Abstract
The effect of red (RL, 660 nm) and blue (BL, 450 nm) light on phy mutant tomato plants was studied. The rates of photosynthesis (Pn) and transpiration, the efficiency of the primary photochemical processes of photosynthesis, the contents of flavonoids and phenolic compounds, the low-molecular-weight antioxidant capacity (Trolox equivalent antioxidant capacity (TEAC)) of leaf extracts, and the expression of light-dependent genes were evaluated. Under RL, BL, and white fluorescent light (WFL), the Pn values decreased in the order: WT > phyb2 > phyaphyb2 > phyaphyb1phyb2, except for the Pn in phyb2 on BL. Phyb2 also had a larger number of stomata under BL and, as a result, it reached maximum transpiration. The noticeable accumulation of flavonoids and phenolic compounds was observed only in the phyb2 and phyaphyb2 mutants upon irradiation with BL, which agrees with the increased TEAC in the leaf extracts. We suggest that the increased antioxidant activity under PHYB2 deficiency and the maintenance of high photosynthesis under BL are based on an increase in the expression of the early signaling transcription factors genes BBX, HY5. The largest decrease in the content of flavonoids and TEAC was manifested with a deficiency in PHYB1, which is probably the key to maintaining the antioxidant status in BL plants.
1. Introduction
Light controls many aspects of plant development through a complex signaling cascade, which, in addition to the photoreceptors, involves transcription factors (TFs), kinases, calmodulin, reactive oxygen species (ROS), etc. Light ensures the differentiation of chloroplasts under changing conditions, which makes it possible to regulate photosynthetic activity [1]. The phytochromes (PHYs) play one of the key roles in these processes. The phytochromes are red light (RL) and far-red light (FRL) sensors, and they regulate many developmental processes, including seed germination and hypocotyl growth. At least five types of phytochrome have been well identified; among them, the main ones are phytochrome A (PHYA) and phytochrome B (PHYB). PHYA is considered as the primary photoreceptor in the response to FRL, while PHYB plays the main role in the response to RL. The signal from phytochromes is transmitted either through various molecules (second messengers such as Ca2+, cAMP etc.) and/or through certain TFs to which photoreceptors bind in their active form [1,2]. The PHYs are inactive in the cytoplasm under the dark condition, and when exposed to RL, a change in the chromophore leads to rearrangements in the structure of apoproteins, which leads to their translocation to the nucleus [2,3]. In the nucleus, PHYs promote the degradation of phytochrome-interacting factors (PIFs), which suppress other positive photomorphogenesis regulators such as TFs HY5 and Golden2-like (GLKs) [4]. GLKs are required for the differentiation and maintenance chloroplast activity [3,5], while in A. thaliana, they are positively regulated by HY5 [6]. Other well-known receptors, the cryptochromes (CRY), perceive light in the blue and UV ranges of the spectrum. They are involved in the growth processes, de-etiolation of seedlings and circadian rhythms. They include two major cryptochromes (CRY1, CRY2) [7].
Changes in the phytochrome content significantly affect the content and activity of various components of phytochrome signaling as well as the genes for antioxidant enzymes and enzymes used for the biosynthesis of low molecular weight antioxidants and photosynthetic proteins [8]. This shift results in changes in the plant antioxidant status, photosynthesis efficiency, and other metabolic responses [8]. One of the main components of phytochrome signaling is TFs, which are involved in regulating most light-dependent genes. Zinc finger TFs constitute one of the most important families of transcription regulators in plants and play a central role in regulating plant growth and development under normal and stress conditions [9,10]. Among these TFs are B-box domain-containing proteins (BBX) that mediate their effect through interactions with components of the light signaling network, including TFs HY5, HYH and PIFs as well as the ubiquitin ligase COP1 [11]. For example, AtBBX21 and AtBBX22 contribute to the accumulation of transcripts for HY5, which is a key component of phytochrome signaling and can be labeled for proteasomal degradation via COP1-mediated ubiquitination [12]. In contrast, AtBBX24 and AtBBX25 suppress light signaling through interactions with HYH and HY5 [13]. Interestingly, AtBBX28 has been characterized as a light-induced repressor, because it could downregulate the transcriptional activity of HY5, allowing degradation in the dark via COP1 [14]. However, a signaling cascade of the TFs PIF3 and PIF1 has been shown to regulate the transcription of AtBBX23, the product of which interacts with HY5 to induce photomorphogenesis in A. thaliana seedlings [10,15]. These studies show that numerous BBX proteins, along with COP1 and HY5, play a critical role in light-dependent plant development. Additionally, the mechanisms of regulation for photomorphogenesis and the role of BBX in these processes have not been sufficiently studied. The tomato is a major vegetable crop that has a wide range of photoreceptor mutants, and these mutants are widely used in experiments to study the effect of light regulation on various physiological processes, including photosynthesis and light signaling [16].
The aim of this research was to study the role of various phytochromes in light signaling in tomato plants as well as to elucidate the extent to which this signaling determines the formation of flavonoids and other low-molecular weight antioxidants and affects photosynthesis in tomato leaves. We hypothesized that one of the possible reasons for the effect of phytochrome deficiency under the action of blue (BL) and red light (RL) is an increase in the expression of early response genes for TFs—BBX and HY5 and a number of other light-dependent genes, which ultimately leads to the observed phenomena.
2. Materials and Methods
2.1. Plant Materials and Experimental Design
In the experiments, wild-type (WT) Solanum lycopersicum L. plants (Moneymaker cultivar, LA2706) and photoreceptor mutants phyb2 (LA4358), phyaphyb2 (LA4362), and phyaphyb1phyb2 (LA4366) were used. The seeds were obtained from the Tomato Genetics Resource Center (TGRC) (University of California, Davis, CA, USA). The plants were grown for 30 days in a thermostatically controlled chamber with a 12 h photoperiod at a temperature of 23 ± 1 °C during the day and 23 ± 1 °C at night. Then, the plants were cloned using cuttings and grown for 2 weeks up to the age of 45 days. The plants were grown under white fluorescent lamps (WFL) (Philips, Poland) at a light intensity of 250 μmol photons m−2 s−1 in 8 × 8 × 10 cm vessels filled with perlite. Throughout the cultivation season, the plants were watered with a 2-fold diluted Hoagland nutrient solution. The experimental samples were irradiated for 0 d, 1 d and 7 d, 250 ± 15 μmol photons m−2 s−1 with red light (660 nm) and blue light (450 nm) LEDs (Epistar, Taiwan) (Figure S1). The spectral characteristics of the light sources were determined using an AvaSpecULS2048CL-EVO spectrometer (Avantes, The Netherlands). Plants used for the analysis of gene expression were sampled at 0 d, 1 d and 7 d of irradiation. All the remaining analyses, including microscopy, were produced on d 7 of the experiment. Six to ten of the most developed leaves from the second and third tiers were used for the analysis. Over the course of irradiation with light, determinations of the PSII activity and the intensity of photosynthesis and transpiration were performed on the leaves of intact plants; samples were taken simultaneously for microscopic analyses.
2.2. Analysis of Low Molecular Weight Antioxidants
The low-molecular-weight antioxidants were extracted with 80% methanol from leaves ground in liquid nitrogen.
The low-molecular-weight antioxidant capacity (Trolox equivalent antioxidant capacity (TEAC)) was determined spectrophotometrically according to the method described by Re et al. [17] involving the reaction of methanolic extracts with 2,2′-azino-bis [3-ethylbenzothiazoline-6-sulfonic acid] diammonium salt (ABTS) (Sigma-Aldrich, Burlington, MA, USA, CAS number 30931-67-0).
The total phenolics were determined spectrophotometrically using Folin–Ciocalteu phenol reagent (Sigma-Aldrich, Burlington, MA, USA; MDL number MFCD00132625) according to the procedure described by Singleton and Rossi [18]. The total phenolic content was expressed as gallic acid equivalents (GAE) in milligrams per gram of fresh weight (FW).
The total flavonoids were measured according to the methods of Kim et al. [19]. Afterwards, 1000 µL of distilled water, 150 µL of extracted sample and 50 µL of 5% NaNO2 were mixed together. After 6 min, 50 μL of 10% AlCl3 was added, and after another 5 min, 300 μL of 1 M NaOH was added to the mixture. The reaction mixture was homogenized, and after 10 min, the absorbance was measured at 510 nm. The total flavonoids were calculated by constructing a calibration curve using (+)-catechin hydrate (Sigma-Aldrich, Burlington, MA, USA, CAS Number 225937-10-0) and were expressed as milligrams of (+)-catechin per gram of FW [20].
