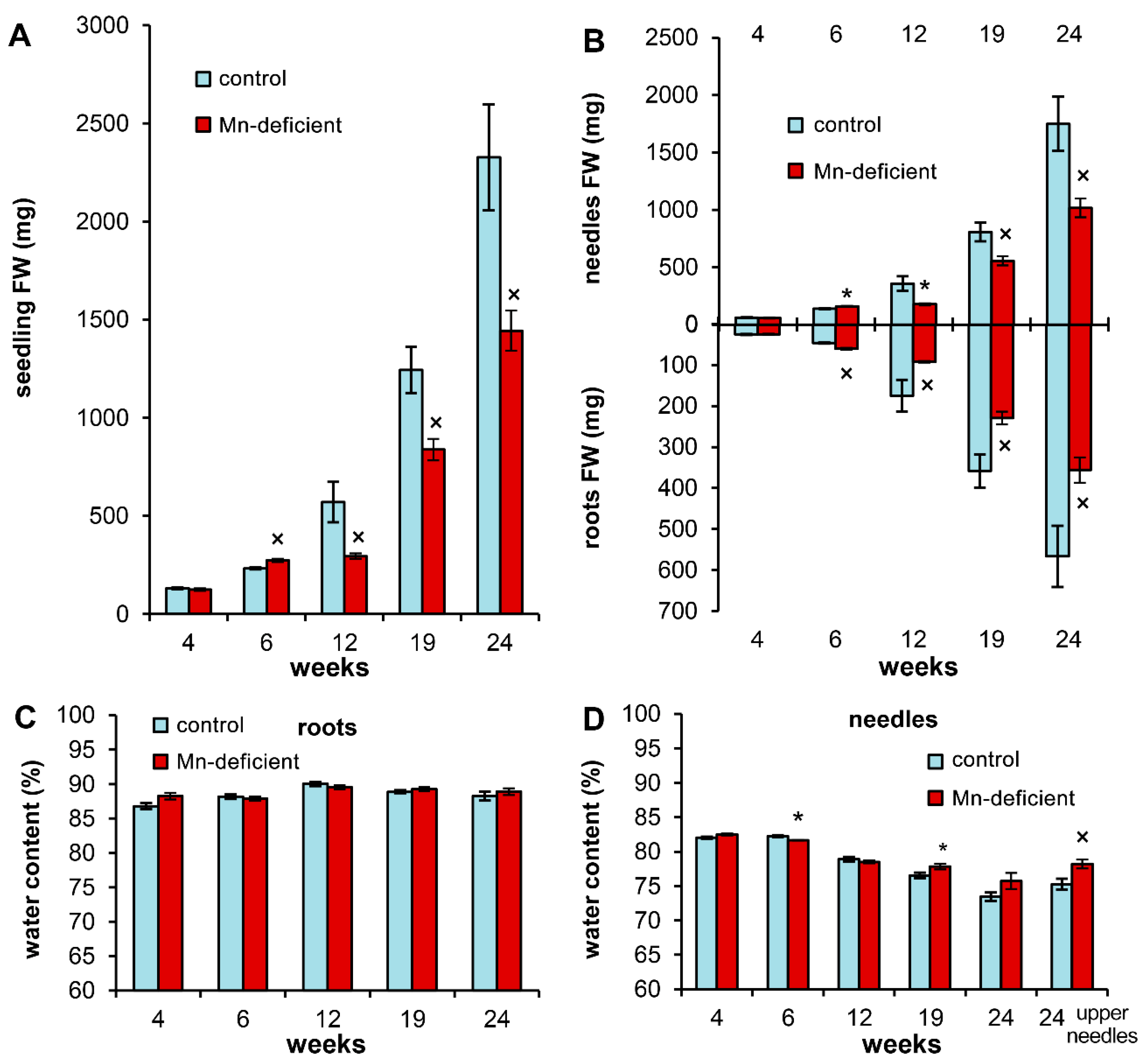
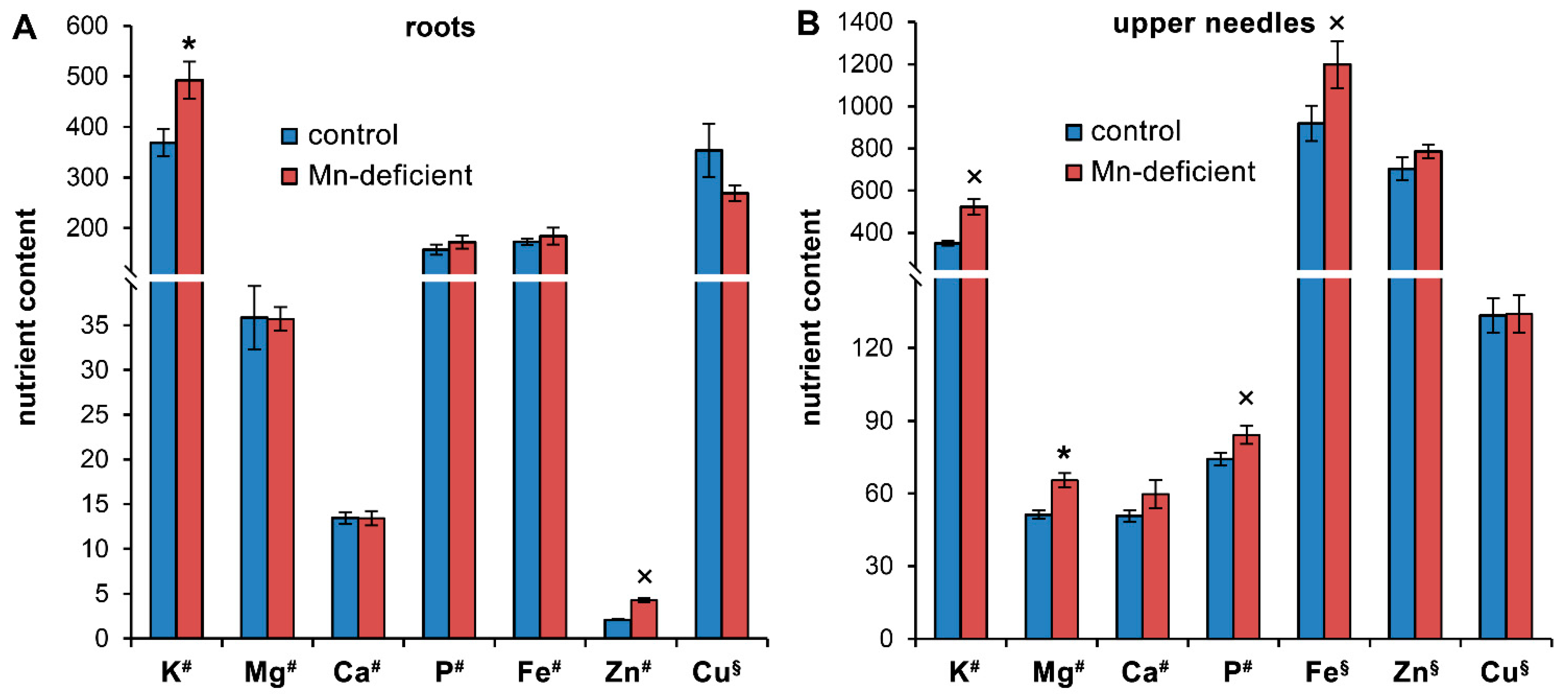
3.2. Growth Disturbances and Ion Homeostasis under Mn Deficiency
3.3. Contents of Photosynthetic Pigments and Lipid Peroxidation Products
3.4. Chlorophyll Fluorescence
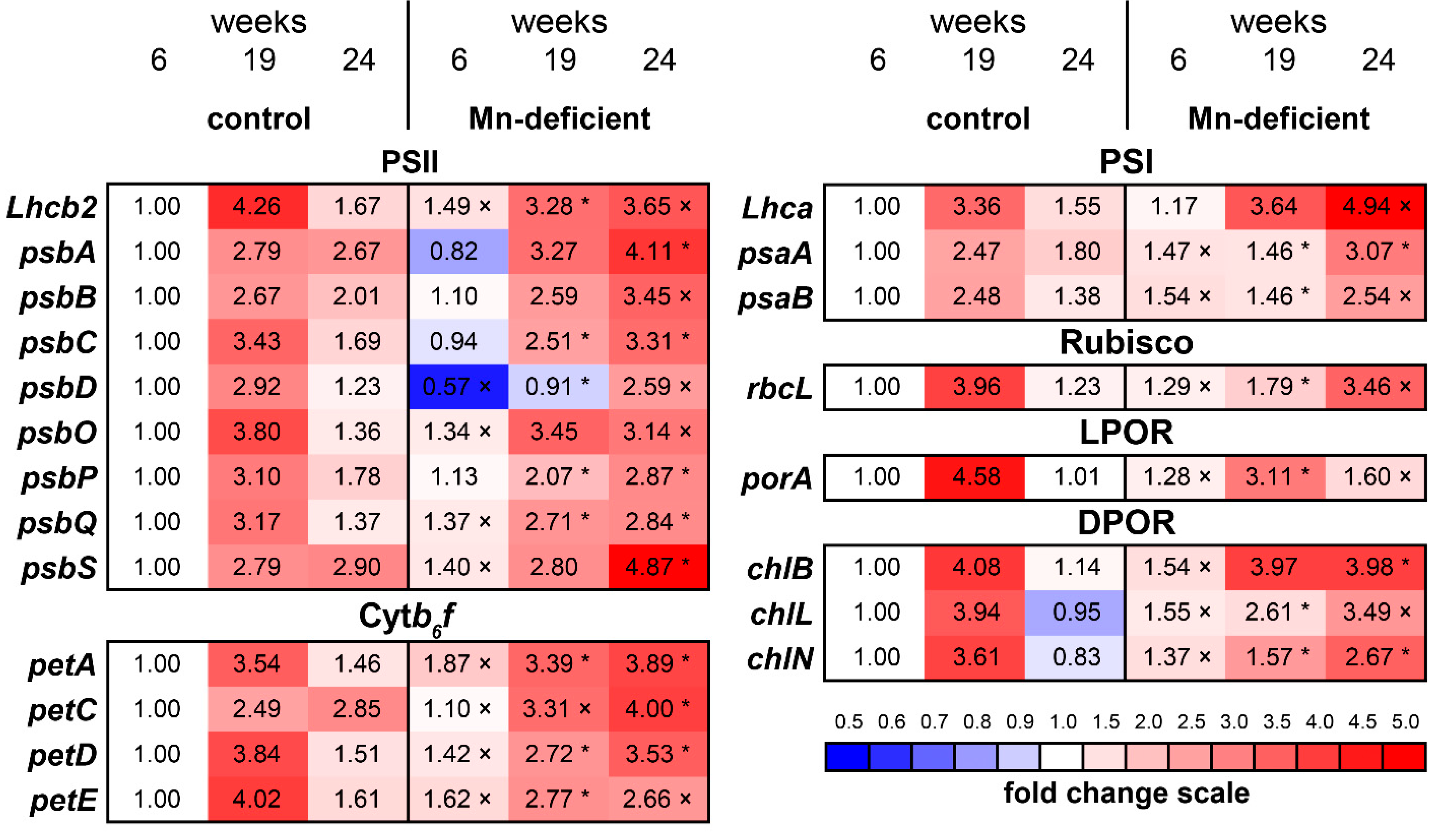
3.5. Transcript Levels of Photosynthetic Genes and Genes Involved in Chlorophyll Biosynthesis
4. Discussion
4.1. Development of Mn Deficiency and Features of Scots Pine Seedling Growth
4.2. Physiological Processes in Scots Pine Seedlings under Mn Deficiency
4.3. Ion Homeostasis under Mn Deficiency
4.4. The Transcript Levels of Photosynthetic Genes under Mn Deficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, B.; Erhagen, B.; Johansson, M.-B.; Nilsson, M.; Stendahl, J.; Trum, F.; Vesterdal, L. Manganese in the Litter Fall-Forest Floor Continuum of Boreal and Temperate Pine and Spruce Forest Ecosystems—A Review. For. Ecol. Manag. 2015, 358, 248–260. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Renger, G.; Hołynńska, M.; Moghaddam, A.N.; Aro, E.-M.; Carpentier, R.; Nishihara, H.; Eaton-Rye, J.J.; Shen, J.-R.; Allakhverdiev, S.I. Manganese Compounds as Water-Oxidizing Catalysts: From the Natural Water-Oxidizing Complex to Nanosized Manganese Oxide Structures. Chem. Rev. 2016, 116, 2886–2936. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Husted, S. The Biochemical Properties of Manganese in Plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Chapter 7-Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. ISBN 978-0-12-384905-2. [Google Scholar]
- Andresen, E.; Peiter, E.; Küpper, H. Trace Metal Metabolism in Plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Römheld, V. Chapter 11-Diagnosis of Deficiency and Toxicity of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 299–312. ISBN 978-0-12-384905-2. [Google Scholar]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese Deficiency in Plants: The Impact on Photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-32714-1. [Google Scholar]
- Reisenauer, H.M. Determination of Plant-Available Soil Manganese. In Manganese in Soils and Plants; Springer: Dordrecht, The Netherlands, 1988; pp. 87–98. [Google Scholar]
- Grey, D.C. A Review of the Role of Manganese in Pine Plantations. S. Afr. For. J. 1988, 145, 42–46. [Google Scholar] [CrossRef]
- Murashkina, M.A.; Koptsik, G.N.; Southard, R.J.; Chizhikova, N.P. Iron, Aluminum, Silicon, and Manganese Compounds in Soils of Forest Ecosystems of the Taiga Zone. Eurasian Soil Sci. 2004, 37, 31–39. [Google Scholar]
- Madgwick, H.A.I.; Jackson, D.S.; Knight, P.J. Above-Ground Dry Matter, Energy, and Nutrient Contents of Trees in an Age Series of Pinus Radiata Plantations. N. Z. J. For. Sci. 1977, 7, 445–468. [Google Scholar]
- Gielen, S.; i Batlle, J.V.; Vincke, C.; Van Hees, M.; Vandenhove, H. Concentrations and Distributions of Al, Ca, Cl, K, Mg and Mn in a Scots Pine Forest in Belgium. Ecol. Model. 2016, 324, 1–10. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Zlobin, I.E.; Kartashov, A.V.; Ivanova, A.I.; Ivanov, V.P.; Marchenko, S.I.; Nartov, D.I.; Kuznetsov, V.V. Mineral Nutrition of Naturally Growing Scots Pine and Norway Spruce under Limited Water Supply. Plants 2022, 11, 2652. [Google Scholar] [CrossRef] [PubMed]
- Juranović Cindrić, I.; Zeiner, M.; Starčević, A.; Stingeder, G. Metals in Pine Needles: Characterisation of Bio-Indicators Depending on Species. Int. J. Environ. Sci. Technol. 2019, 16, 4339–4346. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of Zinc on Scots Pine (Pinus sylvestris L.) Seedlings Grown in Hydroculture. Plant Physiol. Biochem. 2016, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.V.; Ivanova, A.I.; Kartashov, A.V.; Kuznetsov, V.V. Phytotoxicity of Short-Term Exposure to Excess Zinc or Copper in Scots Pine Seedlings in Relation to Growth, Water Status, Nutrient Balance, and Antioxidative Activity. Environ. Sci. Pollut. Res. 2021, 28, 14828–14843. [Google Scholar] [CrossRef] [PubMed]