2.3. Measurements of CO2 Gas Exchange and Transpiration
The photosynthetic rate (Pn) and transpiration rate (Tr) were determined in a closed system under light conditions using an LCPro + portable infrared gas analyzer from ADC BioScientific Ltd. (Hoddesdon, UK) that was connected to a leaf chamber with an area of 6.25 cm2. The CO2 uptake per leaf area (μmol m−2 s−1) was determined. The rate of photosynthesis of the leaves in the second layer from the top was determined at a saturating light intensity of 1000 μmol photons m−2 s−1. After the photosynthesis rate was measured, the light was turned off, and the rate of dark respiration was measured. The measurements were performed at 1000 μmol m−2 s−1 as well as before irradiation.
2.4. Determination of Photochemical Activity
The fluorescent induction curves were measured with a mini-PAM II fluorometer (Walz, Effeltrich, Germany) on plants adapted to the dark (30 min) as described earlier [21]. After a pulse of saturating light, the leaves of plants adapted to 30 min in the dark were kept in the dark for one minute, and then they were exposed to actinic light for 5 minutes, which was followed by saturating light pulses during which the parameters were measured. Blue LEDs (474 nm) were used to provide the measuring light (0.5 μmol photons m−2 s−1), actinic light (150 μmol photons m−2 s−1) and saturating pulses (474 nm, 5000 μmol photons m−2 s−1 and 800 ms duration). The parameter calculations on the basis of fluorescence data were performed using WinControl-3 v3.32 software (Walz, Effeltrich, Germany), and the formulas are taken from [22]. The values for the F0, Fv, Fm, Fm′ and F0′, as well as the PSII maximum (Fv/Fm) and effective Y(II) (Fm′ − Ft)/Fm′ photochemical quantum yields and nonphotochemical quenching (NPQ) (Fm/Fm′ − 1), were determined. Fm and Fm′ are the maximum Chl fluorescence levels under dark- and light-adapted conditions, respectively. Fv is the photoinduced change in fluorescence, and Ft is the level of fluorescence before a saturation impulse is applied. F0 is the initial Chl fluorescence level. Actinic light was switched on for 10 min (I = 125 µmol photons m−2 s−1). The quenching parameters were also determined: NPQ—nonphotochemical fluorescence quenching; Y(NO)—quantum yield of nonregulated nonphotochemical energy dissipation in PSII and Y(NPQ)—quantum yield of regulated nonphotochemical energy dissipation in PSII, Y(NO) + Y(NPQ) + Y(II) = 1.
2.5. RNA Extraction and RT-PCR
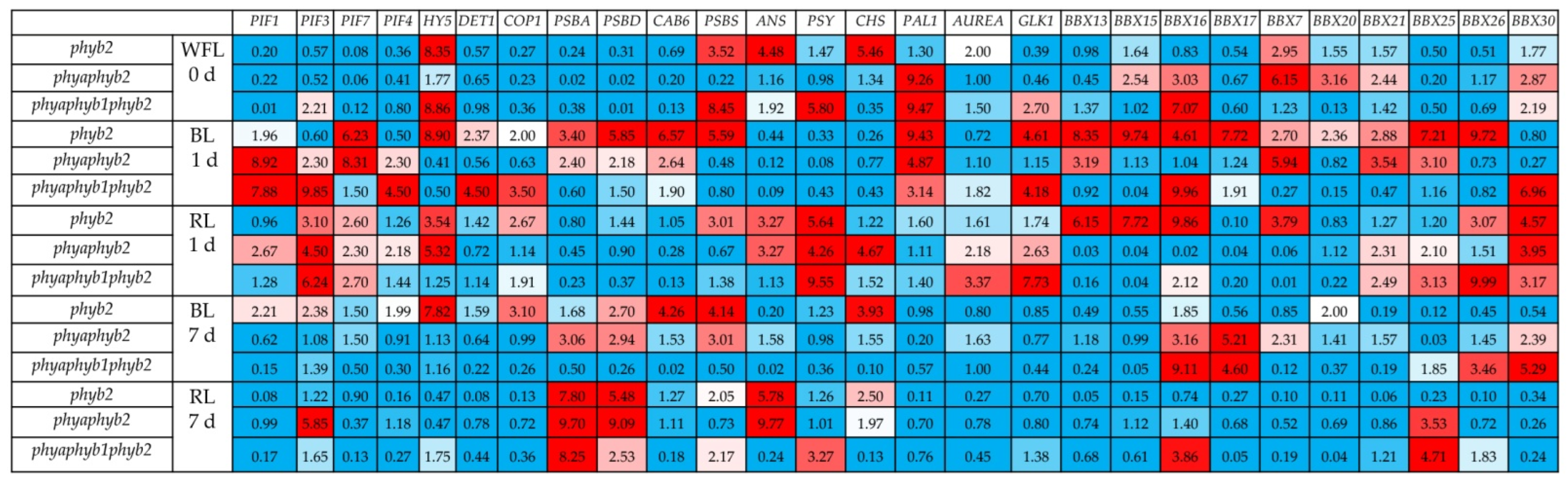
RNA isolation was performed according to the TRIzol method (Sigma-Aldrich, Burlington, MA, USA). The quantity and quality of the total RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA synthesis was performed using an M-MLV Reverse Transcriptase Kit (Fermentas, Waltham, MA, USA), an oligo (dT) 21 primer for nuclear encoding genes and a Random6 universal primer for chloroplast genes. The expression patterns of the genes were assessed using a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Gene-specific primers (Table S1) for anthocyanidin synthase (ANS NM_001374394.1), phytoene synthase (PSY NM_001247883.2), chalcone synthase (CHS NM_001247104.2), transcription factor elongated hypocotyl 5 (HY5 NM_001247891.2), transcription factor phytochrome-interacting factor 4 (PIF4 NM_001308008.1), E3 ubiquitin–protein ligase (COP1 NM_001247118.2), phenylalanine ammonia-lyase 1 (PAL1 XM_004249510.4), chloroplastic photosystem II nonphotochemical quenching protein (PSBS NM_001309257.1), de-etiolated1 (DET1 NM_001247219.2), phytochrome-interacting factor 1b (PIF1b Solyc06g008030), phytochrome-interacting factor 3 (PIF3 Solyc01g102300), phytochrome-interacting factor 7b (PIF7 Solyc06g069600) (primers taken from [23]); photosystem II protein D1(PSBA Q2MIC0), photosystem II D2 protein (PSBD A0A0C5CUN5), chlorophyll ab binding protein 6A (CAB6 P12360), phytochromobilin synthase (Aurea Q588D6), golden 2-like 1 transcription factor (GLK1, I6QQX8), B-box domain containing proteins (BBX13 Solyc04g007210), B-box domain containing proteins (BBX15 Solyc05g009310), B-box domain containing proteins (BBX16 Solyc12g005750), B-box domain containing proteins (BBX17 Solyc07g052620), B-box domain containing proteins (BBX7 Solyc12g006240), B-box domain containing proteins (BBX20 Solyc12g089240), B -box domain containing proteins (BBX21 Solyc04g081020) and B-box domain containing proteins (BBX30 Solyc06g063280) (primers taken from Bu et al., 2021) were selected using nucleotide sequences from the National Center for Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov, accessed on 1 March 2022), https://www.uniprot.org/ accessed on 1 March 2022, https://phytozome-next.jgi.doe.gov/ accessed on 1 March 2022, with Vector NTI Suite 9 software (Invitrogen, Waltham, MA USA). The transcript levels were normalized to the expression of the Actin1 gene. The gene expression in the wild type was given a value of 1. Changes in expression were considered significant with an increase or decrease in expression by at least 2 times relative to the WT control.
2.6. Scanning Electron Microscopy
The fragments (1 cm2) of fresh leaves from the middle part and the edge of the leaf blade were set on 2 cm × 4 cm copper plates. To obtain greater detail of the microstructure at high magnifications, the samples were frozen on a massive metal holder at −20 °C. The plate with a fresh sample was then fixed on the cooling stage of the Deben Coolstage refrigerating unit (UK) at −30 °C. The samples were imaged by a LEO-1430 VP (Carl Zeiss, Berlin, Germany) scanning electron microscope in high-vacuum mode operating at 20 kV with a backscattered electron detector QBSD and a working distance of 8–12 mm (cryoSEM).