- Kavvadias, V.; Miller, H. Manganese and Calcium Nutrition of Pinus Sylvestris and Pinus Nigra from Two Different Origins. I. Manganese. For. Int. J. For. Res. 1999, 72, 35–46. [Google Scholar] [CrossRef]
- Oleksyn, J.; Reich, P.B.; Zytkowiak, R.; Karolewski, P.; Tjoelker, M.G. Needle Nutrients in Geographically Diverse Pinus sylvestris L. Populations. Ann. For. Sci. 2002, 59, 1–18. [Google Scholar] [CrossRef]
- Brække, F.; Salih, N. Reliability of Foliar Analyses of Norway Spruce Stands in a Nordic Gradient. Silva Fenn. 2002, 36, 489–504. [Google Scholar] [CrossRef][Green Version]
- Morrison, I.K.; Armson, K.A. Influence of Manganese on Growth of Jack Pine and Black Spruce Seedlings. For. Chron. 1968, 44, 32–35. [Google Scholar] [CrossRef][Green Version]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of Copper Deficiency and Copper Toxicity on Organogenesis and Some Physiological and Biochemical Responses of Scots Pine (Pinus sylvestris L.) Seedlings Grown in Hydroculture. Environ. Sci. Pollut. Res. 2016, 23, 17332–17344. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Ivanov, V.P.; Marchenko, S.I.; Nartov, D.I.; Kuznetsov, V.V. Long-Term Impact of Cement Plant Emissions on the Elemental Composition of Both Soils and Pine Stands and on the Formation of Scots Pine Seeds. Environ. Pollut. 2018, 243, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The Molybdenum Blue Reaction for the Determination of Orthophosphate Revisited: Opening the Black Box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.-C.; Chaudiere, J. Reactions of 1-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. Chlorophyll A Fluoresc. 2004, 19, 279–319. [Google Scholar]
- Kolosova, N.; Miller, B.; Ralph, S.; Ellis, B.E.; Douglas, C.; Ritland, K.; Bohlmann, J. Isolation of High-Quality RNA from Gymnosperm and Angiosperm Trees. Biotechniques 2004, 36, 821–824. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Vankova, R.; Zlobin, I.E.; Dobrev, P.; Ivanov, Y.V.; Kartashov, A.V.; Kuznetsov, V.V. Comparative Analysis of Abscisic Acid Levels and Expression of Abscisic Acid-Related Genes in Scots Pine and Norway Spruce Seedlings under Water Deficit. Plant Physiol. Biochem. 2019, 140, 105–112. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells 2021, 10, 3284. [Google Scholar] [CrossRef]
- Nicol, L.; Croce, R. The PsbS Protein and Low PH Are Necessary and Sufficient to Induce Quenching in the Light-Harvesting Complex of Plants LHCII. Sci. Rep. 2021, 11, 7415. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Murakami, A. Changes in Levels of Thylakoid Components in Chloroplasts of Pine Needles of Different Ages. Plant Cell Physiol. 1996, 37, 1102–1107. [Google Scholar] [CrossRef]
- Ulrich, A.; Hills, F. Principles and Practices of Plant Analysis. In Soil Testing and Plant Analysis Part II Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1967; pp. 11–24. [Google Scholar]
- Papadakis, I.E.; Giannakoula, A.; Therios, I.N.; Bosabalidis, A.M.; Moustakas, M.; Nastou, A. Mn-Induced Changes in Leaf Structure and Chloroplast Ultrastructure of Citrus volkameriana (L.) Plants. J. Plant Physiol. 2007, 164, 100–103. [Google Scholar] [CrossRef]
- Long, L.; Pedas, P.R.; Kristensen, R.K.; Schulze, W.X.; Husted, S.; Zhang, G.; Schjoerring, J.K.; Yuan, L. High Light Intensity Aggravates Latent Manganese Deficiency in Maize. J. Exp. Bot. 2020, 71, 6116–6127. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Ponomarev, E.I.; Ivanova, G.A.; Dvinskaya, M.L.; Coogan, S.C.; Flannigan, M.D. Wildfires in the Siberian Taiga. Ambio 2021, 50, 1953–1974. [Google Scholar] [CrossRef] [PubMed]
- Zlobin, I.E.; Kartashov, A.V.; Pashkovskiy, P.P.; Ivanov, Y.V.; Kreslavski, V.D.; Kuznetsov, V.V. Comparative Photosynthetic Responses of Norway Spruce and Scots Pine Seedlings to Prolonged Water Deficiency. J. Photochem. Photobiol. B Biol. 2019, 201, 111659. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Tsai, Y.-H.; Wen, T.-N.; Wu, Y.-C.; Curie, C.; Schmidt, W. Systems-Wide Analysis of Manganese Deficiency-Induced Changes in Gene Activity of Arabidopsis Roots. Sci. Rep. 2016, 6, 35846. [Google Scholar] [CrossRef] [PubMed]