2.7. Statistics
The fluorescence and CO2 gas exchange measurements were performed in four biological replicates. The scanning electron microscopy was performed in six biological replicates. Each plant sample fixed in liquid nitrogen was treated as a biological replicate; therefore, there were three biological replicates for the determination of Trolox equivalent antioxidant capacity, total phenol and flavonoids content as well as for gene expression analyses. For each of these experiments, at least three parallel independent measurements were performed. The significance of the differences among the groups was calculated by one-way analysis of variance (ANOVA) followed by Duncan’s method using SigmaPlot 12.3 (Systat Software Inc., San Jose, CA, USA). Letters indicate significant differences between the WT and the mutants (p < 0.05). The data are shown as the arithmetic means ± standard errors.
3. Results
3.1. Low-Molecular Weight Antioxidant Capacity
The highest antioxidant activity was observed in the phyb2 and phyaphyb2 mutants under WFL, while in the triple mutant and WT, this indicator was lower. Under RL, the antioxidant activity was the lowest in phyb2 among all the variants, and the highest value was in the double mutant. The impact of BL was significantly different in terms of nonenzymatic antioxidant activity in all the studied samples. The activity of antioxidants increased in all the variants; however, the highest value was in the phyb2 mutant (50.5), which was 2.6 times higher than in the WT under these conditions, and for the phyb2 mutant itself, it was the highest among the studied types of light (Table 1).

Table 1.
Effect of WFL, BL and RL light on photosynthetic rate Pn (µmol CO2 m−2 s−1), transpiration rate Tr (µmol H2O m−2 s−1), Trolox equivalent antioxidant capacity (TEAC), µM Trolox/g FW, gallic acid equivalents (GAE), mg/g FW, and content of flavonoids mg catechin/g FW in the leaves of tomato plants mutant for the genes encoding the main phytochromes. Different letters indicate significant differences (p < 0.05) between the experimental treatments.
3.2. Total Phenolics Content
When growing plants on WFL, the highest content of phenolic compounds was observed in the phyb2 (2.1 times higher than control) and phyaphyb2 (41.5% higher than control) mutants, while there were no differences between the WT and the triple mutant. Under RL, phyb2 had the lowest (compared to WT) content of phenolic compounds (0.69), while the double mutant had the highest content (1.3). The effect of BL was different in that in all the variants, the content of phenolic compounds increased relative to other types of light; however, the largest increase was in the phyb2 (2.5 times) and phyaphyb2 (2.1 times relative to control) mutants (Table 1).
3.3. Flavonoid Content
The increase in flavonoid content in comparison with the control was also observed in the phyb2 (by 65.9%) and double (by 34.1%) mutants under the WFL. RL caused an increase in flavonoids in the double mutant by an average of 72.9% relative to the other options. Exposure to BL resulted in a 2.8-fold increase in flavonoid content in phyb2 and a 2.2-fold increase in phyaphyb2 relative to the control and triple mutant (Table 1).
3.4. Measurements of CO2 Gas Exchange and Transpiration
When plants were irradiated with WFL and RL, the Pn values decreased in the order WT > phyb2 > phyaphyb2 > phyaphyb1phyb2. However, under BL conditions, the decrease in Pn changed somewhat: WT = phyb2 > phyaphyb2 > phyaphyb1phyb2.
The transpiration rates in the WFL variant were comparable for the WT and the double mutant 0.3–0.4 µM H2O m−2 s−1, while the rates for the triple and phyb2 mutant were significantly lower (0.1–0.2). In phyb2, transpiration under RL was 4 times higher than that under WFL; in other variants, RL caused a decrease in transpiration, but the greatest decrease was observed in the WT and the triple mutant, and in phyb2 and the double mutant, the values were comparable at 0.38–0.48. The greatest changes in transpiration were observed under BL, since in this case, transpiration was higher relative to other irradiation options. In addition, in the WT and the triple mutant, it was comparable (0.5), and its largest increase was in the phyb2 mutant (2.5 times higher than the WT control) (Table 1).
3.5. Determination of Photochemical Activity
The maximum quantum yield of PSII remained stable in the studied plants on WFL and BL, and a decrease in this indicator was observed under RL; the largest decrease was in the triple mutant (0.794). The effective quantum yield was the highest for the WFL double mutant (0.50), and under BL, the phyb2 mutant had the highest value (0.54), while the triple mutant had the lowest value of 0.34. RL caused a decrease in the Y(II) index, with the greatest decrease observed in the triple mutant (0.31); in other plants, the Y(II) index on BL was comparable. The nonphotochemical quenching indices were the lowest in the phyb2 mutant under BL (NPQ 0.63; Y(NPQ) 0.17), while in other variants, this indicator was significantly higher; for example, the NPQ in the WT under BL exceeded 1. The highest values of the Y(NPQ) indices were in the triple mutant under RL (0.33) (Table 2).

Table 2.
Influence of WFL, BL and RL on the main indicators fluorescence chlorophyll Y(II)—PSII effective quantum yield; NPQ—nonphotochemical fluorescence; Y(NO)—quantum yield of nonregulated nonphotochemical energy dissipation in PSII; Y(NPQ)—quantum yield of regulated nonphotochemical energy dissipation in PSII; and Fv/Fm—PSII maximum quantum yield in leaves from tomato mutant plants on the genes of major phytochromes. Different letters indicate significant differences (p < 0.05) between the experimental treatments.
3.6. Gene Expression
Initially, at the beginning of the experiment, the phyb2 mutant showed increased expression of the HY5 (8.3 times), PSBS (3.5 times), ANS (4.5 times), CHS (5.5 times), and Aurea (2.0 times) and BBX7 (almost 3 times) genes relative to the WT control. The transcript levels of the other genes changed insignificantly. The double mutant initially had a high level of expression for the PAL1 (by 9.2 times), BBX15, BBX16, BBX20, BBX21, BBX30 (by 2.5–3 times), and BBX7 (by 6.1 times) genes relative to the control WT. The transcript levels of other genes in the double mutant changed insignificantly. In the triple mutant, the expression of HY5, PSBS, PSY, PAL1, and BBX16 increased seven to nine times relative to the WT control. Additionally, the triple mutant initially had a high level of ANS, GLK1, and BBX30 expression (an average increase of 2.3 relative to the WT control) (Figure 1).
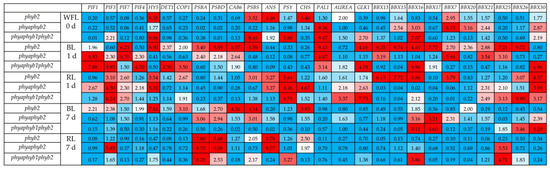
Figure 1.
Influence of WFL, BL and RL on the levels of the anthocyanin synthase genes ANS, phytoene synthase PSY, chalcone synthase CHS, transcription factor elongated hypocotyl 5 HY5, transcription factor phytochrome-interacting factors PIF1, PIF3, and PIF4, E3 ubiquitin–protein ligase COP1, phenylalanine ammonia-lyase 1 PAL1, chloroplastic photosystem II nonphotochemical quenching protein PSBS, de-etiolated1 DET1, photosystem II protein D1 psbA, photosystem II D2 protein psbD, chlorophyll ab binding protein 6A cab6, phytochromobilin synthase Aurea, golden 2-like 1 transcription factor GLK1, B-box domain containing proteins BBX13, BBX15, BBX16, BBX17, BBX7, BBX20, BBX21, BBX25, BBX26 and BBX30 effect on leaves of mutant tomato plants on genes for the major phytochromes, WFL 0 d—starting point experiment, 1 d—1 day of light exposure, and 7 d—seven days of exposure to light. The transcript levels were normalized to the expression of the Actin1 gene. The gene expression in the WT was used as one unit. Changes in expression were considered significant, with an increase (red light) or decrease (blue light) in expression by at least two times relative to the WT control.