- Henriques, F.S. Gas Exchange, Chlorophyll a Fluorescence Kinetics and Lipid Peroxidation of Pecan Leaves with Varying Manganese Concentrations. Plant Sci. 2003, 165, 239–244. [Google Scholar] [CrossRef]
- Wei Yang, T.J.; Perry, P.J.; Ciani, S.; Pandian, S.; Schmidt, W. Manganese Deficiency Alters the Patterning and Development of Root Hairs in Arabidopsis. J. Exp. Bot. 2008, 59, 3453–3464. [Google Scholar] [CrossRef]
- Anderson, J.M.; Pyliotis, N.A. Studies with Manganese-Deficient Spinach Chloroplasts. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1969, 189, 280–293. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of Photosystem II under Environmental Stress. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Allen, M.D.; Kropat, J.; Tottey, S.; Del Campo, J.A.; Merchant, S.S. Manganese Deficiency in Chlamydomonas Results in Loss of Photosystem II and MnSOD Function, Sensitivity to Peroxides, and Secondary Phosphorus and Iron Deficiency. Plant Physiol. 2007, 143, 263–277. [Google Scholar] [CrossRef]
- Wingsle, G.; Gardeström, P.; Hällgren, J.-E.; Karpinski, S. Isolation, Purification, and Subcellular Localization of Isozymes of Superoxide Dismutase from Scots Pine (Pinus sylvestris L.) Needles 1. Plant Physiol. 1991, 95, 21–28. [Google Scholar] [CrossRef]
- Eisenhut, M.; Hoecker, N.; Schmidt, S.B.; Basgaran, R.M.; Flachbart, S.; Jahns, P.; Eser, T.; Geimer, S.; Husted, S.; Weber, A.P. The Plastid Envelope CHLOROPLAST MANGANESE TRANSPORTER1 Is Essential for Manganese Homeostasis in Arabidopsis. Mol. Plant 2018, 11, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative Stress and Antioxidant Responses of Mulberry (Morus alba) Plants Subjected to Deficiency and Excess of Manganese. Acta Physiol. Plant. 2013, 35, 3345–3356. [Google Scholar] [CrossRef]