During the first 24 h of irradiation, RL caused an increase in PIF3 expression in all the variants, in particular, in phyb2 by 3.1 times, in phyaphyb2 by 4.5 times, and in the triple mutant by 6.2 times relative to the WT. The expression of PIF1 under RL increased (by 2.6 times) only in phyb2 and did not change in the other mutants. The expression of PIF4 under RL slightly increased in the double mutant but did not change in the other variants. The level of HY5 transcripts under RL did not change in the triple mutant and increased by 3.5 times in phyb 2 and 5.3 times in phyaphyb2.
The expression of COP1 under RL increased by 2.7 times in phyb2 and did not change in the other mutants. The expression level of the Aurea gene under RL increased by 2.1 times in the double mutant and by 3.4 times in the triple mutant, while it did not change in the phyb2 mutant. The expression of BBX13, BBX15, and BBX16 increased under RL in phyb2 from 6.2 to 9.9 times. It is noteworthy that no increase in the expression of these genes was noted in the other mutants, with the exception of the triple mutant, in which the expression of the BBX16 gene increased twofold relative to the WT. The level of BBX17 transcripts decreased in all the variants under RL relative to the WT. The level of BBX7 transcripts increased (3.8 times) only in the phyb2 mutant under RL; simultaneously, the BBX30 expression was 3.1 to 4.6 times higher than the control in all the variants. The highest expression of BBX26 was observed in the triple mutant under RL. The expression levels of the study proteins PSII PSBA, PSBD, CAB6, and PSBS decreased in all the variants under RL, with the exception of a 3.0-fold increase in PSBS expression in phyb2 relative to the WT. The expression of ANS increased under RL by 3.3 times in phyb2 and the double mutant, while no changes in expression were noted in the triple mutant. The level of PSY transcripts increased under RL in all the variants, but the highest increase was in the triple mutant by more than nine times relative to the control. CHS expression increased 4.7 times only in the double mutant after 24 h of RL irradiation, and the expression level of the PAL1 gene did not change in all the variants under RL (Figure 1).
Under BL irradiation on the first day of the experiment, PIF1 expression increased by 8.9- and 7.9-fold in the double and triple mutants, respectively, but not in phyb2. The level of PIF3 transcripts under BL increased in the double mutant by 2.3, and in the triple mutant, it increased by 9.85 times relative to the control. The expression level of PIF7 was high in the phyb and phyaphyb2 mutants, while it did not change in the triple mutant. The expression level of PIF4 increased on the first day of BL irradiation in the double and triple mutants but decreased in phyb2. Under BL, HY5 expression increased only in the phyb2 mutant (by 8.9 times relative to the control). The expression of DET1 after 24 h under BL increased 2.4-fold in phyb2 and 4.5-fold in the triple mutant but decreased in the double mutant. The level of COP1 transcripts increased 2.0-fold in phyb2 and 3.5-fold in the phyaphyb1phyb2 mutant, while it decreased in the double mutant. The Aurea expression did not change under BL during the first day. The level of GLK1 transcripts increased under BL in the phyb2 and triple mutants by an average of 4.4 times but did not change in the double mutant. The expression of all the studied TF BBX genes, with the exception of BBX30, significantly increased in the phyb2 mutant. In the double mutant under BL, the expression of BBX13, BBX7, BBX21 and BBX25 increased, while the expression of the remaining BBX did not change. In addition, the expression of TF BBX16 was observed in the triple mutant, which was 9.6 times higher than the control, and BBX30 expression was almost 7 times higher than the control (8.7 times for phyb2 and 25.8 times for phyaphyb2) (Figure 1).
Seven days after the start of the experiment, in the phyb2 mutant under BL, the expression of the PIF1, PIF3, PIF4, COP1, psbD, and BBX20 genes remained at a level two to three times higher than that in the control. The expression of the CAB6, PSBS, and CHS genes was four times higher than that in the control, and the expression of the HY5 gene was 7.8 times higher. In the double mutant, after 7 days of exposure to BL, high expression was retained in the psbA, PSBD, PSBS, BBX16 and BBX30 genes (two to three times higher than WT), and the expression of BBX17 was 5.2 times higher than that of the WT. In the triple mutant on day 7 of the experiment, only the expression of the BBX16, BBX17, BBX26, and BBX30 genes remained three to nine times higher than that in the WT control. Under RL, after 7 days of the experiment, the phyb2 mutant retained expression only in PSBA, PSBD, PSBS, CHS, and ANS (two to seven times higher than the WT control). In the double mutant, on the 7th day of the experiment under RL, expression was preserved in PIF3, PSBS, PSBD, ANS, and BBX25 (from 3.5 to 9.7 times relative to the WT control). The triple mutant under RL retained the expression of PSBA, PSBD, PSBS, PSY, BBX16, and BBX25 on day 7 of the experiment (at two to eight times higher relative to the WT control) (Figure 1).
3.7. Scanning Electron Microscopy
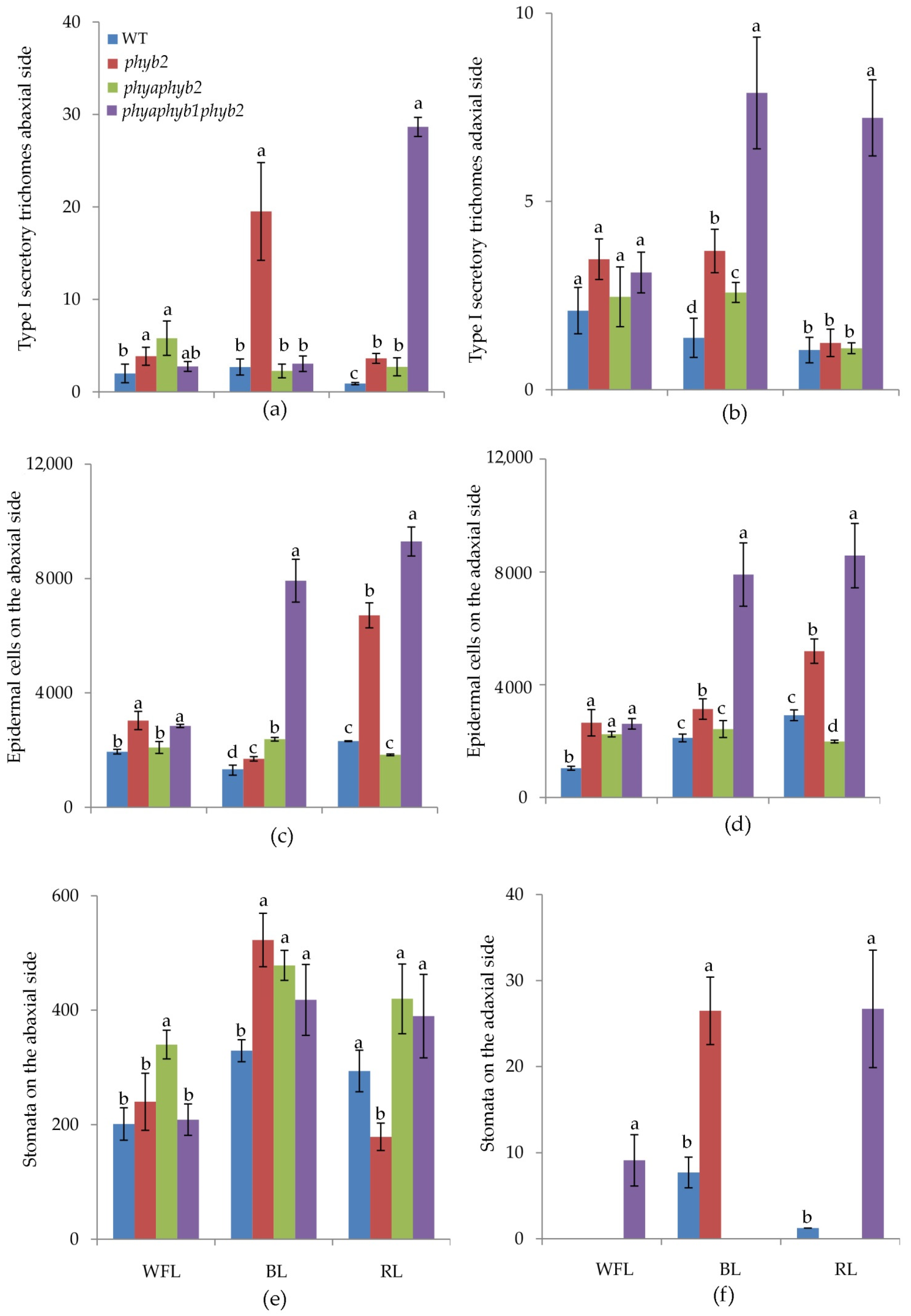
BL caused an increase in the number of trichomes of the first type in the phyb2 mutant on the abaxial side of the leaves (Figure 2a and Figure 3f); along with this increase, the number of trichomes also increased in the triple mutant, but they were underdeveloped (Figure 2a and Figure 3h).
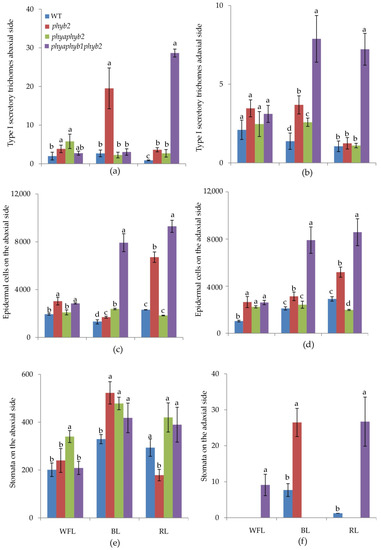
Figure 2.
Effect of WFL, BL, and RL on the number of type I secretory trichomes (a,b); number of epidermal cells (c,d) and number of stomata (e,f) per mm2 on abaxial (a,c,e) and adaxial (b,d,f) sides of leaves. Different letters indicate significant differences (p < 0.05) between the experimental treatments. The means ± standard errors, n = 6.
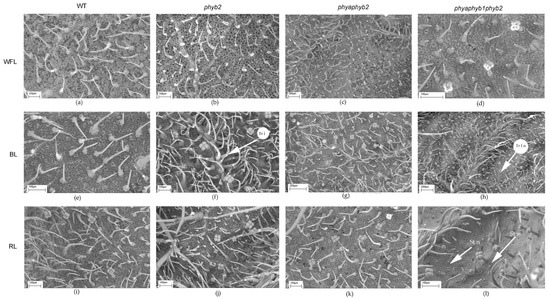
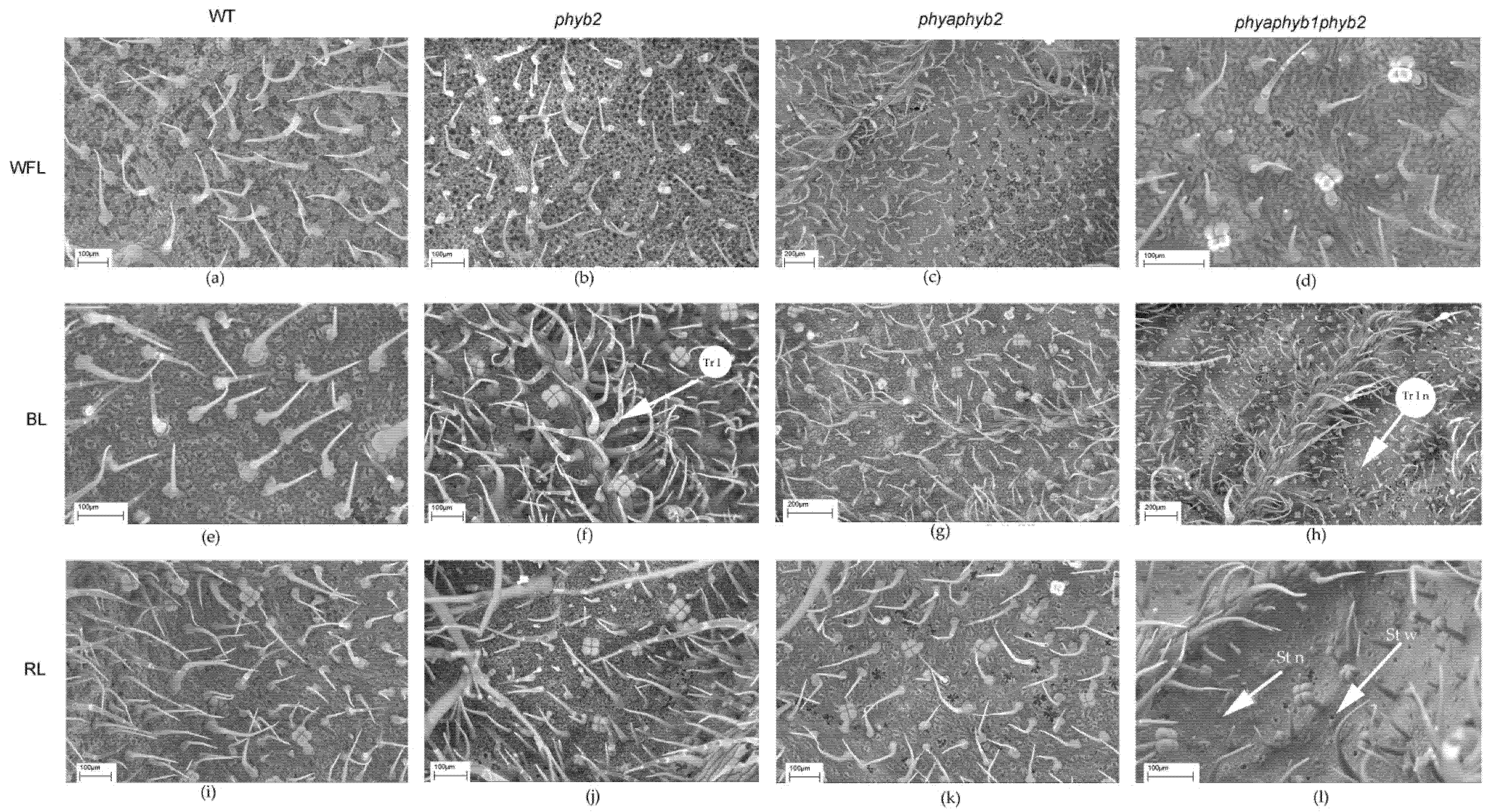
Figure 3.
SEM photograph of the abaxial surface of tomato leaf mutants for the main phytochrome genes: WT (a,e,i); phyb2 (b,f,j); phybaphyb2 (c,g,k) and phyaphyb1phyb2 (d,h,l) under the action of WFL (a–d), BL (e–h) and RL (i–l). Tr I—trichomes of type I; Tr I n—underdeveloped trichomes of type I; St n—underdeveloped stomata; and St w—stomata embedded in the epidermis and their guard cells were filled with deposits of epicuticular wax.
One of the features of the triple mutant was an increase in the number of epidermal cells on both sides of the leaf under BL and RL light (Figure 2c,d, Figure 3h,I and Figure 4h,i), while this increase was not observed under WFL (Figure 2c,d and Figure 4d).