- Ifuku, K.; Noguchi, T. Structural Coupling of Extrinsic Proteins with the Oxygen-Evolving Center in Photosystem II. Front. Plant Sci. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.; Venkatesh, J.; Daneshi, R.F.; Gururani, M.A. Photosystem II Extrinsic Proteins and Their Putative Role in Abiotic Stress Tolerance in Higher Plants. Plants 2018, 7, 100. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A New Paradigm for the Action of Reactive Oxygen Species in the Photoinhibition of Photosystem II. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef]
| Variant | 4th Week | 6th Week |
|---|---|---|
| Control | 113.7 ± 12.9 | 228.3 ± 14.3 |
| Mn-deficient | 53.9 ± 2.8 * | 70.3 ± 2.3 × |
| Parameter | Control | Mn-Deficient | ||
|---|---|---|---|---|
| 4th Week | 6th Week | 4th Week | 6th Week | |
| Primary root length, mm | 163.8 ± 6.0 | 222.3 ± 3.3 | 157.1 ± 8.4 | 244.4 ± 4.7 × |
| The distance from the tip of the main root to the first lateral root, mm | 64.4 ± 3.4 | 61.8 ± 1.9 | 67.1 ± 6.0 | 65.6 ± 2.1 × |
| The number of first-order lateral roots, pieces | 35.2 ± 2.2 | 53.6 ± 1.3 | 28.1 ± 4.3 | 50.8 ± 1.6 |
| Average hypocotyls length, mm | 30.1 ± 0.7 | 29.4 ± 0.3 | 28.3 ± 1.5 | 30.4 ± 0.5 |
| Average needle length, mm | 28.3 ± 0.7 | 33.5 ± 0.3 | 27.6 ± 1.0 | 35.1 ± 0.5 × |
| Number of needles, pieces | 19.4 ± 0.6 | 34.4 ± 0.5 | 17.9 ± 0.8 | 35.9 ± 0.8 |
| Parameter | Control | Mn-Deficient | ||||
|---|---|---|---|---|---|---|
| 6th Week | 19th Week | 24th Week | 6th Week | 19th Week | 24th Week | |
| Chl a, mg/g DW | 7.09 ± 0.20 | 7.22 ± 0.58 | 4.99 ± 0.31 | 6.51 ± 0.19 | 6.93 ± 0.29 | 3.13 ± 0.12 * |
| Chl b, mg/g DW | 2.92 ± 0.09 | 2.97 ± 0.24 | 2.09 ± 0.15 | 2.80 ± 0.08 | 3.23 ± 0.14 | 1.50 ± 0.02 × |
| Carotenoids, mg/g DW | 1.12 ± 0.03 | 1.06 ± 0.08 | 0.78 ± 0.04 | 1.06 ± 0.03 | 1.11 ± 0.04 | 0.60 ± 0.01 * |
| Chl a/Chl b | 2.43 ± 0.02 | 2.43 ± 0.01 | 2.39 ± 0.03 | 2.32 ± 0.02 * | 2.15 ± 0.04 × | 2.09 ± 0.09 × |
| Car/Chls | 0.112 ± 0.001 | 0.104 ± 0.003 | 0.110 ± 0.002 | 0.114 ± 0.002 | 0.109 ± 0.002 | 0.130 ± 0.005 * |