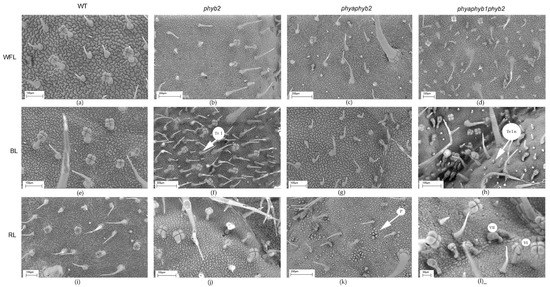
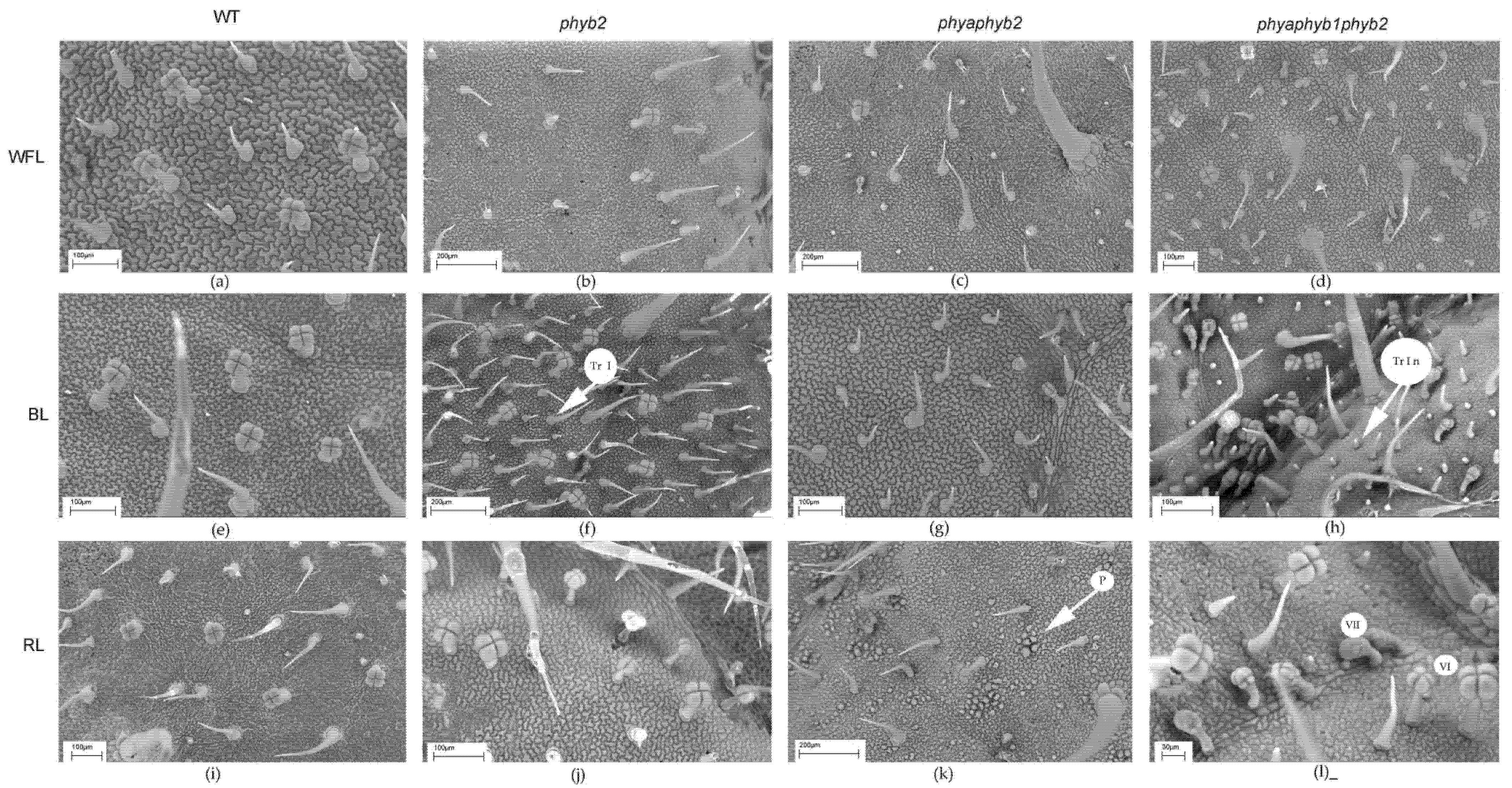
Figure 4.
SEM photograph showing the adaxial surface of tomato leaf mutants for the main phytochrome genes: WT (a,e,i); phyb2 (b,f,j); phybaphyb2 (c,g,k) and phyaphyb1phyb2 (d,h,l) under the action of WFL (a–d), BL (e–h) and RL (i–l). Tr I—trichomes of type I; Tr I n—underdeveloped trichomes of type I; VI—trichomes of type VI; VII—trichomes of type VII; and P—papillose formations on the outer surface of the main cells of the epidermis.
Light affected both the number of stomata and their location. Under BL, all mutants showed an increase in the number of stomata on the abaxial side of the leaves (Figure 2e and Figure 3f,g,h). Moreover, RL caused a decrease in the number of stomata in the phyb2 mutant (Figure 2e and Figure 3j), which was not observed in other plants. The triple mutant also had an increase in the number of stomata (Figure 2e), with many stomata being laid in the epidermis but remaining underdeveloped, especially under RL (Figure 3l). Another feature of the phyb2 mutant was the formation of a large number of stomata on the adaxial side of the leaf under BL (Figure 2f).
RL on the double mutant caused papillose formations on the outer surface of the main cells of the epidermis, which are characteristic only of this mutant (Figure 4k).
In the triple mutant, BL and RL caused an increase in the number of secretory multicellular trichome types III, VI, and VII (Figure 4h,l), which was not observed in the other plants. In addition, a large number of underdeveloped trichomes were observed in the triple mutant (Figure 3h,l and Figure 4h,l). Other stomata were embedded in the epidermis, and their guard cells were filled with deposits of epicuticular wax (Figure 3l).
4. Discussion
The RL receptor phytochrome interacts with a large amount of TF and is able to regulate photosynthesis, the formation of chloroplasts and the synthesis of photosynthetic pigments as well as the formation of secondary metabolites in the leaves and fruits of plants [24]. These components include TF PIF, HY5, COP1, DET1, BBX, GLK1 and many others. For example, TF HY5 regulates the accumulation of anthocyanin by directly binding to the promoters of genes to biosynthesize these pigments [25]. DET1 deficiency under high light conditions affects the accumulation of flavonoids in tomato plants [26]. In this work, we evaluated the effect of phytochrome deficiency on photosynthesis and the accumulation of low molecular weight antioxidants and the expression of light-dependent genes in tomato leaves exposed to red and blue light. In particular, it was important to understand how phytochromes and related TFs can be involved in regulating the content of phenolic compounds, primarily flavonoids, in tomato plants under RL and BL.
It is known that changes in the phytochrome system and its signaling components lead to rapid regulation of the expression of genes encoding photosynthetic proteins and antioxidant enzymes, which affect photosynthetic activity and the antioxidant status, changing the content of low-molecular weight antioxidants and antioxidant enzyme activity [7,8]. In addition, phytochrome-induced effects depend on the light quality in which plants grow [27].
Upon activation by light, PHY phosphorylates the COP1 E3 ubiquitin ligase, leading to its inactivation. In the light, TF HY5 (which is negatively regulated by COP1 in the dark) activates the expression of genes associated with photomorphogenesis as well as the biosynthesis of some secondary metabolites [25]. In fact, in phytochrome-deficiency mutants, we observed a change in the flavonoid content (Table 1). Additionally, the greatest changes were observed under BL in phyb2 and phyaphyb2. In addition, during the first 24 h under BL, phyb2 showed an increase in HY5 expression against the background of a decrease in the expression of PIF3, PIF4 and COP1, which did not occur in other variants (Figure 1). It is likely that a decrease in the phytochrome signaling in the phyb2 mutant under BL provides an enhancing of positive factors of photomorphogenesis, such as HY5 and GLK1, which not only activates the flavonoids biosynthesis but also increased the transpiration rate (Table 1). In addition, the phyb2 mutant under BL showed the maximum number of stomata, both on the lower and upper sides of the leaf, which also contributes to the activation of transpiration (Table 1; Figure 2, Figure 3 and Figure 4).
The observations made here are additionally confirmed by fluorescent parameters; in particular, the phyb2 mutant under BL showed the highest effective quantum yield (Y(II)) and the lowest nonphotochemical quenching NPQ and Y(NPQ) (Table 2). All this was accompanied by an increase in the expression of the main genes (psbA, psbD, CAB6 and psbS) of PSII proteins over the first 24 h of the experiment, which was not observed in all other variants (Figure 1). This increase probably indicates the plasticity of the photosynthetic apparatus of the phyb2 mutant, which is higher than in the WT.
The phytochrome family of tomato consists of five genes, PHYA, PHYC, and three members of the PHYB subfamily, PHYE and two paralogs of PHYB that have arisen through independent duplication (PHYB and PHYD in A. thaliana, PHYB1 and PHYB2 in S. lycopersicum). It is likely that PHYC and PHYE can partly replace key PHYA, PHYB1 and PHYB2 in the synthesis of phenolic compounds; furthermore, a difference in the content of these compounds between WT and mutants was not substantial. Under BL, the role of all phytochromes was reduced, and a difference between WT and mutants was more significant. In addition, the photosynthetic proteins D1 (psbA) and D2 (psbD), whose gene expression is important for the light-induced recovery of PSII, and the antenna complex gene (CAB6), which is important for the functioning of the antenna Chl–protein complex, were minimum in a triple mutant grown under RL and especially BL. It is likely that this is one of the main reasons for the strong decrease in photosynthetic activity in a triple mutant with the deficiency of key phytochromes (PHYA, PHYB1 and PHYB2). We can see that PHYB1 plays a key role in this decrease. It is likely that phytochromes can affect photosynthesis by the control of photosynthetic proteins.
Apparently, TF BBX in the phyb2 mutant can be a positive regulator of photomorphogenesis and it can be the gene involved in the primary response to changes in the quality of light, triggering the biosynthesis of flavonoids and activating photosynthesis and antioxidant potential in the phyb2 mutant under BL. This observation is consistent with the fact that when plants are irradiated with TFs, BBX can bind to the promoter regions of HY5 and activate its transcription [24]. Moreover, BBX21 and HY5 can bind to the promoters of other BBX genes and activate their transcription [28]. Thus, BBX and HY5 TFs form a regulatory cluster capable of controlling plant photomorphogenesis. In this study, we were able to detect an increase in the expression of several key BBXs under BL in the phyb2 mutant. Thus, within the first 24 h of the experiment, the levels of BBX13, BBX15, BBX16, BBX17, BBX7, BBX20, BBX21, BBX25, and BBX26 transcripts increased from two to nine times relative to the WT control (Figure 1). It should be noted that the expression of only one gene, BBX30, decreased in the phyb2 mutant. In addition, the expression of BBX30 increased 7-fold relative to the WT in the triple mutant (Figure 1). In addition, we observed an increase in the expression of PAL1 under BL, and it is one of the main enzymes required for flavonoid synthesis (there was a 9-fold increase in the expression in phyb2 relative to the WT) (Figure 1). We hypothesize that BBX, directly or indirectly through HY5, can upregulate PAL1 expression under BL.
A question arises as to what can affect the activation of BBX itself under BL conditions when phytochrome signaling is reduced. Previously, it was shown that BBX4, BBX23 and BBX29 in tomato plants were only expressed in response to RL, while BBX7, BBX13 and BBX25 were expressed in response to FRL [29]. These results indicate that BBX proteins can directly interact with phytochrome photoreceptors. In addition, PHYB was found to directly interact with BBX and positively regulate its accumulation under RL in A. thaliana [30], indicating that photoreceptors can directly control some BBX. In our experiments, we were able to show that PHYB1 is a receptor that is involved in light signaling, which determines the biosynthesis of flavonoids under BL conditions, because the deficiency of these phytochromes in the triple mutant leads to a decrease in the amount of phenolic compounds and flavonoids (Table 1). It is also known that TF GLK may be involved in the PHY-mediated regulation of BBX, which is confirmed by the presence of binding domains in the promoter regions of the BBX, HY5, and PIF genes [10]. Interestingly, in our experiments, the highest expression of GLK1 was observed in the triple mutant under RL against the background of increasing expression of BBX21, BBX25, BBX26, BBX30, and PIF3 (Figure 1). However, the amount of flavonoids in this variant did not increase (Table 1). It can be assumed that BBX21, BBX25, BBX26, and BBX30 are weakly involved in flavonoid biogenesis in the triple mutant. This assumption is supported by the fact that BBX25 expression was preserved by day 7 only in the triple mutant under RL and BL, and the contents of flavonoids was reduced (Figure 1, Table 1).
Most of the flavonoids contained in the tomato leaf are synthesized in special secretory cells called secretory trichomes [31]. The tomato has several types of secretory trichomes (types 1, 6 and 7), which are most often located on the abaxial side of the leaves. Confirming our results, under BL, the highest number of type 1 secretory trichomes was found in phyb2 mutants on the abaxial side of the leaves (Figure 2). Additionally, a large number of secretory trichomes were found in the triple mutant under RL; however, most of them were underdeveloped and most likely not able to accumulate flavonoids (Figure 3). We suggest that trichomes in the phyb2 mutant may be one of the possible sites for the accumulation of flavonoids. In addition, the double mutant under RL showed papillose formations of epidermal cells on the adaxial side of the leaves, which were not found in any other mutant (Figure 4). It can be assumed that this is one of the possible mechanisms for reducing the penetration of RL into the leaf mesophyll. The change in epidermal cell size in the double mutant could theoretically indicate the involvement of PHYA in cell elongation, which occurs due to a change in the ratio of the main phytohormones [32,33]. This assumption is supported by data on the number of epidermal cells. Thus, the highest number of cells on both the abaxial and adaxial sides of the leaves was found in the triple mutant, and their number was four times higher than that in the WT and other mutants. This result indicates a change in the hormonal status of the triple mutant and a possible increase in the level of cytokinins relative to auxins [34].
The content of flavonoids in our experiments correlates with the antioxidant capacity of leaf extracts (r = 0.99, data not shown). Before starting the main experiments, we performed a screening analysis of a large group of tomato light-receptor mutants under conditions of light of different spectral composition. The results obtained are presented in Table S2. It was shown that blue light that had the greatest effect on the accumulation of flavonoids in leaves, and phyb1 mutations led to a decrease in the flavonoids content. The highest nonenzymatic activity was observed in the phyb2 mutant under BL (Table 1). BBX proteins also play a vital role in the regulatory networks that control plant adaptations to abiotic stress. Previous studies have shown that both BBX5 and BBX21 positively regulate plant tolerance to drought and salt stress in A. thaliana [35]. We hypothesize that the resistance of plants in previous studies is due precisely to the increased content of flavonoids and that phyb2 plants grown on BL will also be more resistant to some abiotic stresses. Thus, PHYB1 and PHYB2 seem to play different roles under conditions of sufficiently high BL intensity. CRY1 plays an important role in the resistance of plants grown under BL to high-intensity light and UV-B [36]. It can be assumed that PHYB1 and PHYB2 in tomato plants are also important elements of this resistance.
We assume that the obtained results can be used to obtain new more resistant tomato varieties for outdoor cultivation. We also assume that it is possible to create conditions for the translocation of flavonoids from leaves into tomato fruits, which will allow creating products with increased nutritional value.
5. Conclusions
BL had the greatest effect on the accumulation of flavonoids and phenolic compounds in the phyb2 mutant, which was confirmed by an increase in the TEAC of the leaves extracts. We suggest that the observed phenomena are based on the regulation of the expression of components phytochrome signaling genes including TF HY5 and BBX. This expression is markedly reduced in PHYB1 deficiency but increased upon PHYB2 deficiency under BL conditions. We believe that PHYB1 is a key phytochrome because its deficiency in the triple mutant leads to a maximum decrease, low-molecular weight antioxidant capacity represented by flavonoids and also in photosynthetic activity under BL.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11213437/s1, Figure S1: Emission spectra of the light sources used in the experiments; Table S1: Primers for qRT-PCR analysis; Table S2: Screening study of the Solanum lycopersicum main photoreceptors mutants for TEAC (µmol Trolox/g FW), GAE (mg/g FW) and flavonoids (mg catechin/g FW) content under conditions of light of different spectral composition: WFL (white fluorescent lamps with a set of peaks in the visible spectral region), BL (blue light with a peak at 450 nm), RL (red light with a peak at 660 nm), GL (green light with a peak at 525 nm) and WL (blue + red with two maxima at 445 nm and 660 nm).