| Parameter | Roots | Needles | ||
| Control | Mn-Deficient | Control | Mn-Deficient | |
| MDA, nmol/g DW | 81.6 ± 7.4 | 67.2 ± 3.4 | 53.9 ± 2.1 | 57.7 ± 2.3 |
| 4-HNE, nmol/g DW | 183.9 ± 25.5 | 188.6 ± 9.0 | 933.3 ± 114.3 | 1093.8 ± 127.6 |
| Parameter | Control | Mn-Deficient |
|---|---|---|
| Fv/Fm | 0.836 ± 0.004 | 0.629 ± 0.008 * |
| F0 | 175.3 ± 11.3 | 291.7 ± 36.0 * |
| Fm | 1075.0 ± 82.5 | 781.7 ± 92.9 * |
| Y(II) | 0.531 ± 0.013 | 0.328 ± 0.014 * |
| Y(NO) | 0.281 ± 0.011 | 0.406 ± 0.011 * |
| Y(NPQ) | 0.188 ± 0.016 | 0.266 ± 0.013 * |
| qP | 0.715 ± 0.016 | 0.792 ± 0.017 * |
| ETRII | 27.7 ± 0.6 | 17.2 ± 0.7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.V.; Pashkovskiy, P.P.; Ivanova, A.I.; Kartashov, A.V.; Kuznetsov, V.V. Manganese Deficiency Suppresses Growth and Photosynthetic Processes but Causes an Increase in the Expression of Photosynthetic Genes in Scots Pine Seedlings. Cells 2022, 11, 3814. https://doi.org/10.3390/cells11233814
Ivanov YV, Pashkovskiy PP, Ivanova AI, Kartashov AV, Kuznetsov VV. Manganese Deficiency Suppresses Growth and Photosynthetic Processes but Causes an Increase in the Expression of Photosynthetic Genes in Scots Pine Seedlings. Cells. 2022; 11(23):3814. https://doi.org/10.3390/cells11233814
Chicago/Turabian StyleIvanov, Yury V., Pavel P. Pashkovskiy, Alexandra I. Ivanova, Alexander V. Kartashov, and Vladimir V. Kuznetsov. 2022. "Manganese Deficiency Suppresses Growth and Photosynthetic Processes but Causes an Increase in the Expression of Photosynthetic Genes in Scots Pine Seedlings" Cells 11, no. 23: 3814. https://doi.org/10.3390/cells11233814
APA StyleIvanov, Y. V., Pashkovskiy, P. P., Ivanova, A. I., Kartashov, A. V., & Kuznetsov, V. V. (2022). Manganese Deficiency Suppresses Growth and Photosynthetic Processes but Causes an Increase in the Expression of Photosynthetic Genes in Scots Pine Seedlings. Cells, 11(23), 3814. https://doi.org/10.3390/cells11233814