Author Contributions
Conceptualization, P.P. and M.V.; methodology, P.P., V.K. (Vladimir Kreslavskiand), Y.I., T.K., A.R., A.V., A.K.; software, P.P., M.V., Y.I., A.R.; validation, P.P., Y.I. and M.V.; formal analysis, V.K. (Vladimir Kreslavskiand); investigation P.P., V.K. (Vladimir Kreslavskiand). Y.I., T.K., A.R., A.V., A.K.; resources, P.P. V.K. (Vladimir Kuznetsov), S.I.A.; data curation, V.K. (Vladimir Kuznetsov), S.I.A.; writing—original draft preparation, P.P., M.V., V.K. (Vladimir Kreslavskiand), V.K. (Vladimir Kuznetsov); writing—review and editing, P.P., V.K. (Vladimir Kreslavskiand), V.K. (Vladimir Kuznetsov); visualization, P.P., M.V., Y.I.; supervision, P.P., V.K. (Vladimir Kreslavskiand), V.K. (Vladimir Kuznetsov), S.I.A.; project administration, P.P., V.K. (Vladimir Kreslavskiand), V.K. (Vladimir Kuznetsov), S.I.A.; funding acquisition, P.P., V.K. (Vladimir Kreslavskiand), V.K. (Vladimir Kuznetsov), S.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, grants numbers: 122042700044-6, 122050400128-1, 122041100071-1, and 122042500074-5. The data in Table 2 and values of Pn in Table 1 were obtained with partial financial support by the Russian Science Foundation (Russian Federation, Project no. 22-74-10086).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors express their gratitude to the dispatching service of the K.A. Timiryazev Institute of Plant Physiology, as represented by Dmitry Kuznetsov, for their help in growing the plants for these experiments as well as to the student of the Tomsk State University Department of Biology, Ecology, Soil Science, Agriculture and Forestry Ksenia Pak for help in obtaining data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Lupi, A.C.D.; Lira, B.S.; Gramegna, G.; Trench, B.; Alves, F.R.R.; Demarco, D.; Peres, L.E.P.; Purgatto, E.; Freschi, L.; Rossi, M. Solanum lycopersicum GOLDEN 2-LIKE 2 transcription factor affects fruit quality in a light-and auxin-dependent manner. PLoS ONE 2019, 14, e0212224. [Google Scholar] [CrossRef]
- Song, Y.; Yang, C.; Gao, S.; Zhang, W.; Li, L.; Kuai, B. Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol. Plant. 2014, 7, 1776–1787. [Google Scholar] [CrossRef]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; He, K.; Stolc, V.; Lee, H.; Figueroa, P.; Gao, Y.; Tongprasit, W.; Zhao, H.; Lee, I.; Deng, X.W. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007, 19, 731–749. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. BBA-Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Monteiro, C.C.; Campos, M.L.; Melo, H.C.; Carvalho, R.F. Phytochromes are key regulators of abiotic stress responses in tomato. Sci. Hortic. 2017, 222, 126–135. [Google Scholar] [CrossRef]
- Kiełbowicz-Matuk, A. Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci. 2012, 185, 78–85. [Google Scholar] [CrossRef]
- Lira, B.S.; Oliveira, M.J.; Shiose, L.; Wu, R.T.A.; Rosado, D.; Lupi, A.C.D.; Freschi, L.; Rossi, M. Light and ripening-regulated BBX protein-encoding genes in Solanum lycopersicum. Sci. Rep. 2020, 10, 19235. [Google Scholar] [CrossRef]
- Song, Z.; Bian, Y.; Liu, J.; Sun, Y.; Xu, D. B-box proteins: Pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020, 62, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Holm, M.; Deng, X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 7655–7660. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Crocco, C.D.; Johansson, H.; Datta, S.; Hettiarachchi, C.; Holm, M.; Botto, J.F. The Arabidopsis B-box protein BBX25 interacts with HY5, negatively regulating BBX22 expression to suppress seedling photomorphogenesis. Plant Cell 2013, 25, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Jiang, Y.; Li, J.; Yan, T.; Fan, L.; Liang, J.; Chen, Z.J.; Xu, D.; Deng, X.W. B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell 2018, 30, 2006–2019. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, R. Light signaling differentially regulates the expression of group IV of the B-box zinc finger family. Plant Signal. Behav. 2017, 12, e1365213. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Strokina, V.V.; Pashkovskiy, P.P.; Balakhnina, T.I.; Voloshin, R.A.; Alwasel, S.; Kosobryukhov, A.A.; Allakhverdiev, S.I. Deficiencies in phytochromes A and B and cryptochrome 1 affect the resistance of the photosynthetic apparatus to high-intensity light in Solanum lycopersicum. J. Photochem. Photobiol. B 2020, 210, 111976. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Vankova, R.; Zlobin, I.E.; Dobrev, P.; Kartashov, A.V.; Ivanova, A.I.; Ivanov, V.P.; Marchenko, S.I.; Nartov, D.I.; Ivanov, Y.V.; et al. Hormonal responses to short-term and long-term water deficit in native Scots pine and Norway spruce trees. Environ. Exp. Bot. 2022, 195, 104789. [Google Scholar] [CrossRef]
- Stetsenko, L.A.; Pashkovsky, P.P.; Voloshin, R.A.; Kreslavski, V.D.; Kuznetsov, V.V.; Allakhverdiev, S.I. Role of anthocyanin and carotenoids in the adaptation of the photosynthetic apparatus of purple-and green-leaved cultivars of sweet basil (Ocimum basilicum) to high-intensity light. Photosynthetica 2020, 58, 890–901. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence: A Signature of Photosynthesis, 1st ed.; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 279–319. [Google Scholar] [CrossRef]
- Rosado, D.; Gramegna, G.; Cruz, A.; Lira, B.S.; Freschi, L.; de Setta, N.; Rossi, M. Phytochrome Interacting Factors (PIFs) in Solanum lycopersicum: Diversity, evolutionary history and expression profiling during different developmental processes. PLoS ONE 2016, 11, e0165929. [Google Scholar] [CrossRef]
- Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020, 228, 1748–1753. [Google Scholar] [CrossRef]
- Liu, C.C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.Q.; Zhou, Y.H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.; Khudyakova, A.; Ashikhmin, A.; Bolshakov, M.; Kozhevnikova, A.; Kosobryukhov, A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Effect of high-intensity light on the photosynthetic activity, pigment content and expression of light-dependent genes of photomorphogenetic Solanum lycopersicum hp mutants. Plant Physiol. Biochem. 2021, 167, 91–100. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Ryazansky, S.; Kartashov, A.; Voloshin, R.; Khudyakova, A.; Kosobryukhov, A.A.; Kreslavski, V.D.; Kuznetsov, V.V.; Allakhverdiev, S.I. Effect of red light on photosynthetic acclimation and the gene expression of certain light signaling components involved in the microRNA biogenesis in the extremophile Eutrema salsugineum. J. Biotechnol. 2021, 325, 35–42. [Google Scholar] [CrossRef]
- Zhao, X.; Heng, Y.; Wang, X.; Deng, X.W.; Xu, D. A positive feedback loop of BBX11-BBX21-HY5 promotes photomorphogenic development in Arabidopsis. Plant Commun. 2020, 1, 100045. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Yan, J.; Zhang, Y.; Zhou, S.; Sun, X.; Yang, Y.; Ahammed, G.J.; Liu, Y.; Qi, M.; et al. Genome-wide characterization of B-box gene family and its roles in responses to light quality and cold stress in tomato. Front. Plant Sci. 2021, 12, 698525. [Google Scholar] [CrossRef]
- Heng, Y.; Lin, F.; Jiang, Y.; Ding, M.; Yan, T.; Lan, H.; Zhou, H.; Zhao, X.; Xu, D.; Deng, X.W. B-box containing proteins BBX30 and BBX31, acting downstream of HY5, negatively regulate photomorphogenesis in Arabidopsis. Plant Physiol. 2019, 180, 497–508. [Google Scholar] [CrossRef]
- McDowell, E.T.; Kapteyn, J.; Schmidt, A.; Li, C.; Kang, J.H.; Descour, A.; Shi, F.; Larson, M.; Schilmiller, A.; An, L.; et al. Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 2010, 155, 524–539. [Google Scholar] [CrossRef]
- Alallaq, S.; Ranjan, A.; Brunoni, F.; Novák, O.; Lakehal, A.; Bellini, C. Red light controls adventitious root regeneration by modulating hormone homeostasis in Picea abies seedlings. Front. Plant Sci. 2020, 11, 586140. [Google Scholar] [CrossRef]
- Bantis, F.; Panteris, E.; Dangitsis, C.; Carrera, E.; Koukounaras, A. Blue light promotes vascular reconnection, while red light boosts the physiological response and quality of grafted watermelon seedlings. Sci. Rep. 2021, 11, 21754. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells 2021, 10, 3284. [Google Scholar] [CrossRef]
- Nagaoka, S.; Takano, T. Salt tolerance-related protein STO binds to a Myb transcription factor homologue and confers salt tolerance in Arabidopsis. J. Exp. Bot. 2003, 54, 2231–2237. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Khudyakova, A.Y.; Strokina, V.V.; Shirshikova, G.N.; Pashkovskiy, P.P.; Balakhnina, T.I.; Kosobryukhov, A.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Impact of high irradiance and UV-B on the photosynthetic activity, pro-/antioxidant balance and expression of light-activated genes in Arabidopsis thaliana hy4 mutants grown under blue light. Plant Physiol. Biochem. 2021, 167, 153–162. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).